Abstract
Several classes of compounds that have no intrinsic activity on aminergic systems nonetheless enhance the potency of aminergic receptor ligands three-fold or more while significantly increasing their duration of activity, preventing tachyphylaxis and reversing fade. Enhancer compounds include ascorbic acid, ethylenediaminetetraacetic acid, cortico-steroids, opioid peptides, opiates and opiate antagonists. This paper provides the first review of aminergic enhancement, demonstrating that all enhancers have a common, inobvious molecular motif and work through a common mechanism that is manifested by three common characteristics. First, aminergic enhancers bind directly to the amines they enhance, suggesting that the common structural motif is reflected in common binding targets. Second, one common target is the first extracellular loop of aminergic receptors. Third, at least some enhancers are antiphosphodiesterases. These observations suggest that aminergic enhancers act on the extracellular surface of aminergic receptors to keep the receptor in its high affinity state, trapping the ligand inside the receptor. Enhancer binding produces allosteric modifications of the receptor structure that interfere with phosphorylation of the receptor, thereby inhibiting down-regulation of the receptor. The mechanism explains how enhancers potentiate aminergic activity and increase duration of activity and makes testable predictions about additional compounds that should act as aminergic enhancers.
Keywords: aldosterone, allosteric, allostery, antiphosphodiesterase, ascorbic acid, corticosteroid, dopamine, down-regulation, EDTA, enhancement, enhances, epinephrine, GPCR, histamine, increased potency, molecular complementarity, morphine, naloxone, naltrexone, norepinephrine, opiate, opioid, phosphodiesterase inhibitors, potentiates, potentiation, serotonin, supersensitivity, vitamin C.
INTRODUCTION
G protein-couple receptors (GPCR) comprise a class of seven-transmembrane proteins that retain a high degree of sequence similarity [1]. In consequence, it can be difficult to develop drugs that have a high degree of selectivity for one specific receptor subtype over others [2]. The recent discovery of extracellular allosteric sites that can be modulated in tandem with a specific GPCR subtypes represents useful drug development targets [3-5]. Until very recently, however, extracellular allosteric sites in GPCR have been demonstrated only on muscarinic and adenosine receptors [3-7]. Since 2004, however, we have demonstrated that ascorbate enhances adrenergic and histaminergic ligand potency through an extracellular mechanism involving binding of the enhancer to the first extracellular loop of the relevant GPCR [8-11]. The existence of this extracellular binding site for ascorbic acid on aminergic GPCR, in conjunction with other evidence related to the effects of ascorbic acid and other aminergic enhancers on aminergic function, suggests a specific mechanism for enhancer activity that may have implications for drug development as profound as those associated with the discovery of the benzodiazepines.
A review of the literature on aminergic systems reveals that, in addition to ascorbic acid, several other classes of compounds have also been characterized as significantly enhancing aminergic GPCR activity. These compounds include folic acid, ethylenediaminetetraacetic acid (EDTA), opiate drugs and their antagonists, opioid peptides, corticosteroids, members of the citric acid cycle, and various flavenoids. All of these compounds have been demonstrated to produce significant (three- to ten-fold) enhancement of the potency and duration of activity of aminergic compounds while having no intrinsic activity themselves on aminergic systems. We define an aminergic enhancer, therefore, as a compound with no intrinsic pharmacological effect on the receptor or tissue upon which it is tested, but which increases the potency and/or duration of activity of an aminergic compound when present concurrently with that compound. Enhancers have two additional effects as well: they prevent tachyphylaxis (the down-regulation of receptors due to repeated exposure to a compound) and fade (the loss of efficacy resulting from continuous exposure to a compound). All told, the effects of aminergic enhancers hold great therapeutic promise.
We report here that the seemingly disparate set of compounds that display aminergic enhancement share a simple molecular motif and most certainly work through one, common mechanism involving binding to the first extracellular loop of aminergic GPCR. The nature of the site suggests a novel, specific, allosteric mechanism of action by which enhancement is effected through antiphosphodiesterase activity. Finally, the relationship of structure and function between enhancers and the compounds they enhance, as well as the way in which this structure-function relationship has been integrated into aminergic GPCR suggests the nature of the natural selection process that may have been at work during the evolution of this class of receptors. Understanding this natural selection process may provide clues as to the nature of enhancers for other classes of GPCR.
In short, by reviewing the forty year history of experiments regarding aminergic GPCR enhancement, we are able to suggest a specific mechanism of action for these enhancers that makes a series of testable predictions concerning enhancer structure, an allosteric mechanism that retards and reverses phosphodiesterase activity, and how these structure-function relationships evolved.
Ascorbic Acid
The best-characterized aminergic enhancer is ascorbic acid (vitamin C). Ascorbate has been shown to have no contractile effect on rabbit or porcine thoracic aortic rings, nor any relaxile effects on guinea pig or porcine trachealis at concentrations up to ten-fold higher than physiological normal [8-12]. Ascorbate at physiological concentrations of 15 to 50 µM enhances submaximal contractions, and at 150 to 500 µM concentrations (about 3 to 10 times physiological normal for human beings) shifts the dose response curve of adrenergic agonists such as norepinephrine (NE), epinephrine, ephedrine, albuterol, isoproterenol, and phenylpropanolamine 0.5 to 1.0 log units to the left [8-13]. The effect is seen only at submaximal concentrations of adrenergic compounds and has no effect on the maximal contraction that can be achieved. In other words, ascorbate increases the potency but not the efficacy of these aminergic compounds. Under the same conditions, the duration of contraction is extended three- to ten-fold [8-13]. Ascorbate creates the same increased potency and duration of action when combined with histamine at any submaximal concentration [10].
Although it was originally hypothesized that aminergic enhancement was due to the antioxidant properties of ascorbate [13], it has been demonstrated using multiple methods and protocols that oxidation is not a significant factor in these experiments, accounting for less than five percent of the observed enhancement [9, 10]. In fact, enhancement is observed in adrenergic compounds such as albuterol, isoproterenol, ephedrine, phenylpropanolamine, and histamine that do not oxidize to a measurable extent over the time course of the experiments. Moreover, as we will show below, enhancement can also be produced by classes of compounds such as opiates and corticosteroids that are not antioxidants.
Experiments demonstrating enhancement by ascorbate have been performed not only in vitro, but in vivo. Dillon, et al. [11] demonstrated increased potency of albuterol in horses with heaves (a model of chronic obstructive pulmonary disease). In a sheep model of asthma, the increase in potency of albuterol was more than ten-fold [11]. Houston, et al. [14] found that intraduodenal administration of ascorbate along with isoproterenol potentiated the isoproterenol effect more than two-fold. Grossmann, et al. [15] reported that in human subjects, ascorbate enhances by almost three-fold the relaxation of veins induced by phenylephrine. Mak and Newton [16] and Shinke, et al. [17] similarly observed a significant increase in the inotropic effects of dobutamine in human subjects when it was co-infused along with ascorbate. And Monahan, Eskurza and Seals [18, 19] reported that bringing the serum ascorbate level up from an average of 40 µM to 1 mM in healthy human patients significantly increased cardiovagal baroreflex sensitivity to endogenous amines. These human studies suggest that ascorbate enhancement of adrenergic and histaminergic drugs is safe and has real clinical potential.
EDTA
A second class of aminergic enhancing compounds is exemplified by the chelator ethylenediaminetetraacetic acid (EDTA). EDTA produces no contraction or relaxation of smooth muscle preparations at any dose thus far tested, but at micromolar concentrations has the same enhancing effects as ascorbate on adrenergic compounds [9, 12, 13]. The enhancement effect of EDTA on aminergic receptors is a particularly important finding since it is common practice for people isolating this class of receptors to use high concentrations of EDTA in their preparation of their receptors and these high concentrations of EDTA often remain during binding and second-messenger assays. The presence of EDTA during these assays may very likely increase apparent binding constants, drug potency, and duration of activity. The observation that EDTA enhances adrenergic compounds in a similar fashion to ascorbate also suggests that the mechanism of action of such enhancers must involve an extracellular mechanism, since EDTA has no known receptor or transporter, and is so highly charged that it is unable to pass through cell membranes.
Opiates, Opiate Antagonists and Opioid Peptides
The likelihood that aminergic receptor enhancement involves an extracellular mechanism is further supported by evidence that opiates such as morphine, dextromethorphan and levorphanol and opiate antagonists such as naloxone also exhibit aminergic enhancing effects. These studies also prove that this enhancement is not mediated by opiate receptors, but by aminergic receptors.
Puri, Cochin and Volicer [20] found that a mixture of morphine with any submaximal dose of dopamine resulted in significantly increased dopamine activity in rat corpus striatum compared with dopamine alone. Morphine had no effect by itself. Marti [21] went on to demonstrate that pre-treatment of guinea pig ileum with morphine shifted the dose response curve to norepinephrine in guinea pig ileum about half a log unit to the left. This enhancement could not be blocked by the opiate antagonist naloxone. Rae and De Moraes [22] confirmed all of Marti’s findings, demonstrating in addition that naloxone not only failed to block morphine’s effect, but could itself enhance norepinephrine. Akabori and Barraclough [23, 24] and He, Molnar and Barraclough [25] found that morphine enhanced norepinephrine-induced luteinizing hormone (LH) release compared with NE by itself but that morphine by itself had no effect on LH release. Kindman, Kates and Ginsburg [26] similarly demonstrated that 10 µM morphine had no contractile effects on rabbit myocardium, but shifted the dose response curve of isoproterenol leftward between three- and five-fold. Like Rae and de Moraes [22], they found that opiate antagonists such as naloxone and naltrexone had an identical potentiating effect. Since both opiate agonists and antagonists are effective, they concluded that their enhancing effects were independent of specific stereochemistry and could not be opiate receptor-mediated.
The finding that opiate antagonists are as effective as opiates in enhancing adrenergic compounds has been replicated by many investigators, strongly suggesting that Kindman, Kates and Ginsburg [26] were correct in concluding that adrenergic enhancement is not opiate-receptor mediated. Lechner, Gurll and Reynolds [27] may have been the first to recognize that naloxone potentiates both endogenous and exogenous monoamines, demonstrating that adrenalectomy eliminated circulatory responses to naloxone during treatment of hemorrhagic shock. They also demonstrated that naloxone increased the response to any given dose of exogenous alpha- or beta-agonist three-fold or more. Akabori and Barraclough [24] found that norepinephrine-induced release of luteinizing hormone could be enhanced by naloxone, which had no releasing effect by itself, but strangely, NE-stimulated release of luteinizing hormone-releasing hormone (LHRH) could be enhanced by morphine but not naloxone [28]. This is the only known instance of an enhancer potentiating one adrenergic system but not another. Allgood, Gurll and Reynolds [29] showed that the ability of naloxone to correct cardiovascular effects of endotoxic shock is dependent on circulating endogenous amines. The mechanism of this response was further explored by Caffrey, et al. [30, 31], who demonstrated that naloxone increases the response to epinephrine (EPI) in isolated canine arteries by more than 100% and that the response can selectively be eliminated by alpha antagonists. The inotropic potentiation of isoproterenol by naloxone was confirmed by Lechner [32], Gu, et al. [33] and Park, et al. [34]. McCubbin, et al. [35] found that naltrexone, another opiate antagonist, also significantly enhanced physiological responses to exogenous epinephrine in a rat model of diabetes.
Despite the findings by Marti [21] and Kindman, Kates and Ginsburg [26] that naloxone does not block morphine-induced enhancement of adrenergic compounds, and despite the evidence just reviewed showing that naloxone and naltrexone are themselves enhancers, three methodologically flawed reports have suggested that opiate antagonists can block opiate enhancement of adrenergic compounds. One confusing report by Parra, et al. [36] argues that opiate antagonists block the enhancement effect of morphine on NE-induced contractions of rat aorta and that the enhancement effect is therefore mediated through opiate receptors. This finding appears to be a methodological artifact resulting from pretreating their tissues with enough opiate antagonists to maximize the response to any dose of adrenergic compounds before adding an opiate. In consequence, the opiate could not further enhance the adrenergic effect. In addition, Parra and his colleagues failed to run a control to test for the effects of opiate antagonists themselves on adrenergics, apparently assuming there would be no effect. A similar methodological problem calls into question two reports by Lee and Berkowitz [37,38] that claim naloxone antagonism of the opiate pentazocine. Tissues were once again pretreated with opiate antagonists before addition of an opiate agonist, and no controls for the opiate antagonists were performed. The Lee and Berkowitz studies also suffer from having been performed at ED95 NE concentrations (10-7 M), which are two orders of magnitude higher than the NE concentrations utilized by most of the other studies described here. Since enhancement by ascorbate (see references above) has been found to increase the potency of drugs but not their efficacy, working near the maximal effective dose of an adrenergic or histaminergic compound will disguise any effects of potential enhancers.
Opioid peptides also function as aminergic enhancers. As noted above, Deyo, Swift and Miller [39, 40] reported that Leu- and Met-enkephalins enhanced dopamine activity in the rat median eminence. Tagaya, et al. [41] also demonstrated that the µ-opiate receptor agonist, DAMGO (D-Ala2, N-MePhe4, Gly-ol-enkephalin), had no relaxant effect on canine tracheal smooth muscle by itself, did not alter acetylcholine-induced contraction, but did augment the dose response to isoproterenol 1.9 to 4.3-fold depending on the concentration of DAMGO. DAMGO had no effect on relaxation caused by nitroprusside, verapamil, 8-cyclic-GMP, or methyl xanthenes, demonstrating that its enhancement effect is adrenergic-specific. Parra, et al. [36] found similarly that DAMGO had no effect on isolated rat aortic strips but increased the tension produced by any submaximal dose of NE. They also claim that opiate antagonists prevent DAMGO’s enhancing effects, but these experiments have the same methodological problems (pre-exposure of tissues to an opiate antagonist without antagonist controls for enhancement) described above regarding their reports of antagonist elimination of morphine enhancement.
Corticosteroids
Some, though probably not all, corticosteroids may have aminergic enhancing effects as well, which may have implications for understanding the well-known side effects of steroids including hypertension, mania, and increased aggression - all symptoms of increased aminergic activity.
Purdy, Weber, and Drayer [42-44] reported that aldosterone alone had no contractile effect on rabbit ear arteries or thoracic aorta, but the addition of 1, 10 and 100 uM aldosterone to rabbit thoracic aortic rings partially contracted with NE to a steady-state of 1.5-3.5 g caused a further contraction of 0.09, 0.47 and 0.80 g, respectively. Aldosterone also increased the duration of NE-induced contractions about 2.5-fold. This enhancement was blocked by the adrenergic antagonist phentolamine [42-44]. The fact that these experiments involve the use of aldosterone at several orders of magnitude above its physiological concentrations makes these results of questionable utility for drug therapy, but do not alter the utility of the results for understanding the structural requirements for aminergic enhancer activity and may have some application to understanding renal physiology, where local concentrations of aldosterone may be significantly higher than blood or plasma levels.
Of greater clinical relevance, Gu, et al. [33] found that in human subjects, corticosterone enhanced epinephrine-induced cardiac contractions, but had no effect on cardiac activity by itself. Corticosterone also decreased epinephrine uptake. And additional evidence supporting the role of some steroid compounds as aminergic enhancers comes from human trials involving prednisolone, budesonide, and hydrocortisone, all of which significantly potentiated the asthma drugs formoterol and salmeterol [45-47].
Folic Acid
Folic acid (vitamin B9) has also been shown to enhance adrenergic and serotonergic activity. Spector [48-50] found that while neither folic acid nor norepinephrine alone affect the respiration of rat brain synaptosomes, the combination produced a significant increase in respiration. As would be expected for a receptor enhancer, folic acid decreased reuptake of NE [51]. More recently, Brocardo, et al. [52] showed that folic acid alone has no psychostimulant effect, but co-administered orally or intravenously potentiates endogenous serotonin and norepinephrine. Doses of folic acid that have no effect on endogenous amines nonetheless potentiate exogenous fluoxetine. This potentiation is eliminated by serotonin receptor antagonists (both 5HT1A and 5HT2A/2C) and alpha-1- and alpha-2-adrenoceptor receptor antagonists.
Ascorbate, opioids, opiates, opiate antagonists, aldosterone and folic acid all satisfy the criteria for being aminergic enhancers. None has any independent effect on smooth muscle or other aminergically activated tissues or functions, but each potentiates any sub-maximal dose of amine or aminergic drug and increases its duration of action. The potentiation in each case is dependent on the presence of the amine and can be blocked by an appropriate aminergic receptor antagonist demonstrating that the potentiation is receptor mediated.
Acetoacetate and Pyruvate
Two members of the citric acid cycle have also been found to enhance aminergic functions. Pyruvate and acetoacetate have both been identified as enhancers of isoproterenol [53-55]. Like other enhancers, pyruvate and acetoacetate shift the dose response curve of isoproterenol activity on myocardium to the left and very significantly increase the duration of its activity. As with ascorbate [9], the effects of pyruvate and acetoacetate continue even after it has been washed off of the tissue it has enhanced [55]. Squires has asserted [53-55] that this enhancement is due to the antioxidant effects of acetoacetate and pyruvate, but present no independent tests of rates of oxidation to demonstrate this assertion. We have shown that isoproterenol does not oxidize measurably over the time course of these experiments [9], so that it is impossible that acetoacetate or pyruvate enhance adrenergic compounds by preventing oxidation of adrenergic compounds. Squires [53-55] suggests as an alternative hypothesis that pyruvate and acetoacetate may act indirectly by altering the redox potential of the ascorbate-glutathione system thereby altering phosphorylation of the adrenergic receptor, a point that we will take up below, but adds that their data demonstrate that non-redox mechanisms must also be at work. In light of the fact that other enhancers described here are not antioxidants, we believe that these non-redox mechanisms are likely to be of greater significance to understanding aminergic enhancement, though the role of phosphorylation in enhancement is certainly of great importance and we will take it up further below.
Flavonoids
One additional class of compounds may also function as aminergic receptor enhancers, but the data are less compelling than for the previous set of compounds. These additional compounds are flavonoids such as quercetin and fisetin. Kappusany and Das [56] demonstrated that quercetin and fisetin potentiated epinephrine- and isoproterenol- but not theophylline-stimulated phosphodiesterase activity in rat adipocytes. This potentiation could be blocked by addition of adrenergic antagonists. Naidu, et al. [57] showed that quercetin has an analgesic effect that is blocked by alpha-adrenergic and dopaminergic antagonists, and potentiated in the presence of alpha-adrenergic and dopaminergic agonists. And Kaur, et al. [58] demonstrated that quercetin has depressive effects that are mediated through presynaptic adrenergic receptors. The difficulty with these reports is that each suggests that, unlike the other enhancers just described, flavonoids may have intrinsic pharmacological activity affecting the receptors and tissues upon which the tests were performed. The studies just cited do not provide sufficient controls to differentiate intrinsic pharmacological activity from enhancer effects and thus, while they are suggestive, do not prove such activity. Their roles as enhancers should be considered merely possible in the discussion that follows.
Preventing Tachyphylaxis and Reversing Fade
Besides increasing the potency and the duration of activity of aminergic compounds, enhancers can also affect aminergic activity in two other unusual and important ways: they prevent tachyphylaxis and reverse fade in muscle preparations and in human subjects.
Tachyphylaxis is the decreasing response to repeated doses of a drug due to receptor down-regulation. Qin, et al. [59] found that ascorbate prevented desensitization of beta adrenergic receptors following induced myocardial infarction in a rabbit model. Tan, McFarlane and Lipworth [45,46,50] demonstrated that concurrent use of prednisolone and hydrocortisone along with formoterol or salmeterol prevented the development of aminergic receptor subsensitivity (tachyphylaxis). Lipworth and Aziz [47] then extended these findings to budesonide. Dillon, et al., [11] reported that ascorbate also prevents tachyphylaxis in vitro using porcine airway smooth muscle relaxed repeatedly by epinephrine.
Similarly, three classes of enhancers have been reported to reverse fade, the time-dependent loss of response to a drug that occurs when the drug is present continuously. Parra, et al. [36] found that the enkephalin compound DAMGO could restimulate an NE contraction after it had faded away. Tan, et al. [45, 46, 60] showed that the corticosteroids prednisolone and hydrocortisone could reverse adrenergic subsensitivity (due either to fade or tachyphylaxis) to formoterol and salmeterol in human airway. Shinke, et al. [17] report that ascorbate can reactivate dobutamine in human subjects after its cardiovascular effects have faded away and Dillon, et al. [11] similarly reported that ascorbate can restimulate an epinephrine contraction of guinea pig tracheal smooth muscle that has faded away. It is important to emphasize that in each of these cases, the enhancer in question was shown to have no effect by itself on the tissue to which it was applied. Since reversal of fade is always on the order of seconds to minutes, it can only be explained by a reversal of short-term down-regulation mechanisms involved in aminergic receptor control.
The practical implications of prevention of tachyphylaxis and the reversal of fade are very significant for aminergic drug safety and activity. Tachyphylaxis results in the necessity to use increasing doses of drugs resulting in increased side effects and, in the most serious cases, loss of drug efficacy. Two striking examples are the development of resistance to both rescue and long-acting bronchodilators by patients with asthma and the development of rhinitis medicamentosa (or “rebound effect”) in the chronic use of decongestants in people with colds and chronic sinusitis. Similar effects have been found for many blood pressure medications and neurotropics as well. Means to prevent the development of tachyphylaxis and reverse fade would have tremendous benefits for patients requiring repeated or chronic dosing with aminergic drugs.
Non-Enhancers and Non-Enhanced Compounds
Though the studies summarized above report on a wide range of compounds that can enhance aminergic activity, most studies report as well on compounds that have no enhancing effects. These non-enhancers act as negative controls that help to define the boundaries of enhancer structure. For example, dehydroascorbic acid (DHA) does not enhance norepinephrine activity, nor does riboflavin [12]. Marti [21] demonstrated that reserpine antagonizes norepinephrine-induced contractions of guinea pig ileum. Indeed, all adrenergic and aminergic receptor antagonists can be included as non-enhancing compounds since these have been used to block potentiation by enhancers (see below).
The studies summarized above also report enhancement of a very wide range of adrenergic and histaminergic agonists and antagonists. These include epinephrine, norepinephrine, dopamine, ephedrine, phenylpropanolamine, albuterol, isoproterenol, phenylephrine, dobutamine, fluoxetine, histamine and antihistamines. This range of adrenergic compounds, in particular, suggests that enhancement may be general to the entire class of adrenergic agonists and antagonists.
Despite the wide range of adrenergic and histaminergic compounds that can be enhanced, it is important to stress that the enhancers reviewed above are specific to particular classes of compounds and not others. Tagaya, et al. [41] demonstrated that the enhancer enkephalin was specific for adrenergic compounds but did not enhance acetylcholine, nitroprusside, verapamil, 8-cyclic-GMP, or methyl xanthenes. Dillon, et al. [9-12] have similarly demonstrated that ascorbate enhances adrenergic and histaminergic compounds but does not enhance contractions due to potassium, serotonin, angiotensin II, acetylcholine, or carbachol. These data are extremely important for limiting the possible mechanisms by which enhancers produce their common effects since all of these compounds produce contraction or relaxation of smooth muscle, but only two classes - adrenergics and histaminergics - are potentiated by all of the enhancers reviewed here.
Common Molecular Motif Among Enhancers
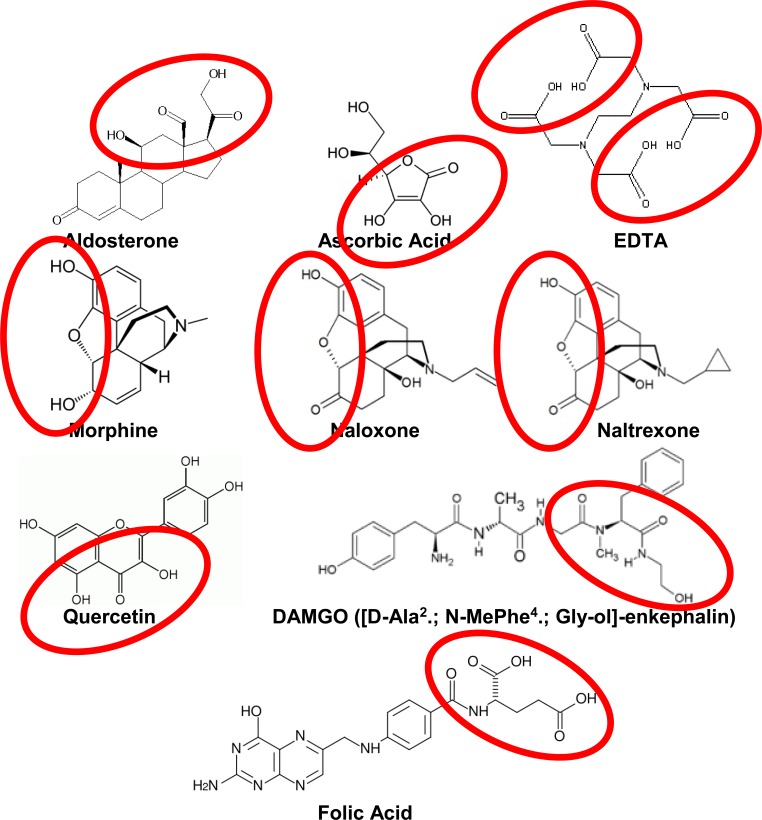
Structural comparisons reveal that only one common molecular motif is present among all known aminergic enhancers (Fig. 1) and is absent among all known non-enhancers (Fig. 3). The common enhancer motif consists of at least three hydrogen-bonding moieties consisting of hydroxyl (OH) groups and carbonyls (double-bonded O) lying within a distance of 4 to 6 angstroms (400 to 600 pm) of each other. The order of the carbonyls and hydroxyls is probably immaterial as the order can be varied in many of the compounds by tautomerization (Fig. 1). It is likely that the set of carbonyls and hydroxyls must be able to adopt a planar conformation with relation to each other, since the two hydroxyls and carbonyl in ascorbic acid, morphine, naloxone, naltrexone and quercetin are all constrained by their ring structures in this way. This constraint suggests that the binding site for enhancers must involve amino acids that can make hydrogen bonds to hydroxyls and carbonyls presented by such ring structures. All of the other known enhancers (enkephalins, folic acid, aldosterone and EDTA) are flexible enough to adopt a conformation of hydroxyl and carbonyl moieties that fits such a planar motif and so are at least theoretically capable of adapting to the same shape of binding site as, say, ascorbic acid (Fig. 2). No known non-enhancer has this motif or can adopt it. (Fig. 3)
Fig. (1).
Common Motif In Enhancer Structures. All known enhancers have a pair of hydroxyls and a carbonyl in a linear array within 4-6 angstroms (400-600 pm) of each other (circled).
Fig. (3).
The Common Motif Identified In Enhancers In Figure 1 Is Lacking In All Known Non-Enhancers.
Fig. (2).
Overlays of Enhancers Demonstrating Shared Arrays of Hydroxyls and Carbonyls. Left: Overlay of morphine (black) and aldosterone (green). Right: Overlay of naloxone (red) and EDTA (blue).
The presence of a common molecular motif argues strongly for a single mechanism of action for all of the enhancers and suggests that this mechanism involves binding to a common target. The common enhancer motif also makes it possible to predict other possible aminergic enhancers in advance of their testing. Some of these are shown in Fig. (4). A search for the simplest molecules that contain the enhancer motif has yielded several possibilities including malonic acid, malic acid, citric acid, isocitric acid, oxaloacetic acid, aconitic acid, oxoalosuccinic acid, and tartaric acid (Fig. 4). We have not found any reports of malonic acid enhancement of aminergic agonists, perhaps because it has never been tested. In any case, malonic acid acts as a competitive inhibitor of succinate dehydrogenase and would be unsafe to use as an aminergic enhancer. We can find no evidence that any of the other compounds have been characterized as enhancers or potentiators of aminergic activities or functions, either. These predicted compounds therefore provide the possibility for testing the accuracy of the motif proposed here. Notably, these compounds are all part of the citric acid cycle and therefore likely to be extremely well tolerated physiologically even at relatively high concentrations, making them attractive starting points for drug development.
Fig. (4).
Predicted Aminergic Enhancers. Based on the common motif introduced in Fig. (1); we predict that the compounds illustrated here will also enhance adrenergic compounds.
Mechanism of Enhancement: Entrapment and Allosteric G-Protein Inhibition
The recognition that several classes of relatively distinct compounds all produce very similar enhancing effects on adrenergic and histaminergic drug activity raises the obvious question of the mechanism or mechanisms by which these common effects are achieved. The problem can be subdivided into at least three more circumscribed questions. First, are there any features of the classes of compounds described above that can explain their common effects. Second, where are these effects initiated in the cells upon which they act: are they mediated by a single receptor, multiple receptors, through a common second messenger, or some other mechanism? And third, how can the mechanism or mechanisms involved in answering the previous questions explain the increased potency and duration of activity of enhancers?
Previous investigators have eliminated a number of possible mechanisms that might have accounted for enhancement. For example, Gu, et al. [33] found that naloxone-induced enhancement of epinephrine could not be accounted for by increased uptake of epinephrine. They report that opiate compounds and their antagonists, in fact, decrease adrenergic reuptake. The fact that EDTA works as an enhancer also seriously limits the possible mechanisms to extracellular ones, as EDTA has no known receptor or transporter and is far too polar to pass through a cell membrane. Thus, the mechanism of aminergic GPCR enhancement appears to be extracellular and probably receptor mediated.
It is also very unlikely that enhancement is due to some sort of “cross-talk” between different classes of receptors or by effects of enhancers on intracellular mechanisms that then alter receptor function. One critical argument against such cross-talk again focuses on EDTA, which has no known receptor and no known intracellular activity. The principle of Occam’s razor also argues against opiate receptors, corticosteroid receptors, ascorbate transporters and folate transporters all sharing an equivalent interaction with aminergic GPCR involving a molecular mechanism also shared by pyruvate, acetoacetate and flavonoids. Moreover, the fact that a compound made by tethering norepinephrine to ascorbate retains enhancer activity [8] makes it very hard to imagine that this compound can act via an ascorbate uptake system. Even if the EDTA and tethered compound data could be explained and a mechanism that would enable such promiscuous cross-talk could be imagined, it would also have to account for how both opiate agonists and antagonists result in enhancement of aminergic GPCR when they have opposite effects on the opiate receptors themselves. Rather than assuming some sort of extremely complicated cross-talk between various systems, it is much simpler to begin with the premise that since all known enhancers share a common molecular motif, they all work through a single mechanism, and that the mechanism is mediated through the aminergic receptor itself.
Several lines of evidence strongly support an allosteric, aminergic receptor-mediated mechanism of enhancement. In the first place, the effects of several different classes of aminergic enhancers can be blocked by adrenergic antagonists. Enhancement of NE by aldosterone was blocked by the adrenergic antagonist phentolamine [42-44]. Caffrey, et al. [30,31] demonstrated that the naloxone potentiation of epinephrine can selectively be eliminated by alpha antagonists. Fluoxetine enhancement by folic acid could be blocked by both alpha-1- and alpha-2-adrenoceptor receptor antagonists [52].
Results of binding studies also argue for an aminergic receptor-mediated enhancement mechanisms. Ascorbate increases about ten-fold the binding of (-)[125I]cyanopindolol (ICYP), a beta adrenergic-specific ligand, to beta adrenergic receptors [61,62] and similarly increases the binding of dopamine to the dopamine D1 receptor [63]. Conversely, ascorbate blocks the binding of the D1-specific receptor agonists 125I-SCH 23982 [63] and [3H]-SKF R-38393 [64] and the antagonist 125I-SCH 23390 [65] suggesting that an ascorbate binding site may exist on the D1 receptor that is close enough to the agonist binding site to block the binding of compounds that are significantly larger than dopamine itself.
A receptor-mediated mechanism for enhancement is further supported by physicochemical experiments demonstrating that ascorbate binds to the adrenergic receptor, specifically at a site defined at least in part by the first extracellular loop [8-11]. Significantly, this loop shares very high homology with sequences of the sodium dependent vitamin C transport proteins supporting a shared affinity for vitamin C [10, 11]. Notably, the first extracellular loop of aminergic receptors has not previously been suggested to have any function other than purely structural, a conclusion we now challenge.
Further evidence that ascorbate potentiates aminergic compounds at an external site on the aminergic receptors has been provided by the synthesis of a novel tethered compound linking ascorbate to norepinephrine by means of a four-unit polyethylene tether. This tethered compound demonstrated in vitro efficacy, producing the same type of long-duration contractions observed previously only when both norepinephrine and ascorbate were present simultaneously [8]. This tethered compound was also shown to bind to the first extracellular loop of the adrenergic receptor [8].
The binding of ascorbate to the external surface of aminergic receptors does not, of course, explain how enhancers potentiate aminergic compounds, but it does provide clues that severely limit the possibilities. One possibility is essentially mechanical and the other allosteric, and both may operate simultaneously.
The mechanical possibility may involve trapping the aminergic compound in the receptor in its high affinity conformation. In the absence of an enhancer, binding of aminergic agonists and antagonists will be reversible and dependent solely on the binding constant determined by the affinity of the receptor for the compound. Because the enhancer binding site is exterior to the aminergic ligand, the binding of an enhancer to the site indicated by physicochemical studies could retard the release of aminergic compounds once bound into the receptor (Fig. 5). This trapping mechanism assumes that binding of enhancers to aminergic receptors is facilitated by prior binding of aminergic compounds into their receptor binding sites. Otherwise, one would expect the enhancers at high concentrations such as were used in most of the experiments reported above to antagonize aminergic activity. This mechanical mechanism should be testable by examining on- and off-rates of the orthosteric agonist in the presence and absence of enhancers.
Fig. (5).
Binding Site of Enhancers. Probable binding site of enhancers (orange) in relation to aminergic agonists (red) [based on studies by Dillon, et al. (8-11)].
Allostery represents the second mechanism by which enhancers may potentiate aminergic receptor activity. The mechanism by which enhancement is mediated must involve allostery as it is possible to decrease the concentration of agonist and yet obtain increased potency in the presence of enhancers. This cross-over effect was first observed in allosteric enzyme regulation and has since been recognized to be characteristic of all allosteric systems [66].
The specific means by which allostery is implemented in aminergic GPCR remains to be determined, but significant clues exist that constrain the possibilities. Several investigators have proposed that aminergic receptors can exist in high affinity and low affinity states. Maintenance of the high affinity state requires the formation of a disulphide bond between the conserved cysteines of the first and second extracellular loops of aminergic receptors (see Fig. 5) [67-69]. Enhancer binding could protect this disulphide bond. Binding of aminergic agonists is then followed by changes in receptor conformation that result in the release of G-protein from the intracellular portion of the receptor. The release of G-protein permits phosphorylation of the receptor at multiple sites, desensitizing it and initiating a cascade of events resulting in the internalization of the receptor. We propose that enhancer binding to the exterior of the aminergic receptors prevents the release of the G protein, blocking phosphorylation and thereby delaying the down-regulation cascade [11]. A strong argument can be made that the mechanism of enhancer action must be mediated through such a G-protein modifying mechanism because of the data reviewed above concerning the prevention of tachyphylaxis (and thus of receptor internalization) as well as the reversal of fade. The reversal of fade is particularly telling as this reversal takes place on the order of seconds to minutes, and the only step involved in the down regulation of receptors that occurs on a similar time scale is release of the G protein leading to phosphorylation.
The specific allosteric mechanism at work in aminergic enhancement is unknown. Various models of allostery exist. One is the Monod-Wyman-Changeux (MWC) model, which proposes that all allosteric proteins are oligomeric, have an axis of symmetry between the proteins forming the complex, have multiple ligand receptor sites, and exist in multiple stable conformations in the absence of the ligand [70]. Allosteric modulators in such a model increase the probability that the oligomeric complex will exist in either an active or inactive conformation [70] Aminergic GPCR can be oligomeric, forming transient dimers [71-73] but whether these dimers participate in allosteric regulation of agonist binding is a source of debate. As noted above, the second extracellular loop of aminergic GPCR may participate in enhancer binding and this second extracellular loop has also been suggested to participate in dimerization [74] so that binding of enhancers might affect the stability of dimerization. This possibility is speculative, however. Even when dimers form, they do not produce complexes with an axis of symmetry between them. There do appear to be multiple ligand receptor sites, but these exist on the monomers (evidence presented in this paper as well as [75]) and these monomers are able to adopt multiple stable conformations in the absence of the ligand, as will be discussed in more detail below. Finally, some investigators have predicted that, “Another important consequence of the MWC model that is often unappreciated in the GPCR field is the expectation that all ligands, whether they bind to orthosteric or allosteric sites, should display some degree of either agonism or inverse agonism depending on whether they select active or inactive receptor states.” [70] Not only do most GPCR allosteric modulators lack intrinsic activity in the absence of endogenous ligands or agonists [75], the enhancers described in this paper also lack intrinsic activity. In short, models of allostery based on the MWC model [70] do not appear to be likely fits for the kinds of effects that aminergic enhancers display.
The information currently available about aminergic enhancers is better explained within the framework recently suggested by Keov et al. [76] and Wooten, et al. [77], who categorize GPCR into four categories: 1) orthosteric agonism without allosteric modification; 2) positive allostery; 3) negative allostery; and 4) neutral allostery. In each of these cases, the allostery is mediated not through oligomeric GPCR forms (although this possibility is also allowed) but primarily through the existence of separate orthosteric and allosteric binding sites on the same protein chain. In general, binding of the allosteric modulator is significantly less than binding of the orthosteric agonist, which is certainly the case with all of the enhancers described here. In addition, the co-localization of the orthosteric and allosteric binding sites on a single chain makes it possible to design bitopic, linked compounds that can bind to both sites simultaneously, which is not possible if the orthosteric and allosteric sites are on separate chains in an oligomeric structure. As noted above, such a bitopic compound has successfully been synthesized by linking ascorbate to norepinephrine [8]. All of the enhancers, including the bitopic compound, described in this review appear to work through positive allostery. Wooten, et al. [77] propose that such positive allostery may be mediated by either or both of two mechanisms, one of which increases the affinity of the orthosteric compound for its binding site, the other by increasing the efficacy of the orthosteric compound. The data summarized above clearly demonstrate that all of the aminergic enhancers increase efficacy; there is no relevant data as to whether any of the enhancers increase affinity. Kinetic binding studies will be needed to clarify this issue.
Another critical factor in characterizing allosteric mechanisms is the effect of allosteric modulators on probe dependence [76, 77]. Probe dependence is a phenomenon in which different orthosteric agonists or inverse agonists binding to the same GPCR produce a different array of second messenger effects. Allosteric modulators should produce biased second-messenger signaling in combination with orthosteric compounds that display probe dependence. There is no evidence of probe dependence in the enhancer data summarized here, but it is very important to emphasize that relevant studies specifically designed to reveal probe dependence have not yet been carried out. Additionally, Wooten, et al. [77] note that differential metabolism and reuptake of orthosteric and allosteric compounds can also have very significant effects on their interactions. Once again, there is no relevant data available in any of the studies reviewed here that is appropriate to address the metabolic issues, which would provide yet another fruitful avenue for future research. Finally, several investigators [75-78] have noted that there are at least three, relatively distinct regions on GPCR to which allosteric modulators are known to bind: to the extracellar region; the transmembrane region; and the intracellular region. The data summarized here argue strongly for an extracellular allosteric binding site. Many of the enhancers have been demonstrated to bind directly to peptides derived from the first and second extracellular loops of aminergic GPCR [8-12]; several of the enhancers (e.g., EDTA) do not enter cells directly and so must exert their allosteric effects from the extracellular side of the receptor; and binding of the bitopic ascorbate-norepinephrine linked compound has been shown to involve the extracellular portion of the receptor [8]. Combined, the existing data on aminergic enhancers fits the general model of a positive allosteric modulator as characterized by Wooten, et al. [77], although a number of key experiments regarding the possibilities of enhanced affinity, probe dependence, metabolic effects and binding site remain to be carried out. The possibility that enhancers modify dimerization of aminergic GPCR must also be considered.
In addition to acting through an allosteric mechanism, opiates, opiate antagonists and ascorbate have antiphosphodiesterase activity that might also contribute to enhancement effects. Such a dual effect has also been reported for extracellular allosteric enhancers of adenine receptors [3]. In effect, G protein-mediated phosphorylation of GPCR requires a ready source of phosphates that are normally supplied by phosphodiesterases (PDE). Kinases add phosphates to the receptor, preparing it for internalization. The antiphosphodiesterase activity of aminergic enhancers would decrease the availability of kinase-mediated phosphorylation, thereby preventing down-regulation of receptors.
The most definitive study to date on the antiphosphodiesterase activity of an enhancer is of naloxone’s effect on isoproterenol-induced contraction of guinea pig myocardium. Park, et al. [34] report that, “The enhancement of myocardial contractility by naloxone in the presence of stimulation of adenylyl cyclase activity appears to be mediated by inhibition of PDE, specifically PDE III.” Moffat, et al. [79] also reported that a number of opiate drugs including nalorphine, dextromethorphan, codeine and methadone all inhibited cAMP PDE activity in bovine heart preparations at about 1 mM concentrations. Although Moffat, et al. [80] also reported that morphine sulphate did not inhibit cAMP PED even at 10 mM concentrations, [80], Puri, et al. [20], and He, et al. [25] subsequently reported that morphine does display antiphosphodiesterase activity at micromolar concentrations in various adrenergic-stimulated tissue preparations. It is likely, therefore, that Moffat’s preparations were desensitized in some way, and possible that all of the compounds he reported to have inhibitory effects on PDE are more effective than his study suggests. Inhibition of cAMP PDE activity by ascorbate has also been reported at micromolar concentrations by Moffat, et al. [79], Buck and Zadunaisky [81], Tisdale [82], Malamud and Kroll [83], and Shinohara, et al. [84]. As noted above, Squires and his colleagues [53-55] have proposed that pyruvate and acetoacetate may also alter the rate at which the adrenergic receptor is phosphorylated, but without providing evidence for any particular mechanism.
It is likely that a combination of the entrapment and the allosteric inhibition of G-protein activity, perhaps accompanied by antiphosphodiesterase activity as well, is responsible for the increased potency and duration of activity that characterizes aminergic receptors: 1) binding of the enhancer to the aminergic receptor keeps the receptor in its high-affinity state, decreasing the off-rate of the agonist, and thereby increasing drug potency; and 2) the binding of the enhancer to the receptor causes an allosteric modification of the receptor that prevents phosphorylation of the receptor, thereby retarding down-regulation and internalization of the receptor (Fig. 6). The result would be increased potency of any sub-maximal dose of aminergic compound accompanied by greater duration of activity - just what is observed.
Fig. (6).
Model of Enhancer Inhibition of GPCR Phosphorylation. Proposed model of enhancement of aminergic GPCR based on receptor allostery. Top Row: Binding of an amine to a GPCR normally results in allosteric changes in the receptor that initiate a series of molecular signals recruiting receptor kinases (indicated by the change of the intracellular loops from white to black and back again), which phosphorylate the receptor resulting in its down regulation and internalization. Bottom Row: We propose that binding of enhancers to GPCR results in prevention of aminergic release from the receptor accompanied by interference with the allosteric signaling (intracellular loops remain metaphorically in the “black” state) that initiates receptor kinase recruitment. Retention of the amine in the binding site accompanied by retardation of receptor phosphorylation results in prevention of tachyphylaxis. We also predict that during the first steps of receptor down-regulation binding of enhancers to the receptor can induce allosteric changes in the receptor that reverse receptor phosphorylation.; thereby also reversing fade.
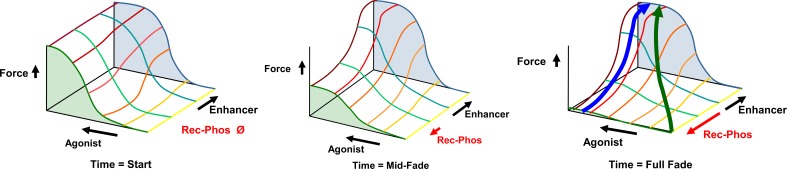
The mechanism proposed in Fig. (6) leads to an energetic model as well, which is illustrated in (Fig. 7). There are at least four factors that need to be considered in modeling the ability of enhancers to reverse fade and prevent tachyphylaxis. One is the concentration of agonist. The second is the force of contraction (or relaxation) caused by the agonist at any given concentration. The third is the effect of any given concentration of enhancer. The enhancer effect will be, as proposed in (Fig. 6), opposite of the effect of phosphorylation. A fourth effect is that of time. Fig. (7) illustrates how these four f actors interact to influence GPCR ardrenergic- or histaminergic-induced smooth muscle contractions (and by extrapolation, relaxations as well). As the agonist increases, the force increases to a maximum. Enhancers do not, themselves, induce any contraction at any concentration, from which it is concluded that they do not cause calcium increases. Enhancers do increase the potency of agonists resulting in greater force at sub-maximal agonist concentrations. During prolonged contractions, in the absence of an enhancer, force fades over time despite the continued presence of agonist, but no fade occurs at high enhancer concentrations. Since fade is thought to occur through phosphorylation of the GPCR receptor, it may be presumed (as proposed above) that the enhancer blocks phosphorylation of the receptor by some means. Notably, the energetic model shown in (Fig. 7) illustrates that there is no energetic barrier to reversal of fade after a continuous contraction when an enhancer is added. The model also suggests that since there is no evidence that any enhancer increases intracellular calcium, the reversal of fade must reverse receptor phosphorylation, which is again consistent with the model proposed above. Similarly, the reversal of tachyphylaxis (loss of force in sequential activations) can be reversed by enhancers when both the agonist and enhancer are added simultaneously at the start of an activation that would otherwise not produce force. In sum, this energetic model is consistent with the evidence summarized above indicating that the reversals of fade and of tachyphylaxis by enhancers are due to the common mechanism of reversing phosphorylation of GPCR receptors.
Fig. (7).
Reversal of Smooth Muscle Fade and Tachyphylaxis by Enhancer Molecules. The graphs show the influence of enhancers on GPCR adrenergic- or histaminergic-induced smooth muscle contractions. Agonist is shown as increasing concentration (arrow) over the yellow-red lines. Force increases are shown on the Z-axis. Enhancer concentration increases over the green-blue lines. Enhancers do not induce any contraction nor do they cause any increase in maximum force (efficacy), from which it is concluded that they do not cause calcium increases. Enhancers increase the potency by 0.5-1.0 log units, resulting in greater force at sub-maximal agonist concentrations. During prolonged contractions, force fades despite the continued presence of agonist, shown by the fall in force at zero enhancer (green shaded area) in the figures from left to right. No fade occurs at high enhancer concentrations (blue shaded area). Fade is thought to occur through phosphorylation of the GPCR receptor, shown in the red arrow. Fade can be reversed in a continuous contraction by the addition of enhancer, shown in the blue arrow in the right hand graph. Since there is no evidence that any enhancer increases intracellular calcium, the reversal of fade must reverse receptor phosphorylation. Similarly, the reversal of tachyphylaxis (loss of force in sequential activations) can also be reversed by enhancers (green arrow in the right hand graph), when both the agonist and enhancer are added simultaneously at the start of an activation that would otherwise not produce force due to tachyphylaxis. The evidence indicates that the reversals of fade and of tachyphylaxis by enhancers are due to the common mechanism of reversing phosphorylation of GPCR receptors.
The mechanism just proposed might also explain the otherwise counter-intuitive report that combinations of adrenergic agonists and antagonists produce increased duration of activity and decreased tachyphylaxis [85]. As noted above, ascorbate is known to block several dopamine receptor antagonists, suggesting that these receptor antagonists bind at least partially into the enhancer site. Combinations of some adrenergic agonists and antagonists may therefore partially or wholly mimic the agonist-enhancer effect.
CONCLUSION
We have reviewed, for the first time, the previously disparate literature concerning enhancement of adrenergic and histaminergic receptors. The effects of enhancement are quite striking. In the presence of enhancers, the potency and duration of activity of aminergic compounds is increased several-fold at any sub-maximal concentration; tachyphylaxis is prevented; fade can be reversed. Enhancers include ascorbate; EDTA; opiates, opioids and their antagonists; corticosteroids; folate; citric acid cycle metabolites; and perhaps flavonoids. Despite the obvious diversity of classes of compounds that can enhance adrenergic and histaminergic receptors, all known enhancers share a common molecular motif comprised of three or more hydrogen bonding moieties consisting of either hydroxyl (OH) groups or carbonyls (double-bonded O) lying within a total linear distance of 4 to 6 angstroms (400 to 600 pm) and which are capable of assuming a planar relationship towards each other. Compounds lacking enhancement activity uniformly lack this molecular motif.
The mechanism or mechanisms by which enhancement is produced has not yet been identified definitively, but existing evidence is sufficient to severely limit the possibilities. The fact that aminergic compounds are enhanced, but not potassium, angiotensin II, acetylcholine, nitroprusside, verapamil, 8-cyclic-GMP, or methyl xanthenes, argues for an aminergic receptor-mediated mechanism. So do data demonstrating that aminergic antagonists can block enhancement effects, which also suggests that enhancers and antagonists share at least some degree of overlap in their binding. Shared overlap of antagonist and enhancer binding, in turn, argues for an extracellular binding site for enhancers. The inference of extracellular binding of enhancers is further supported by three additional lines of evidence. First, EDTA (which does not enter cells) is an active enhancer. Second, ascorbate binds to the first extracellular loop of aminergic receptors. And third, a tethered compound linking norepinephrine to ascorbate has the same enhancement effect as the compounds applied separately [8], arguing for an enhancement binding site very close to the aminergic binding site in the aminergic receptor. It is, of course, possible that various enhancers also have intracellular activity, but it is difficult to imagine pathways by which all of the enhancers (including both opiate agonists AND antagonists) could exert a common effect specifically on aminergic activity through intracellular mechanisms.
Extracellular binding of enhancers can increase potency of aminergic compounds through three, probably synergistic, mechanisms. Binding of enhancers to the aminergic receptor appears to be at a site that would protect the disulphide bond between the first and second extracellular loops that is essential to maintaining the receptor in its high affinity state. Enhancer binding may also create a “cap” that “traps” the aminergic compound in the receptor, decreasing its off rate. Delay of tachyphylaxis and reversal of fade necessitate an allosteric mechanism by which enhancer binding interferes with, or reverses, kinase-mediated phosphorylation of the receptor, retarding receptor down-regulation. Since ascorbate, opiates and opiate antagonists are each known to have antiphosphodiesterase activity, enhancers could interfere with the supply of phosphate to kinases, decreasing the rate of receptor phosphorylation.
Because the literature reviewed here has not been integrated previously, approaches to aminergic receptor enhancement have not been coordinated and many gaps need filling. Among the most outstanding of these is the detailed working out of the mechanism or mechanisms by which enhancement is effected. No studies currently exist of the effects of any of the enhancers described here on rates of adrenergic receptor internalization or phosphorylation. It is not known whether the initial steps of G-protein release followed by receptor phosphorylation can be reversed by addition of enhancers, as is proposed here as a mechanism for reversing fade. It is not known whether the prevention of phosphorylation is the direct means by which enhancers retard tachyphylaxis. If these predictions are verified, it will still be necessary to work out how binding of an enhancer modifies changes in receptor structure and signaling to prevent phosphorylation. If these predictions are not verified, then another set of mechanisms consistent with the data reviewed here will need to be postulated and tested. These are huge gaps to fill, but the research directions just suggested will be well worth the effort if they lead to the kinds of dramatic breakthroughs in pharmacological treatment of diseases that followed the discovery of benzodiazapines.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Trumpp-Kallmeyer S, Hoflack J, Bruinvels A, Hibert M. Modeling of G-protein-coupled receptors: application to dopamine, adrenaline, serotonin, acetylcholine, and mammalian opsin receptors. J. Med. Chem. 1992;35(19):3448–3462. doi: 10.1021/jm00097a002. [DOI] [PubMed] [Google Scholar]
- 2.Bylund D B, Eikenberg D C, Hieble J P, Langer S Z, Lefkowitz R J, Minneman K P, Molinoff P B, Ruffolo R R , Jr, Trendelenburg U. International Union of Pharmacology nomenclature of adrenoceptors. Pharmacol. Rev. 1994;46:121–136. [PubMed] [Google Scholar]
- 3.Valant C, Aurelio L, Urmaliya V B, White P, Scammells P J, Sexton P M, Christopoulos A. Delineating the mode of action of adenosine A1 receptor allosteric modulators. Mol. Pharmacol. 2010;78:444–455. doi: 10.1124/mol.110.064568. [DOI] [PubMed] [Google Scholar]
- 4.Gilchrist A. Modulating G-protein-coupled receptors, from traditional pharmacology to allosterics. Trends Pharmacol. Sci. 2007;28:431–437. doi: 10.1016/j.tips.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Bridges T M, Lindsley C W. G-protein-coupled receptors, from classical modes of modulation to allosteric mechanisms. A.C.S. Chem. Biol. 2008;3:530–541. doi: 10.1021/cb800116f. [DOI] [PubMed] [Google Scholar]
- 6.Yanamala N, Klein-Seetharaman J. Allosteric modulation of G protein coupled receptors by cytoplasmic, transmembrane and extracellular ligands. Pharmaceuticals. 2010;3:3324–3342. doi: 10.3390/ph3103324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.May L T, Avlani V A, Sexton P M, Christopoulos A. Allosteric modulation of G protein-coupled receptors. Curr. Pharm. Des. 2004;10:2003–2013. doi: 10.2174/1381612043384303. [DOI] [PubMed] [Google Scholar]
- 8.Root-Bernstein R S, Dillon P F, Hollingsworth R. A tethered ascorbate-norepinephrine compound 4-UT displays long-acting adrenergic activity on rabbit aortic smooth muscle. Drug. Res. Develop. 2008;69:242–250. [Google Scholar]
- 9.Dillon P F, Root-Bernstein R S, Lieder C M. Antioxidant-independent ascorbate enhancement of catecholamine-induced contractions of vascular smooth muscle. Am. J. Physiol. Heart Circ. Physiol. 2004;286:H2353–H2360. doi: 10.1152/ajpheart.00968.2003. [DOI] [PubMed] [Google Scholar]
- 10.Dillon P F, Root-Bernstein R S, Lieder C M. Ascorbate enhancement of H1 histamine receptor sensitivity coincides with ascorbate oxidation inhibition by histamine receptors. Am. J. Physiol. Cell. Physiol. 2006;291:C977–C984. doi: 10.1152/ajpcell.00613.2005. [DOI] [PubMed] [Google Scholar]
- 11.Dillon PF., Root-Bernstein R, Robinson NE., Abraham WM, Berney C. Receptor-mediated enhancement of beta adrenergic drug activity by ascorbate in vitro and in vivo. PLoS One. 2010;5:e15130. doi: 10.1371/journal.pone.0015130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Root-Bernstein R S, Dillon P F. Fostering adventure research A case study of the discovery that ascorbic acid enhances adrenergic drug activity. Drug Develop. Res. 2002;57:58–74. [Google Scholar]
- 13.Maxwell L C, Herlihy J T, Riedel GL. Effects of ascorbic acid and EDTA on vascular concentration-response to catecholamines. Microvasc. Res. 1983;26:81–88. doi: 10.1016/0026-2862(83)90057-2. [DOI] [PubMed] [Google Scholar]
- 14.Houston J B, Wilkens H J, Levy G. Potentiation of isoproterenol effect by ascorbic acid. Res. Commun. Chem. Pathol. Pharmacol. 1976;14:643–650. [PubMed] [Google Scholar]
- 15.Grossmann M, Dobrev D, Himmel H M, Ravens U, Kirch W. Ascorbic acid-induced modulation of venous tone in humans. Hypertension. 2001;37:949–954. doi: 10.1161/01.hyp.37.3.949. [DOI] [PubMed] [Google Scholar]
- 16.Mak S, Newton G E. Vitamin C augments the inotropic response to dobutamine in humans with normal left ventricular function. Circulation. 2001;103:826–830. doi: 10.1161/01.cir.103.6.826. [DOI] [PubMed] [Google Scholar]
- 17.Shinke T, Shite J, Takaoka H, Hata K, Inoue N, Yoshikawa R, Matsumoto H, Masai H, Watanabe S, Ozawa T, Otake H, Matsumoto D, Hirata K, Yokoyama M. Vitamin C restores the contractile response to dobutamine and improves myocardial efficiency in patients with heart failure after anterior myocardial infarction. Am. Heart J. 2007;154:645.e1–645. doi: 10.1016/j.ahj.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Monahan K D, Eskurza I, Seals D R. Ascorbic acid increases cardiovagal baroreflex sensitivity in healthy older men. Am. J. Physiol. Heart Circ. Physiol. 2004;286(6):H2113–H2117. doi: 10.1152/ajpheart.01054.2003. [DOI] [PubMed] [Google Scholar]
- 19.Jablonski K L, Seals D R, Eskurza I, Monahan K D, Donato A J. High-dose ascorbic acid infusion abolishes chronic vasoconstriction and restores resting leg blood flow in healthy older men. J. Appl. Physiol. 2007;103:1715–1721. doi: 10.1152/japplphysiol.00533.2007. [DOI] [PubMed] [Google Scholar]
- 20.Puri S K, Cochin J, Volicer L. Effect of morphine sulfate on adenylate cyclase and phosphodiesterase activities in rat corpus striatum. Life Sci. 1975;16:759–768. doi: 10.1016/0024-3205(75)90352-5. [DOI] [PubMed] [Google Scholar]
- 21.Marti M C. The effects of chronic morphine administration to mice on the contractile activity in vitro of the vas deferens without and with morphine. Eur. J. Pharmacol. 1982;78:439–447. doi: 10.1016/0014-2999(82)90486-1. [DOI] [PubMed] [Google Scholar]
- 22.Rae G A, De Moraes S. Supersensitivity to noradrenaline in vas deferens from morphine-dependent mice is confirmed. Eur. J. Pharmacol. 1983;86:347–352. doi: 10.1016/0014-2999(83)90183-8. [DOI] [PubMed] [Google Scholar]
- 23.Akabori A, Barraclough C A. Effects of morphine on LH secretion and catecholamine turnovers in the hypothalamus of estrogen-treated rats. Brain Res. 1986;362:221–226. doi: 10.1016/0006-8993(86)90447-6. [DOI] [PubMed] [Google Scholar]
- 24.Akabori A, Barraclough C A. Gonadotropin responses to naloxone may depend upon spontaneous activity in noradrenergic neurons at the time of treatment. Brain Res. 1986;362:55–62. doi: 10.1016/0006-8993(86)91398-3. [DOI] [PubMed] [Google Scholar]
- 25.He J-R, Molnar J, Barraclough CA. Morphine amplifies norepinephrine , NE, -induced LH release but blocks NE-stimulated increases in LHRH mRNA levels, comparison of responses obtained in ovariectomized estrogen-treated normal and androgen-sterilized rats. Mol. Brain Res. 1993;20:71–78. doi: 10.1016/0169-328x(93)90111-2. [DOI] [PubMed] [Google Scholar]
- 26.Kindman L A, Kates R E, Ginsburg R. Opioids potentiate contractile response of rabbit myocardium to the beta adrenergic agonist isoproterenol. J. Cardiovasc. Pharmacol. 1991;17:61–67. doi: 10.1097/00005344-199101000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Lechner R B, Gurll N J, Reynolds D G. Naloxone potentiates the cardiovascular effects of catecholamines in canine hemorrhagic shock. Circ. Shock . 1985;16:347–361. [PubMed] [Google Scholar]
- 28.He J-R, Barraclough C A. Morphine but not naloxone enhances luteinizing hormone-releasing hormone neuronal responsiveness to norepinephrine. J. Neuroendocrinol. 1992;4:92–99. doi: 10.1111/j.1365-2826.1992.tb00350.x. [DOI] [PubMed] [Google Scholar]
- 29.Allgood S C, Gurll N J, Reynolds D G. Naloxone requires circulating catecholamines to attenuate the cardiovascular suppression of endotoxic shock. J. Surg. Res. 1988;44:73–81. doi: 10.1016/0022-4804(88)90125-4. [DOI] [PubMed] [Google Scholar]
- 30.Caffrey J L, Hathorne L F, Carter G C, Sinclair R J. Naloxone potentiates contractile responses to epinephrine in isolated canine arteries. Circ. Shock. 1990;31:317–332. [PubMed] [Google Scholar]
- 31.Caffrey J L, Stoll S T, Sinclair R J, Barron B A. +Naloxone enhances vascular contractile responses to added epinephrine. Prog. Clin. Biol. Res. 1990;328:375–378. [PubMed] [Google Scholar]
- 32.Lechner R B. Naloxone potentiates inotropic but not chronotropic effects of isoproterenol in vitro. Circ. Shock. 1993;39:226–230. [PubMed] [Google Scholar]
- 33.Gu H, Gaugl J F, Barron B A, Caffrey J L. Naloxone enhances cardiac contractile responses to epinephrine without altering epinephrine uptake from plasma. Circ. Shock. 1990;32:257–271. [PubMed] [Google Scholar]
- 34.Park W K, Chang C H, Chae J E, Kim M H, Cho Y L, Ahn D S. Phosphodiesterase inhibition by naloxone augments the inotropic actions of beta-adrenergic stimulation. Acta Anaesthesiol. Scand. 2009;53:1043–1051. doi: 10.1111/j.1399-6576.2009.02023.x. [DOI] [PubMed] [Google Scholar]
- 35.McCubbin J A, Surwit R S, Kuhn C M, Cochrane C, Feinglos M N. Naltrexone potentiates glycemic responses during stress and epinephrine challenge in genetically obese mice. Psychosom. Med. 1989;51:441–448. doi: 10.1097/00006842-198907000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Parra L, Pérez-Vizcaíno F, Alsasua A, Martín M I, Tamargo J. Mu- and delta-opioid receptor-mediated contractile effects on rat aortic vascular smooth muscle. Eur. J. Pharmacol. 1995;277:99–105. doi: 10.1016/0014-2999(95)00067-u. [DOI] [PubMed] [Google Scholar]
- 37.Lee C H, Berkowitz B A. Stereoselective and calcium-dependent contractile effects of narcotic antagonist analgesics in the vascular smooth muscle of the rat. J. Pharmacol. Exp. Ther. 1976;198:347–356. [PubMed] [Google Scholar]
- 38.Lee C H, Berkowitz B A. Calcium antagonist activity of methadone. L-acetylmethadol and L-pentazocine in the rat aortic strip. J. Pharmacol. Exp. Ther. 1977;202:646–653. [PubMed] [Google Scholar]
- 39.Deyo S N, Swift R M, Miller R J. Morphine and endorphins modulate dopamine turnover in rat median eminence. Proc. Natl. Acad. Sci. U. S. A. 1979;76:3006–3009. doi: 10.1073/pnas.76.6.3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deyo S N, Swift R M, Miller R J, Fang V S. Development of tolerance to the prolactin-releasing action of morphine and its modulation by hypothalamic dopamine. Endocrinlogy. 1980;106:1469–1474. doi: 10.1210/endo-106-5-1469. [DOI] [PubMed] [Google Scholar]
- 41.Tagaya E, Tamaoki J, Chiyotani A, Konno K. Stimulation of opioid mu-receptors potentiates beta adrenoceptor-mediated relaxation of canine airway smooth muscle. J. Pharmacol. Exp. Ther. 1995;275:1288–1292. [PubMed] [Google Scholar]
- 42.Purdy R E, Weber M A. Enhancement and prolongation of vascular smooth muscle contraction by aldosterone. Blood Vessels. 1983;20:34–43. doi: 10.1159/000158457. [DOI] [PubMed] [Google Scholar]
- 43.Purdy R E, Weber M A, Drayer JI. Vasoconstrictor effects of aldosterone in isolated vascular tissue. Clin. Exp. Hypertens. A. 1982;4:1583–1591. doi: 10.3109/10641968209061626. [DOI] [PubMed] [Google Scholar]
- 44.Weber M A, Purdy R E, Drayer J I. Interactions of mineralocorticoids and pressor agents in vascular smooth muscle. Hypertesion. 1983;5:141–146. doi: 10.1161/01.hyp.5.2_pt_2.i41. [DOI] [PubMed] [Google Scholar]
- 45.Tan K S, Grove A, McLean A, Gnosspelius Y, Hall I P, Lipworth B J. Systemic corticosteroid rapidly reverses bronchodilator subsensitivity induced by formoterol in asthmatic patients. Am. J. Respir. Crit. Care Med. 1997;156:28–35. doi: 10.1164/ajrccm.156.1.9610113. [DOI] [PubMed] [Google Scholar]
- 46.Tan K S, McFarlane L C, Lipworth B J. Concomitant administration of low-dose prednisolone protects against in vivo beta2-adrenoceptor subsensitivity induced by regular formoterol. Chest. 1998;113:34–41. doi: 10.1378/chest.113.1.34. [DOI] [PubMed] [Google Scholar]
- 47.Lipworth B J, Aziz I. Bronchodilator response to albuterol after formoterol and effects of acute corticosteroid administration. Chest. 2000;117:156–162. doi: 10.1378/chest.117.1.156. [DOI] [PubMed] [Google Scholar]
- 48.Spector R G. Influence of folic acid on noradrenaline stimulation of rat brain synaptosomes. Br. J. Pharmacol. 1971;43:438P. [PMC free article] [PubMed] [Google Scholar]
- 49.Spector R G. Effects of formyl tetrahydrofolic acid and noradrenaline on the oxygen consumption of rat brain synaptosome-mitochondrial preparations. Br. J. Pharmacol. 1972;44:279–85. doi: 10.1111/j.1476-5381.1972.tb07264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spector R G. Influence of folic acid on excitable tissues. Nat. New Biol. 1972;240:247–249. doi: 10.1038/newbio240247b0. [DOI] [PubMed] [Google Scholar]
- 51.Bourne M G, Sharma S K, Spector R G. The effects of folate on neurotransmitter uptake into rat cerebral cortex slices. Biochem. Pharmacol. 1976;25:1917–1918. doi: 10.1016/0006-2952(76)90201-x. [DOI] [PubMed] [Google Scholar]
- 52.Brocardo P S, Budni J, Kaster M P, Santos A R, Rodrigues A L. Folic acid administration produces an antidepressant-like effect in mice, evidence for the involvement of the serotenergic and noradrenergic systems. Neuropharmacology. 2008;54:464–473. doi: 10.1016/j.neuropharm.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 53.Tejero-Taldo M I, Sun J, Caffrey J L, Mallet R T. Pyruvate potentiates κ-adrenergic inotropism of stunned guinea-pig myocardium. J. Mol. Cell. Cardiol. 1998;30:2327–2339. doi: 10.1006/jmcc.1998.0792. [DOI] [PubMed] [Google Scholar]
- 54.Tejero-Taldo M I, Caffrey J L, Sun J, Mallet R T. Antioxidant properties of pyruvate mediate its potentiation of κ-adrenergic inotropism in stunned myocardium. J. Mol. Cell. Cardiol. 1999;31:1863–1872. doi: 10.1006/jmcc.1999.1020. [DOI] [PubMed] [Google Scholar]
- 55.Squires J E, Sun J, Caffrey J L, Yoshishige D, Mallet R T. Acetoacetate augments adrenergic inotropism of stunned myocardium by an antioxidant mechanism. Am. J. Physiol. Heart Circ. Physiol. 2003;284:H1340–H1347. doi: 10.1152/ajpheart.00473.2002. [DOI] [PubMed] [Google Scholar]
- 56.Kuppusamy U R, Das N P. Potentiation of beta-adrenoceptor agonist-mediated lipolysis by quercetin and fisetin in isolated rat adipocytes. Biochem. Pharmacol. 1994;47:521–529. doi: 10.1016/0006-2952(94)90184-8. [DOI] [PubMed] [Google Scholar]
- 57.Naidu P S, Singh A, Kulkarni S K. D2-dopamine receptor and alpha2-adrenoreceptor-mediated analgesic response of quercetin Indian J. Exp. Biol. 2003;41:1400–1404. [PubMed] [Google Scholar]
- 58.Kaur R, Chopra K, Singh D. Role of alpha2 receptors in quercetin-induced behavioral despair in mice. J. Med. Food. 2007;10:165–168. doi: 10.1089/jmf.2005.063. [DOI] [PubMed] [Google Scholar]
- 59.Qin F, Yan C, Patel R, Liu W, Dong E. Vitamins C and E attenuate apoptosis, beta-adrenergic receptor desensitization, and sarcoplasmic reticular Ca2+ ATPase downregulation after myocardial infarction. Free Radic. Biol. Med. 2006;40:1827–1842. doi: 10.1016/j.freeradbiomed.2006.01.019. [DOI] [PubMed] [Google Scholar]
- 60.Aziz I, McFarlane L C, Lipworth B J. Concomitant inhaled corticosteroid resensitises cardiac beta2-adrenoceptors in the presence of long-acting beta2-agonist therapy. Eur. J. Clin. Pharmacol. 1998;54:377–381. doi: 10.1007/s002280050478. [DOI] [PubMed] [Google Scholar]
- 61.Molenaar P. Kompa A R, Roberts S J, Pak H S, Summers R J. Localization of [125I]cyanopindolol binding in guinea-pig heart, characteristics of non-beta-adrenoceptor related binding in cardiac pacemaker and conducting regions. Neurosci. Lett. 1992;136:118–122. doi: 10.1016/0304-3940(92)90662-q. [DOI] [PubMed] [Google Scholar]
- 62.Self G J, Rigby P J, Passarelli M C, Goldie R G. Localization of [125I]cyanopindolol binding in guinea-pig heart, characteristics of non-κ-adrenoceptor related binding in cardiac pacemaker and conducting regions. Eur. J. Pharmacol. 1990;176:169–176. [Google Scholar]
- 63.Kimura K, Sidhu A. Ascorbic acid inhibits 125I-SCH 23982 binding but increases the affinity of dopamine for D1 dopamine receptors. J. Neurochem. 1994;63:2093–2098. doi: 10.1046/j.1471-4159.1994.63062093.x. [DOI] [PubMed] [Google Scholar]
- 64.Tolbert L C, Morris P E , Jr, Spollen J J, Ashe S C. Stereospecific effects of ascorbic acid and analogues on D, and D, agonist binding. Life Sci. 1992;51:921–930. doi: 10.1016/0024-3205(92)90400-j. [DOI] [PubMed] [Google Scholar]
- 65.Wiener H L, Lajtha A, Sershen H. Ascorbic acid inhibits I'H] SCH-23390 binding to striatal dopamine D, receptors . J. Recept. Res. 1989;9:331–339. doi: 10.3109/10799898909066062. [DOI] [PubMed] [Google Scholar]
- 66.Newsholme E A, Start C, editors. John Wiley & Sons New York. 1981. Regulation in Metabolism. pp. 16–17. [Google Scholar]
- 67.Rubenstein L A, Lanzara R G. Activation of G protein-coupled receptors entails cysteine modulation of agonist binding. J. Molec. Struc. 1998;430:57–71. [Google Scholar]
- 68.Shi L, Javitch JA. The binding site of aminergic G protein-coupled receptors, the transmembrane segments and second extracellular loop. Ann. Rev. Pharmacol. Toxicol. 2002;42:437–467. doi: 10.1146/annurev.pharmtox.42.091101.144224. [DOI] [PubMed] [Google Scholar]
- 69.Rubenstein L A, Zauhar R J, Lanzara R G. Molecular dynamics of a biophysical model for beta2-adrenergic and G protein-coupled receptor activation. J. Mol. Graph. Model. 2006;25:396–409. doi: 10.1016/j.jmgm.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 70.Canals M, Sexton PM, Christopoulos A. Allostery in GPCRs: 'MWC' revisited. Trends Biochem. Sci. 2011;36(12):663–672. doi: 10.1016/j.tibs.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 71.Johnston JM, Wang H, Provasi D, Filizola M. Assessing the relative stability of dimer interfaces in g protein-coupled receptors. PLoS Comput. Biol. 2012;8(8):e1002649. doi: 10.1371/journal.pcbi.1002649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dorsch S, Klotz KN, Engelhardt S, Lohse MJ, Bunemann M. Analysis of receptor oligomerization by FRAP microscopy. Nat. Methods. 2009;6:225–230. doi: 10.1038/nmeth.1304. [DOI] [PubMed] [Google Scholar]
- 73.Fung JJ, Deupi X, Pardo L, Yao XJ, Velez-Ruiz GA. Ligand regulated oligomerization of beta(2)-adrenoceptors in a model lipid bilayer. EMBO J. 2009;28:3315–3328. doi: 10.1038/emboj.2009.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang J, Chen S, Zhang JJ, Huang XY. Crystal structure of oligomeric κ1-adrenergic G protein-coupled receptors in ligand-free basal state. Nat. Struct. Mol. Biol. 2013;20(4):419–425. doi: 10.1038/nsmb.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.May LT, Leach K, Sexton PM, Christopoulos A. Allosteric modulation of G protein coupled receptors. Annu. Rev. Pharmacol. Toxicol. 2007;47:1–51. doi: 10.1146/annurev.pharmtox.47.120505.105159. [DOI] [PubMed] [Google Scholar]
- 76.Keov P, Sexton PM, Christopoulous A. Allosteric modulation of G-protein coupled receptors: A pharmacological perspective. Neuropharmacology. 2011;60:24–35. doi: 10.1016/j.neuropharm.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 77.Wooten D, Christopoulos A, Sexton PM. Emerging paradigms in GPCR allostery: implications for drug discovery. Nat. Rev. Drug Discov. 2013;12:630–643. doi: 10.1038/nrd4052. [DOI] [PubMed] [Google Scholar]
- 78.Yanamala N, Klein-Seetharaman J. Allosteric modulation of g protein coupled receptors by cytoplasmic, transmembrane and extracellular ligands. Pharmaceuticals. 2010;3:3324–3342. doi: 10.3390/ph3103324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moffat A C, Patterson D A, Curry A S, Owen P. Inhibition in vitro of cyclic adenosine 3'4'-monophosphate phosphodiesterase activity by drugs. Eur. J. Toxicol. 1972;5:160–162. [PubMed] [Google Scholar]
- 80.Gintzler A R, Musacchio J M. Interactions of morphine adenosine adenosine triphosphate and phosphodiesterase inhibitors on the field-stimulated guinea-pig ileum. J. Pharmacol. Exp. Ther. 1975;194:575–582. [PubMed] [Google Scholar]
- 81.Buck M G, Zadunaisky J A. Stimulation of ion transport by ascorbic acid through inhibition of 3', 5'-cyclic-AMP phos-phodiesterase in the corneal epithelium and other tissues. Biochim. Biophys.Acta. 1975;389:251–260. doi: 10.1016/0005-2736(75)90319-3. [DOI] [PubMed] [Google Scholar]
- 82.Tisdale M J. Inhibition of cyclic adenosine 3'4'-monophosphate phosphodiesterase from Walker carcinoma by ascorbic and dehydroascorbic acid. Biochem. Biophys. Res. Commun. 1975;62:877–882. doi: 10.1016/0006-291x(75)90404-0. [DOI] [PubMed] [Google Scholar]
- 83.Malamud D, Kroll Y. Ascorbic acid inhibition of cyclic nucleotide phosphodiesterase activity. Proc. Soc. Exp. Biol. Med. 1980;164:534–536. doi: 10.3181/00379727-164-40911. [DOI] [PubMed] [Google Scholar]
- 84.Shinohara K, Fujiki H, Hidaka Y, Tseng Y K, Murakami H, Omura H. Effect of reductones on cyclic 3', 5'-adenosine monophosphate phosphodiesterase. Acta Vitaminol. Enzymol. 1985;7:99–108. [PubMed] [Google Scholar]
- 85.Lanzara R. Optimal agonist/antagonist combinations maintain receptor response by preventing rapid ß1-adrenergic receptor desensitization. Int. J. Pharmacol. 2005;1:122–131. [Google Scholar]