Abstract
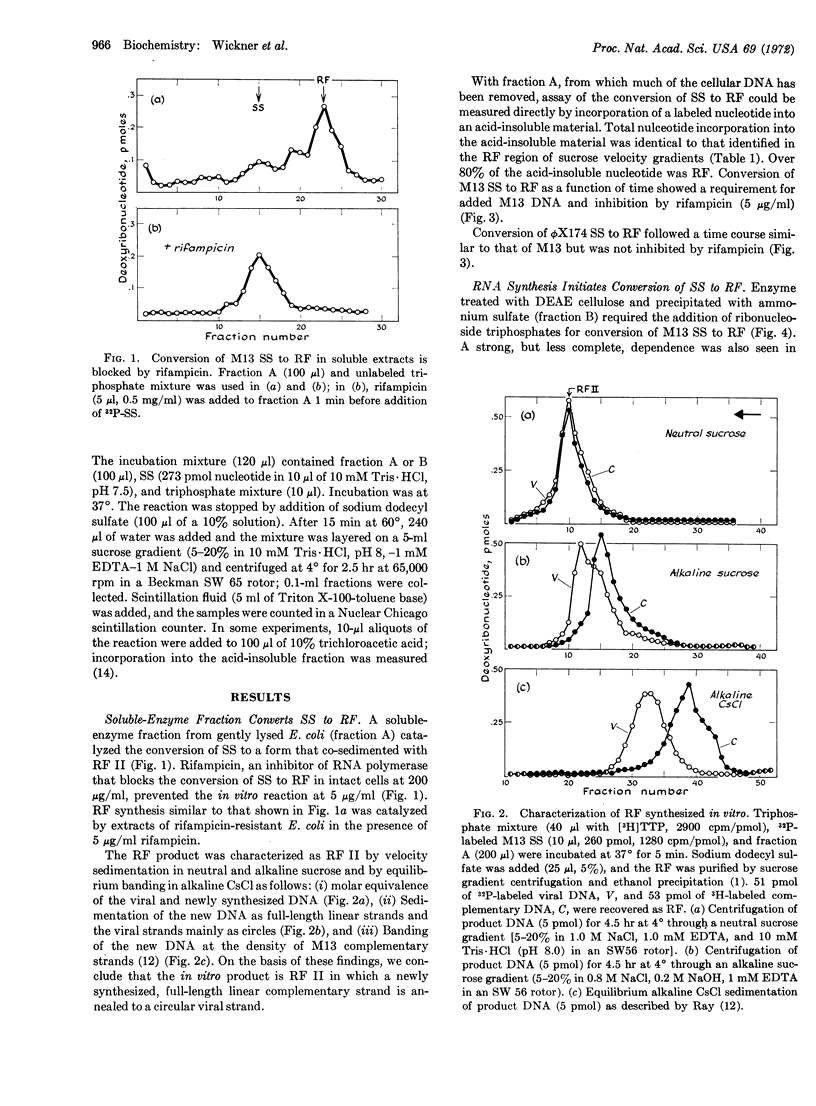
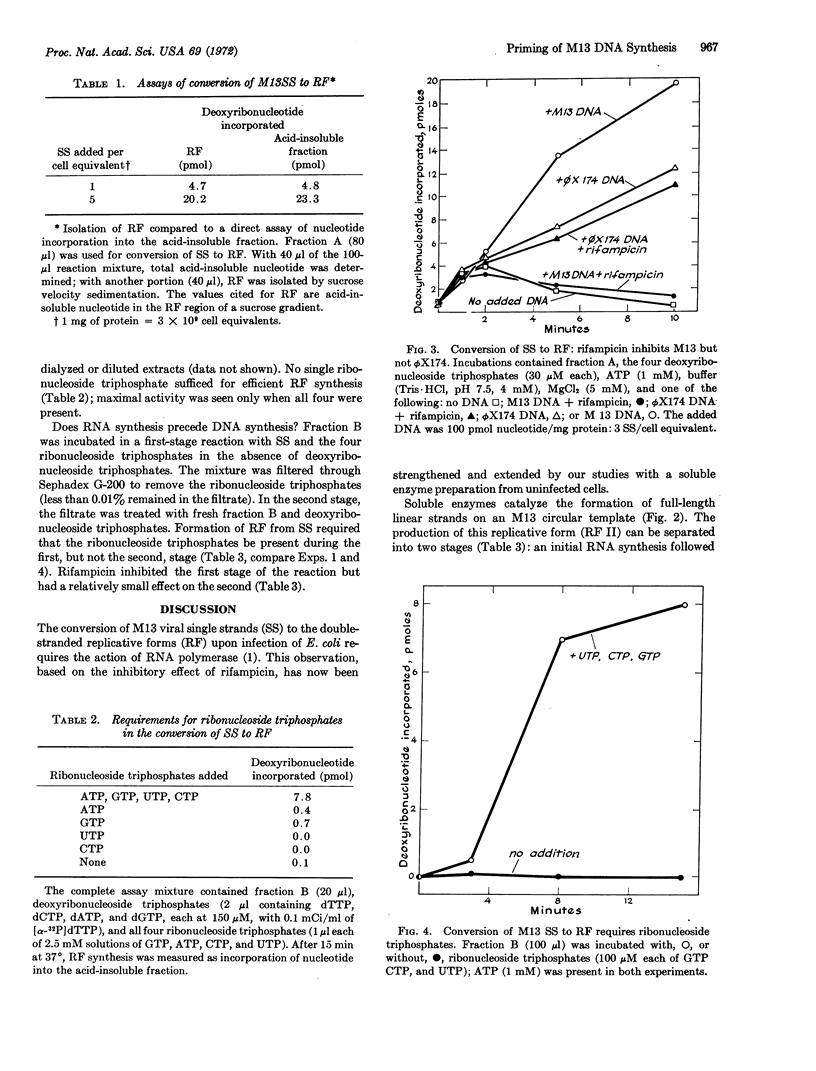
Soluble enzyme fractions from uninfected Escherichia coli convert M13 and ϕX174 viral single strands to their double-stranded replicative forms. Rifampicin, an inhibitor of RNA polymerase, blocks conversion of M13 single strands to the replicative forms in vivo and in vitro. However, rifampicin does not block synthesis of the replicative forms of ϕX174 either in vivo or in soluble extracts. The replicative form of M13 synthesized in vitro consists of a full-length, linear, complementary strand annealed to a viral strand. The conversion of single strands of M13 to the replicative form proceeds in two separate stages. The first stage requires enzymes, ribonucleoside triphosphates, and single-stranded DNA; the reaction is inhibited by rifampicin. The macromolecular product separated at this stage supports DNA synthesis with deoxyribonucleoside triphosphates and a fresh addition of enzymes; ribonucleoside triphosphates are not required in this second stage nor does rifampicin inhibit the reaction. We presume that in the first stage there is synthesis of a short RNA chain, which then primes the synthesis of a replicative form by a DNA polymerase.
Keywords: ϕX174 DNA, DNA initiation, DNA replication, rifampicin
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brutlag D., Schekman R., Kornberg A. A possible role for RNA polymerase in the initiation of M13 DNA synthesis. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2826–2829. doi: 10.1073/pnas.68.11.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsheit A. B., Ray D. S. Replication of bacteriophage M13. VI. Attachment of M13 DNA to a fast-sedimenting host cell component. Virology. 1971 Mar;43(3):647–664. doi: 10.1016/0042-6822(71)90289-3. [DOI] [PubMed] [Google Scholar]
- Francke B., Ray D. S. Fate of parental phi X174-DNA upon infection of starved thymine-requiring host cells. Virology. 1971 Apr;44(1):168–187. doi: 10.1016/0042-6822(71)90163-2. [DOI] [PubMed] [Google Scholar]
- Jovin T. M., Englund P. T., Bertsch L. L. Enzymatic synthesis of deoxyribonucleic acid. XXVI. Physical and chemical studies of a homogeneous deoxyribonucleic acid polymerase. J Biol Chem. 1969 Jun 10;244(11):2996–3008. [PubMed] [Google Scholar]
- Ray D. S. Replication of bacteriophage M13. II. The role of replicative forms in single-strand synthesis. J Mol Biol. 1969 Aug 14;43(3):631–643. doi: 10.1016/0022-2836(69)90364-7. [DOI] [PubMed] [Google Scholar]
- Silverstein S., Billen D. Transcription: role in the initiation and replication of DNA synthesis in Escherichia coli and phiX174. Biochim Biophys Acta. 1971 Oct;247(3):383–390. doi: 10.1016/0005-2787(71)90023-2. [DOI] [PubMed] [Google Scholar]
- Sippel A., Hartmann G. Mode of action of rafamycin on the RNA polymerase reaction. Biochim Biophys Acta. 1968 Mar 18;157(1):218–219. doi: 10.1016/0005-2787(68)90286-4. [DOI] [PubMed] [Google Scholar]
- Steinberg R. A., Denhardt D. T. Inhibition of synthesis of phiX174 DNA in a mutant host thermosensitive for DNA synthesis. J Mol Biol. 1968 Nov 14;37(3):525–528. doi: 10.1016/0022-2836(68)90120-4. [DOI] [PubMed] [Google Scholar]
- Vosberg H. P., Hoffmann-Berling H. DNA synthesis in nucleotide-permeable Escherichia coli cells. I. Preparation and properties of ether-treated cells. J Mol Biol. 1971 Jun 28;58(3):739–753. doi: 10.1016/0022-2836(71)90037-4. [DOI] [PubMed] [Google Scholar]


