Abstract
From (ϕ,Ψ) data for the middle amino acid, n, in the series of tripeptides (n - 1)(n)(n + 1) in six proteins whose three-dimensional structure is known from x-ray studies, (ϕ,Ψ) values for the residues 2-107 of kappa Bence Jones proteins and immunoglobulin light chains were computed. It was assumed that all residues except those in the hypervariable regions 24-34, 50-56, and 89-97, were involved essentially in three-dimensional structure, and hence, their (ϕ,Ψ) angles would mostly be those best satisfying all kappa tri- and constituent di-peptides that occur at those positions. A set of criteria for selecting and averaging (ϕ,Ψ) angles is given. In the hypervariable regions, which are presumed to be involved in site complementarity, the (ϕ,Ψ) angles were selected for a single Bence Jones protein Ag. The three-dimensional models, constructed from the computed (ϕ,Ψ) values by hand and by display computer were very similar, and residues 23 and 88 were sufficiently close for the disulfide bond to form.
Keywords: Bence Jones proteins; tripeptides; (ϕ,Ψ) angles
Full text
PDF
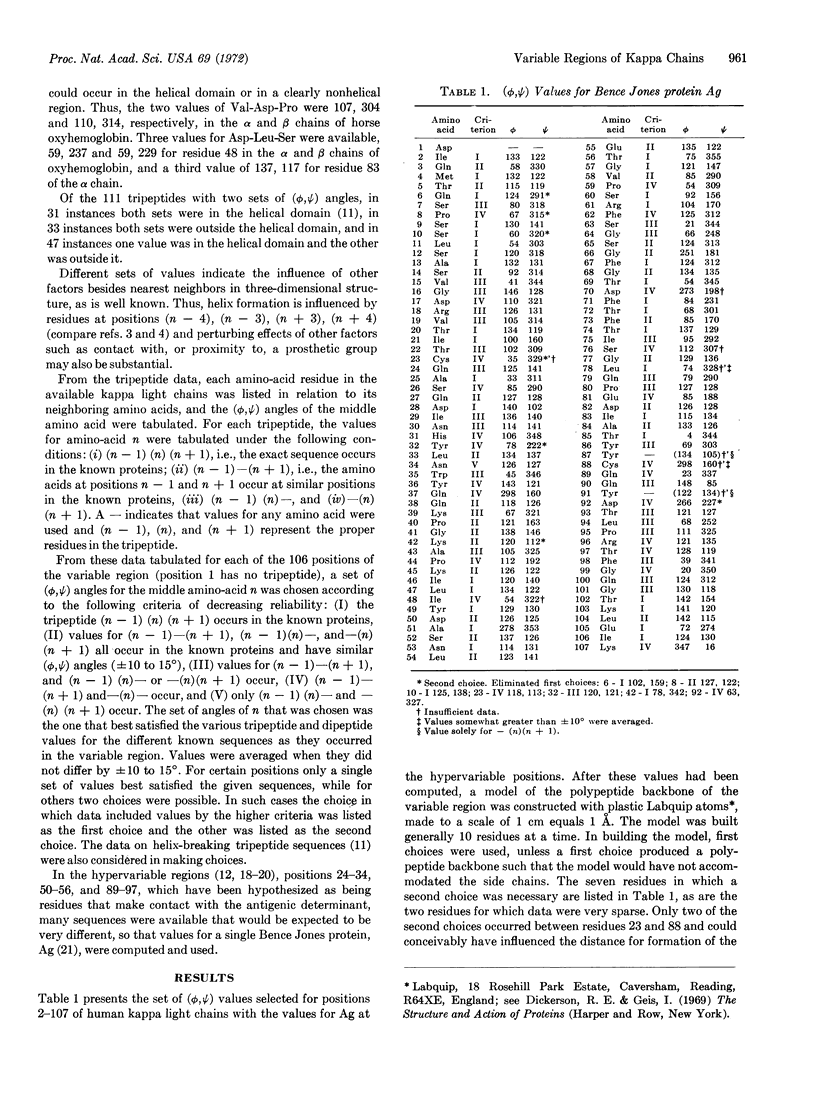
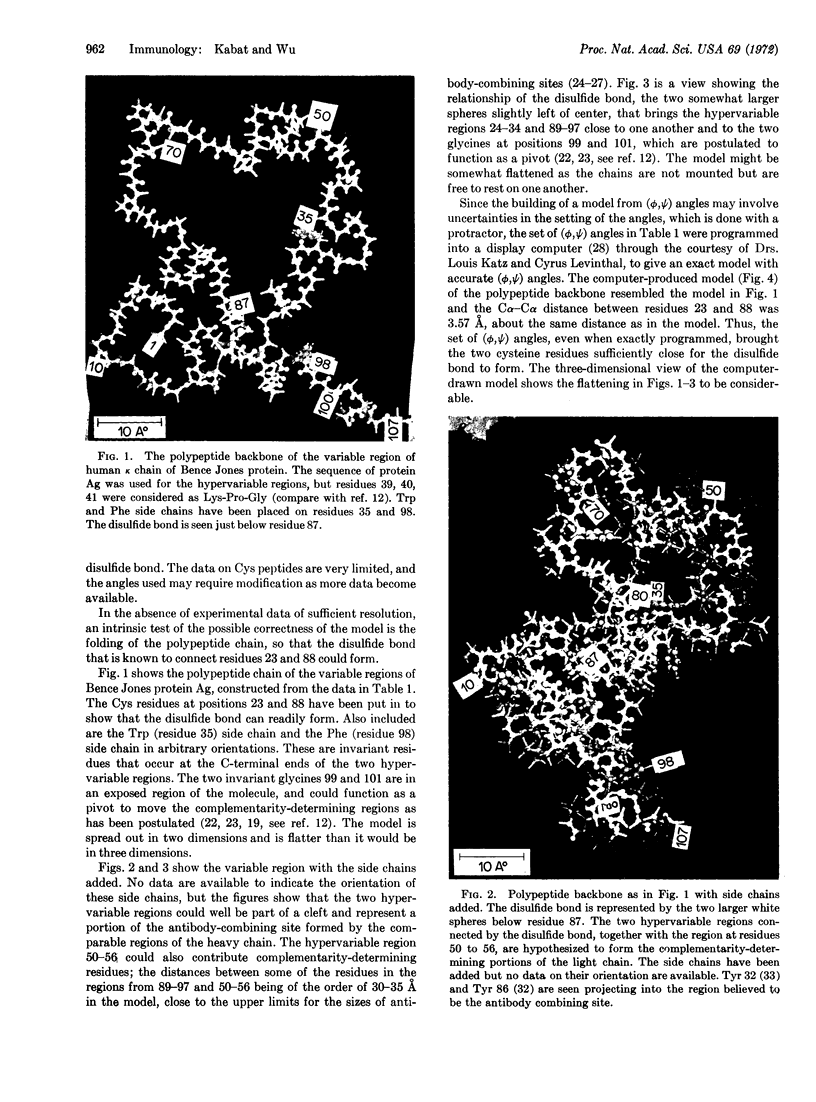


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birktoft J. J., Matthews B. W., Blow D. M. Atomic co-ordinates for tosyl-alpha-chymotrypsin. Biochem Biophys Res Commun. 1969 Jul 7;36(1):131–137. doi: 10.1016/0006-291x(69)90659-7. [DOI] [PubMed] [Google Scholar]
- Björk I., Karlsson F. A., Berggård I. Independent folding of the variable and constant halves of a lambda immunoglobulin light chain. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1707–1710. doi: 10.1073/pnas.68.8.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake C. C., Mair G. A., North A. C., Phillips D. C., Sarma V. R. On the conformation of the hen egg-white lysozyme molecule. Proc R Soc Lond B Biol Sci. 1967 Apr 18;167(1009):365–377. doi: 10.1098/rspb.1967.0034. [DOI] [PubMed] [Google Scholar]
- Frommel D., Litman G. W., Terry W. D., Good R. A., Rosenberg A. Conformational differences of immunoglobulin G subclasses. Biochim Biophys Acta. 1970 Nov 17;221(2):399–402. doi: 10.1016/0005-2795(70)90287-4. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., Metzger H. Affinity labeling of a mouse myeloma protein which binds nitrophenyl ligands. Kinetics of labeling and isolation of a labeled peptide. Biochemistry. 1970 Mar 3;9(5):1267–1278. doi: 10.1021/bi00807a031. [DOI] [PubMed] [Google Scholar]
- Goodman J. W. Immunochemical specificity: recent conceptual advances. Immunochemistry. 1969 Jan;6(1):139–149. doi: 10.1016/0019-2791(69)90185-2. [DOI] [PubMed] [Google Scholar]
- Guzzo A. V. The influence of amino-acid sequence on protein structure. Biophys J. 1965 Nov;5(6):809–822. doi: 10.1016/S0006-3495(65)86753-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirgensons B., Saine S., Ross D. L. The ultraviolet rotatory dispersion and conformation of Bence-Jones proteins. J Biol Chem. 1966 May 25;241(10):2314–2319. [PubMed] [Google Scholar]
- Kabat E. A. Heterogeneity and structure of antibody-combining sites. Ann N Y Acad Sci. 1970 Feb 13;169(1):43–54. doi: 10.1111/j.1749-6632.1970.tb55978.x. [DOI] [PubMed] [Google Scholar]
- Kabat E. A. The nature of an antigenic determinant. J Immunol. 1966 Jul;97(1):1–11. [PubMed] [Google Scholar]
- Kabat E. A. Unique features of the variable regions of Bence Jones proteins and their possible relation to antibody complementarity. Proc Natl Acad Sci U S A. 1968 Feb;59(2):613–619. doi: 10.1073/pnas.59.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotelchuck D., Scheraga H. A. The influence of short-range interactions on protein onformation. II. A model for predicting the alpha-helical regions of proteins. Proc Natl Acad Sci U S A. 1969 Jan;62(1):14–21. doi: 10.1073/pnas.62.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinthal C. Molecular model-building by computer. Sci Am. 1966 Jun;214(6):42–52. doi: 10.1038/scientificamerican0666-42. [DOI] [PubMed] [Google Scholar]
- Lipscomb W. N., Reeke G. N., Jr, Hartsuck J. A., Quiocho F. A., Bethge P. H. The structure of carboxypeptidase A. 8. Atomic interpretation at 0.2 nm resolution, a new study of the complex of glycyl-L-tyrosine with CPA, and mechanistic deductions. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):177–214. doi: 10.1098/rstb.1970.0020. [DOI] [PubMed] [Google Scholar]
- Litman G. W., Good R. A., Frommel D., Rosenberg A. Conformational significance of the intrachain disulfide linkages in immunoglobulins. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1085–1092. doi: 10.1073/pnas.67.3.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B. W., Lovell F. M., Rudko A. D. Prediction of alpha-helical regions in proteins of known sequence. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1519–1526. doi: 10.1073/pnas.60.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pain R. H., Robson B. Analysis of the code relating sequence to secondary structure in proteins. Nature. 1970 Jul 4;227(5253):62–63. doi: 10.1038/227062a0. [DOI] [PubMed] [Google Scholar]
- Periti P. F., Quagliarotti G., Liquori A. M. Recognition of alpha-helical segments in proteins of known primary structure. J Mol Biol. 1967 Mar 14;24(2):313–322. doi: 10.1016/0022-2836(67)90336-1. [DOI] [PubMed] [Google Scholar]
- Poljak R. J., Amzel L. M., Avey H. P., Becka L. N. Structure of Fab' New at 6 A resolution. Nat New Biol. 1972 Feb 2;235(57):137–140. doi: 10.1038/newbio235137a0. [DOI] [PubMed] [Google Scholar]
- Prothero J. W. Correlation between the distribution of amino acids and alpha helices. Biophys J. 1966 May;6(3):367–370. doi: 10.1016/S0006-3495(66)86662-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptitsyn O. B. Statistical analysis of the distribution of amino acid residues among helical and non-helical regions in globular proteins. J Mol Biol. 1969 Jun 28;42(3):501–510. doi: 10.1016/0022-2836(69)90238-1. [DOI] [PubMed] [Google Scholar]
- Robson B., Pain R. H. Analysis of the code relating sequence to conformation in proteins: possible implications for the mechanism of formation of helical regions. J Mol Biol. 1971 May 28;58(1):237–259. doi: 10.1016/0022-2836(71)90243-9. [DOI] [PubMed] [Google Scholar]
- Sarma V. R., Silverton E. W., Davies D. R., Terry W. D. The three-dimensional structure at 6 A resolution of a human gamma Gl immunoglobulin molecule. J Biol Chem. 1971 Jun 10;246(11):3753–3759. [PubMed] [Google Scholar]
- Schiffer M., Edmundson A. B. Use of helical wheels to represent the structures of proteins and to identify segments with helical potential. Biophys J. 1967 Mar;7(2):121–135. doi: 10.1016/S0006-3495(67)86579-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sela M. Antigenicity: some molecular aspects. Science. 1969 Dec 12;166(3911):1365–1374. doi: 10.1126/science.166.3911.1365. [DOI] [PubMed] [Google Scholar]
- Titani K., Shinoda T., Putnam F. W. The amino acid sequence of a kappa type Bence-Jones protein. 3. The complete sequence and the location of the disulfide bridges. J Biol Chem. 1969 Jul 10;244(13):3550–3560. [PubMed] [Google Scholar]
- Weigert M. G., Cesari I. M., Yonkovich S. J., Cohn M. Variability in the lambda light chain sequences of mouse antibody. Nature. 1970 Dec 12;228(5276):1045–1047. doi: 10.1038/2281045a0. [DOI] [PubMed] [Google Scholar]
- Wu T. T., Kabat E. A. An analysis of the sequences of the variable regions of Bence Jones proteins and myeloma light chains and their implications for antibody complementarity. J Exp Med. 1970 Aug 1;132(2):211–250. doi: 10.1084/jem.132.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. T., Kabat E. A. An attempt to locate the non-helical and permissively helical sequences of proteins: application to the variable regions of immunoglobulin light and heavy chains. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1501–1506. doi: 10.1073/pnas.68.7.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff H. W., Tsernoglou D., Hanson A. W., Knox J. R., Lee B., Richards F. M. The three-dimensional structure of ribonuclease-S. Interpretation of an electron density map at a nominal resolution of 2 A. J Biol Chem. 1970 Jan 25;245(2):305–328. [PubMed] [Google Scholar]