Abstract
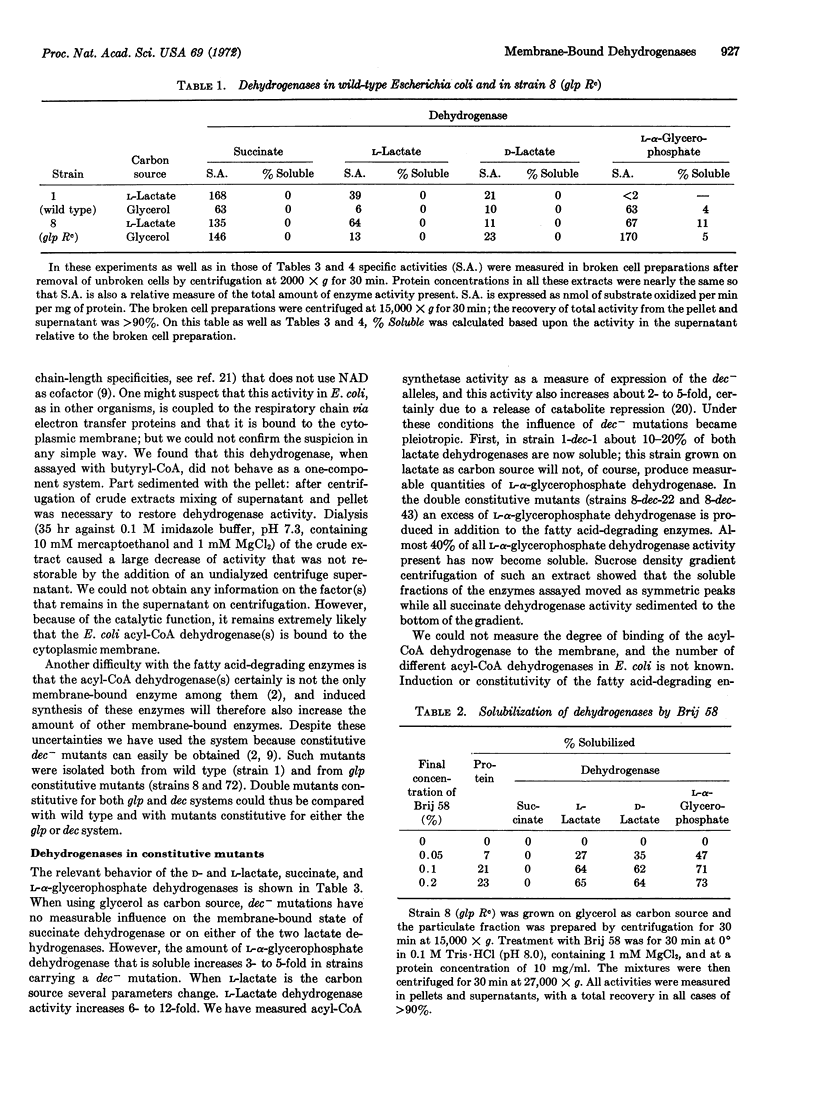
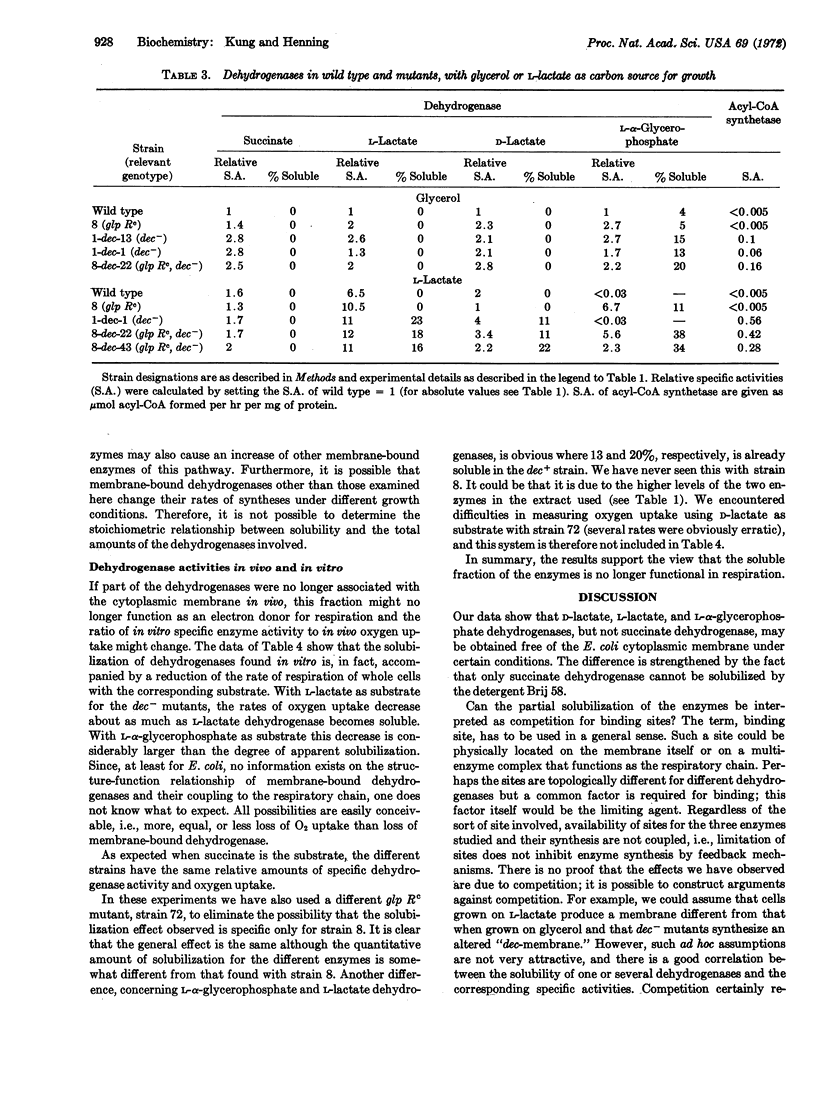
Experiments are reported that demonstrate that in E. coli the pyridine nucleotide-independent D- and L-lactate dehydrogenases and the aerobic L-α-glycerophosphate dehydrogenase are membrane bound. These enzymes differed from succinate dehydrogenase in that they could be solubilized by treatment with nonionic detergent while succinate dehydrogenase could not. The binding of these enzymes to membrane was measured in mutants constitutive for the synthesis of various dehydrogenases: in cells in which the amount of dehydrogenases synthesized was greater than in others, the enzymes described above (except succinate dehydrogenase) were found in part in the soluble fraction of the cell extracts. Experiments of oxygen uptake indicate that when a fraction of the enzymes became soluble, this soluble fraction is no longer functional in respiration. These results indicate that it is possible to prevent membrane attachment of certain dehydrogenases by the excess production of other dehydrogenases; it may be that dehydrogenases compete for identical binding sites.
Keywords: acyl-CoA, glycerophosphate, lactate, succinate, respiration
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARRIGONI O., SINGER T. P. Limitations of the phenazine methosulphate assay for succinic and related dehydrogenases. Nature. 1962 Mar 31;193:1256–1258. doi: 10.1038/1931256a0. [DOI] [PubMed] [Google Scholar]
- Barnes E. M., Jr, Kaback H. R. Beta-galactoside transport in bacterial membrane preparations: energy coupling via membrane-bounded D-lactic dehydrogenase. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1190–1198. doi: 10.1073/pnas.66.4.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boos W., Schaedel P., Wallenfels K. Untersuchungen zur Induktion der Lac-Enzyme. 1. Induktionswirkung und Permeation. Eur J Biochem. 1967 Jun;1(4):382–394. doi: 10.1111/j.1432-1033.1967.tb00086.x. [DOI] [PubMed] [Google Scholar]
- Courtright J. B., Henning U. Malate dehydrogenase mutants in Escherichia coli K-12. J Bacteriol. 1970 Jun;102(3):722–728. doi: 10.1128/jb.102.3.722-728.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzarelli N. R., Freedberg W. B., Lin E. C. Genetic control of L-alpha-glycerophosphate system in Escherichia coli. J Mol Biol. 1968 Feb 14;31(3):371–387. doi: 10.1016/0022-2836(68)90415-4. [DOI] [PubMed] [Google Scholar]
- Fox C. F. A lipid requirement for induction of lactose transport in Escherichia coli. Proc Natl Acad Sci U S A. 1969 Jul;63(3):850–855. doi: 10.1073/pnas.63.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAUGAARD N. D- and L-lactic acid oxidases of Escherichia coli. Biochim Biophys Acta. 1959 Jan;31(1):66–72. doi: 10.1016/0006-3002(59)90439-1. [DOI] [PubMed] [Google Scholar]
- Hennaut C., Hilger F., Grenson M. Space limitation for permease insertion in the cytoplasmic membrane of Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1970 May 22;39(4):666–671. doi: 10.1016/0006-291x(70)90257-3. [DOI] [PubMed] [Google Scholar]
- Kaback H. R., Milner L. S. Relationship of a membrane-bound D-(-)-lactic dehydrogenase to amino acid transport in isolated bacterial membrane preparations. Proc Natl Acad Sci U S A. 1970 Jul;66(3):1008–1015. doi: 10.1073/pnas.66.3.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein K., Steinberg R., Fiethen B., Overath P. Fatty acid degradation in Escherichia coli. An inducible system for the uptake of fatty acids and further characterization of old mutants. Eur J Biochem. 1971 Apr;19(3):442–450. doi: 10.1111/j.1432-1033.1971.tb01334.x. [DOI] [PubMed] [Google Scholar]
- Kline E. S., Mahler H. R. The lactic dehydrogenases of E. coli. Ann N Y Acad Sci. 1965 Jul 31;119(3):905–919. doi: 10.1111/j.1749-6632.1965.tb47451.x. [DOI] [PubMed] [Google Scholar]
- LIN E. C., KOCH J. P., CHUSED T. M., JORGENSEN S. E. Utilization of L-alpha-glycerophosphate by Escherichia coli without hydrolysis. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2145–2150. doi: 10.1073/pnas.48.12.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overath P., Pauli G., Schairer H. U. Fatty acid degradation in Escherichia coli. An inducible acyl-CoA synthetase, the mapping of old-mutations, and the isolation of regulatory mutants. Eur J Biochem. 1969 Feb;7(4):559–574. [PubMed] [Google Scholar]
- Overath P., Raufuss E. M. The induction of the enzymes of fatty acid degradation in Escherichia coli. Biochem Biophys Res Commun. 1967 Oct 11;29(1):28–33. doi: 10.1016/0006-291x(67)90535-9. [DOI] [PubMed] [Google Scholar]
- REED L. J., LEACH F. R., KOIKE M. Studies on a lipoic acid-activating system. J Biol Chem. 1958 May;232(1):123–142. [PubMed] [Google Scholar]
- Reed L. J., Oliver R. M. The multienzyme alpha-keto acid dehydrogenase complexes. Brookhaven Symp Biol. 1968 Jun;21(2):397–412. [PubMed] [Google Scholar]
- SEDAR A. W., BURDE R. M. LOCALIZATION OF THE SUCCINIC DEHYDROGENASE SYSTEM IN ESCHERICHIA COLI USING COMBINED TECHNIQUES OF CYTOCHEMISTRY AND ELECTRON MICROSCOPY. J Cell Biol. 1965 Feb;24:285–295. doi: 10.1083/jcb.24.2.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. Location of the maltose A and B loci on the genetic map of Escherichia coli. J Bacteriol. 1966 Oct;92(4):1083–1089. doi: 10.1128/jb.92.4.1083-1089.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showe M. K., DeMoss J. A. Localization and regulation of synthesis of nitrate reductase in Escherichia coli. J Bacteriol. 1968 Apr;95(4):1305–1313. doi: 10.1128/jb.95.4.1305-1313.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarmy E. M., Kaplan N. O. Kinetics of Escherichia coli B D-lactate dehydrogenase and evidence for pyruvate-controlled change in conformation. J Biol Chem. 1968 May 25;243(10):2587–2596. [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Weeks G., Shapiro M., Burns R. O., Wakil S. J. Control of fatty acid metabolism. I. Induction of the enzymes of fatty acid oxidation in Escherichia coli. J Bacteriol. 1969 Feb;97(2):827–836. doi: 10.1128/jb.97.2.827-836.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



