Radiation therapy is a commonly used and rapidly evolving modality in the treatment of cancer in veterinary practice. It can be used to treat tumors curatively, or to palliate the symptoms of the disease. In the late 1960s, the first linear accelerators were introduced in veterinary practice and radiation therapy has since progressed into a complex field with the emergence of several innovative techniques (1). New technology, such as Multi-Leaf Collimators (MLCs) and On-board Imaging (OBI) systems, has allowed for more complicated techniques leading to better outcomes and less severe side effects (2).
Stereotactic radiation therapy (SRT) is one method used to deliver a curative dose of external beam radiation therapy. This precise and conformal treatment directly targets the radiation at the tumor with rapid dose drop-off, which allows for very high doses of radiation to be administered without increasing toxicity to adjacent normal tissues (3). It can be delivered using a linear accelerator, gamma knife, Cyberknife, or Tomotherapy unit.
Stereotactic radiation therapy, as opposed to conventional curative treatments, is delivered with very few treatments. Whereas conventional radiation treatments for veterinary patients with cancer involve 15 to 20 treatments on a Monday to Friday basis over the course of 3 to 4 weeks, SRT can be done in a few days, usually involving 3 treatments consecutively. The total dose per course of treatment is less than conventional treatments but the dose per fraction is higher, resulting in a biological equivalent dose. For example, a nasal tumor treated conventionally may consist of 54 Gy in 18 fractions, whereas SRT would consist of 27 Gy in 3 fractions. This short treatment regimen allows for fewer anesthetic events without compromising tumor control, which is beneficial for the patient. This is also advantageous to the client as it provides convenience in terms of time and travel.
The concepts of SRT stem from stereotactic radiosurgery techniques used for brain tumors in humans, which have allowed the development of treatment regimens elsewhere in the body (4). At the Western College of Veterinary Medicine, SRT is delivered with a linear accelerator and involves multiple beam angles in combination with dynamic intensity modulated radiation therapy (IMRT) to deliver a conformal dose directly to the tumor. The IMRT modifies the fluence of the radiation beam by shifting the position of the MLCs during treatment (5). This alters the intensity of the dose within the patient, allowing for tight margins around the tumor, regardless of tumor shape. The use of IMRT for SRT treatments also allows for avoidance of critical organs as defined in each treatment plan, so that side effects can be kept to a minimum (5).
Stereotactic radiation therapy is an alternative to invasive or debilitating surgery, especially for deep-seated brain tumors, or tumors that require amputation or radical surgery. It may also be used preoperatively as a means of debulking a tumor deemed unresectable (2). It can be used to treat many types of tumors; however, these tumors must be intact at the time of treatment; SRT cannot be used to treat a tumor that has already been surgically removed. This is the case because the surrounding surgical site would also need to be irradiated leading to severe normal tissue toxicity. Some common tumors treated with SRT include: brain tumors, oral tumors, nasal tumors, mast cell tumors, soft tissue sarcomas, and osteosarcomas.
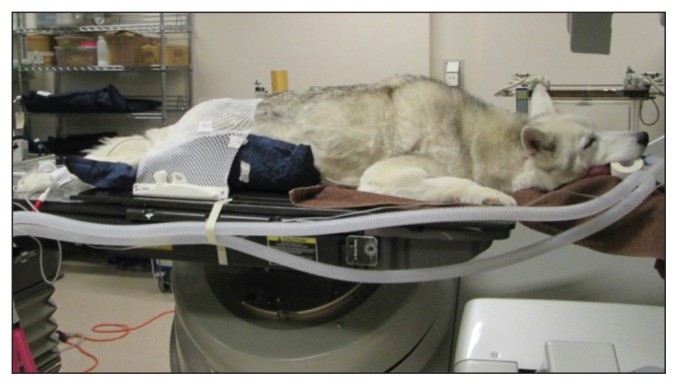
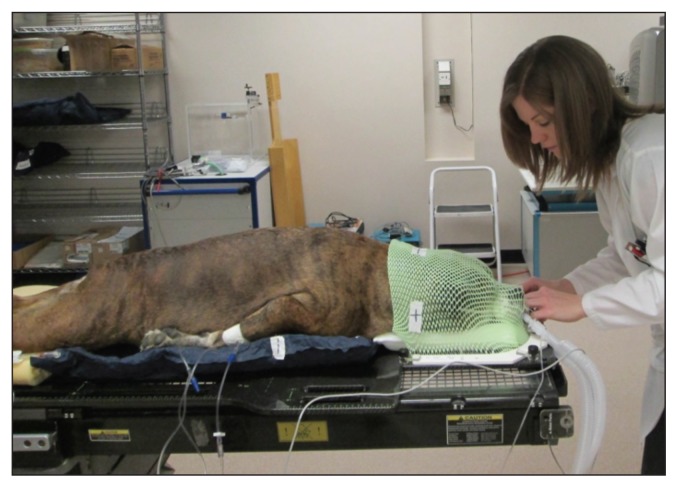
Elaborate immobilization devices are needed for SRT due to the precise nature of the treatment. This equipment is customized to the patient, so that maximum accuracy and reproducibility can be maintained throughout the course of treatment (Figures 1A–B). The equipment usually includes a formed cushion, mouldable thermoplastic shells, and a skull fixation device, depending on the tumor site. The immobilization devices are constructed immediately before the patient’s CT simulation scan so that the patient will be in the same position for the treatment as he/she was for the scan.
Figure 1A.
Pelvic immobilization device constructed for stereotactic radiation therapy.
Figure 1B.
Head and neck immobilization device constructed for SRT.
A 3-dimensional plan is needed prior to SRT; therefore, patients referred to the WCVM radiation oncology service will require a CT simulation scan regardless of whether any diagnostic scanning has been done. The planning for SRT is much more elaborate and complex than conventional plans, and may take more time to perform. Quality assurance checks are essential with SRT, and are carried out at the start of each treatment to ensure the patient is receiving the correct calculated dose.
Image-guided radiation therapy is used to determine any inaccuracies with patient set up, and has been in use since the beginning of radiation oncology (6). The introduction of an On-board Imager (OBI) to a linear accelerator has allowed for the acquisition of kV images and cone-beam computed tomography (CBCT) images before treatment commences. These daily images are taken to ensure the patient is in the same position as in the CT simulation scan and allow the placement of the target volume within millimeters of accuracy.
Due to the conformal nature of SRT, side effects from the treatment tend to be minimal and confined to the tumor and immediate surrounding area (Figures 2B–D). Acute side effects are limited to rapidly dividing tissues and may include erythema, dry and moist desquamation, mucositis, and colitis; depending on where the radiation is targeted (7). The risk of late effects is higher with SRT due to the high dose per fraction (7); however, the risk of these effects occuring can be decreased with careful planning by the radiation oncologist. Mild late side effects may be inevitable and include hyper- or hypopigmentation, alopecia, and fibrosis. More severe late effects may include necrosis, fistula, or stricture. In conventional curative treatments, the plan is designed so that less than 5% of patients will likely suffer these severe side effects over 5 years (8). Although data are limited for SRT, there are calculation models that provide a reliable approximation for tumor doses as well as tolerance doses of critical organs when using less than 10 Gy per fraction (3).
Figure 2B.
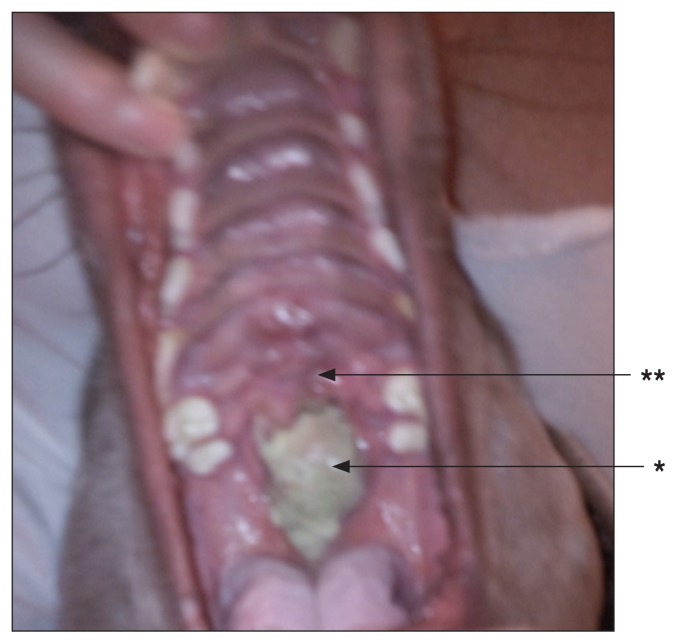
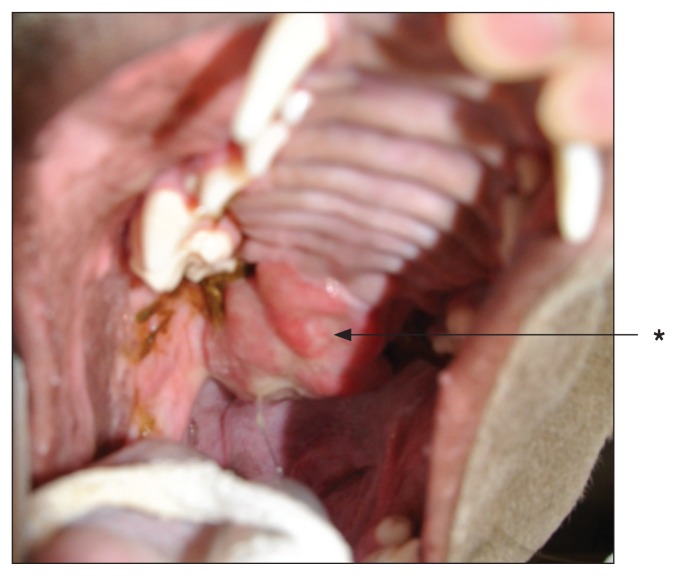
Three weeks post SRT. Note the necrotic tumor tissue (*) with mild mucositis in the immediate surrounding area (**).
Figure 2D.
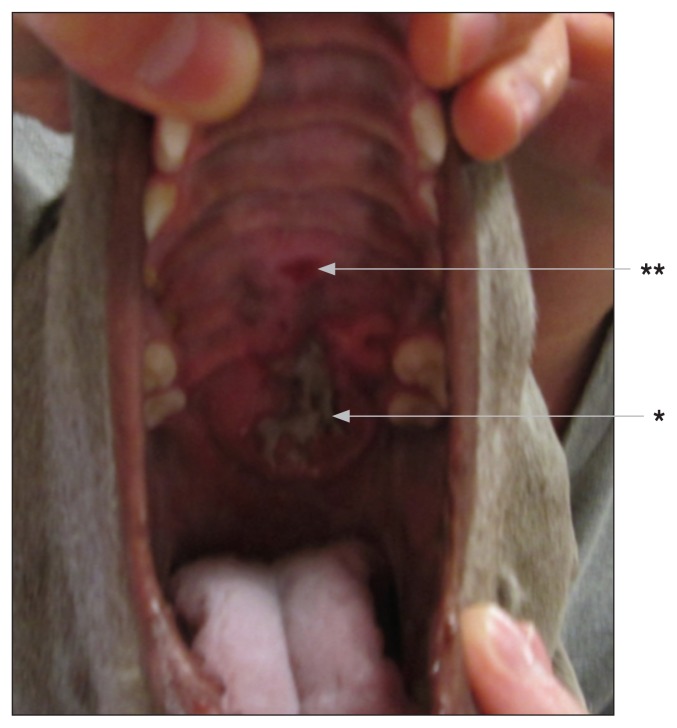
Ten weeks post SRT. There is no evidence of residual disease. Acute side effects have subsided. Appearance of hyperpigmentation (*) and fibrosis (**).
Figure 2A.
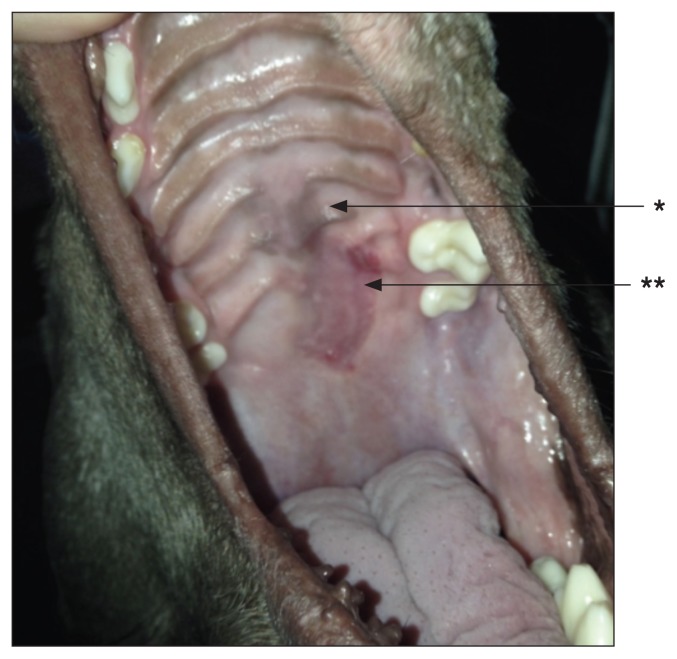
A 2-year-old spayed female Weimaraner with initial presentation of a 3.0 cm × 1.7 cm × 6.2 cm (W × H × L) oral fibrosarcoma (*) of the soft and hard palate with evidence of disease in the mandibular lymph nodes. Surgical resection was determined to have a high risk of an oronasal fistula; therefore, stereotactic radiation therapy was used. The target volume included the tumor with a 1 cm margin, and the submandibular lymph nodes.
Figure 2C.
Four weeks post SRT. Note the tumor cells sloughing away (*). There is mild ulceration of the palette (**).
Footnotes
Use of this article is limited to a single copy for personal study. Anyone interested in obtaining reprints should contact the CVMA office (hbroughton@cvma-acmv.org) for additional copies or permission to use this material elsewhere.
References
- 1.Gillette EL. History of veterinary radiation oncology. Vet Clin N Am Small Anim Pract. 1997;13:1–6. doi: 10.1016/s0195-5616(97)50001-5. [DOI] [PubMed] [Google Scholar]
- 2.Aristu JJ, Ciérvide R, Guridi J, et al. Stereotactic radiation therapy. An Sist Sanit Navar. 2009;32(Suppl 2):61–71. doi: 10.23938/ASSN.0176. [DOI] [PubMed] [Google Scholar]
- 3.Song CW, Park H, Griffin RJ, Levitt SH. Radiobiology of stereotactic radiosurgery and stereotactic body radiation therapy. In: Levitt SH, Purdy JA, Perez CA, Poortsmans P, editors. Technical Basis of Radiation Therapy. 5th ed. New York, New York: Springer-Verlag Berlin Heidelberg; 2012. pp. 51–61. [Google Scholar]
- 4.Song DY, Kavanagh BD, Benedict SH, Schefter T. Stereotactic body radiation therapy: Rationale, techniques, applications, and optimization. Oncology. 2004;18:1419–30. [PubMed] [Google Scholar]
- 5.Lawrence JA, Forrest LJ. Intensity-modulated radiation therapy and helical tomotherapy: Its origin, benefits, and potential applications in veterinary medicine. Vet Clin N Am Small Anim Pract. 2007;37:1151–1165. doi: 10.1016/j.cvsm.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Verellen D, Ridder MD, Storme G. A (short) history of image-guided radiotherapy. Radiother Oncol. 2008;86:4–13. doi: 10.1016/j.radonc.2007.11.023. [DOI] [PubMed] [Google Scholar]
- 7.Harris D, King GK, Bergman PJ. Radiation therapy toxicities. Vet Clin N Am Small Anim Pract. 1997;27:37–46. doi: 10.1016/s0195-5616(97)50004-0. [DOI] [PubMed] [Google Scholar]
- 8.Emami B. Tolerance of normal tissue to therapeutic radiation. [Last accessed October 21, 2014];Reports of Radiotherapy and Oncology. 2013 1:35–48. Available from: journals.sbmu.ac.ir/rro/article/download/4316/3851. [Google Scholar]