Abstract
n
Our objective was to confirm the efficacy and safety of edaravone in amyotrophic lateral sclerosis (ALS) patients. We conducted a 36-week confirmatory study, consisting of 12-week pre-observation period followed by 24-week treatment period. Patients received placebo or edaravone i.v. infusion over 60 min for the first 14 days in cycle 1, and for 10 of the first 14 days during cycles 2 to 6. The efficacy primary endpoint was changed in the revised ALS functional rating scale (ALSFRS-R) scores during the 24-week treatment. Patients were treated with placebo (n = 104) and edaravone (n = 102). Changes in ALSFRS-R during the 24-week treatment were −6.35 ± 0.84 in the placebo group (n = 99) and −5.70 ± 0.85 in the edaravone group (n = 100), with a difference of 0.65 ± 0.78 (p = 0.411). Adverse events amounted to 88.5% (92/104) in the placebo group and 89.2% (91/102) in the edaravone group. In conclusion, the reduction of ALSFRS-R was smaller in the edaravone group than in the placebo group, but efficacy of edaravone for treatment of ALS was not demonstrated. Levels and frequencies of reported adverse events were similar in the two groups.
Keywords: Amyotrophic lateral sclerosis, ALSFRS-R, edaravone, placebo, randomized trial
Introduction
Amyotrophic lateral sclerosis (ALS) is a refractory and progressive disease that causes selective degeneration of upper and lower motor neurons (1). Median survival from onset to death in ALS is reported to vary from 20 to 48 months (2).
Oxidative stress has been considered to be involved in the onset and progression of ALS (3). An established marker of oxidative stress is 3-nitrotyrosine (3NT), formed by reaction of the free radical peroxynitrite with tyrosine residues. A significant increase of 3NT was reported in spinal cord of transgenic mice expressing mutated SOD1 (4) and in autopsied spinal cord of FALS patients with genetic mutation of SOD1 and sporadic ALS (SALS) patients (5). 3NT was found in motor neurons (6,7) and was elevated in cerebrospinal fluid of SALS patients (8). Moreover, oxidative stress induces nuclear translocation and activation of Nrf-2, a transcription factor that generates an anti-oxidative response (9). Nrf-2 translocation also occurs in mutant TDP-43 transfected cultured motor neuron cell lines (10–12). Consequently, drugs that eliminate free radicals might protect motor neurons from oxidative stress and free radical damage in ALS patients.
Edaravone (MCI-186, 3-methyl-1-phenyl-2-pyrazolin-5-one, Mitsubishi Tanabe Pharma Corporation, Tokyo, Japan) is a free radical scavenger approved for treatment of acute cerebral infarction in Japan in 2001 (13). Edaravone eliminates lipid peroxides and hydroxyl radicals during cerebral ischemia and protects nerve cells within or around the ischemic region from free radical damage (14–16). Beneficial effects of edaravone have been reported in wobbler mice with ALS-like symptoms (17) and in ALS-model animals (18,19).
A phase II trial was conducted to investigate the safety and efficacy of edaravone in ALS patients, and found that progression of motor dysfunction was slowed and no clinically significant adverse drug reactions occurred. The level of 3NT was low in cerebrospinal fluid of almost all patients in the phase II trial, suggesting that edaravone could protect neuronal cells from oxidative stress (20). Therefore we designed a clinical trial to confirm the efficacy and safety of edaravone in ALS patients.
Methods
Standard protocol approvals
Twenty-nine sites in Japan participated in the study between May 2006 and September 2008. An institutional review board at each site approved the study protocol. The study was conducted in compliance with Good Clinical Practice (GCP). All participants provided written informed consent before the pre-observation stage. The study sponsor was Mitsubishi Tanabe Pharma Corporation. The study is registered in ClinicalTrials.gov with a registration number NCT00330681.
Patients
Inclusion criteria were: age 20–75 years; diagnosis of ‘definite’, ‘probable’ or ‘probable laboratory-supported’ ALS (21,22) according to the revised Airlie House diagnostic criteria; forced vital capacity (FVC) of at least 70%; duration of disease within three years; and change in revised ALS functional rating scale (ALSFRS-R) (23,24) score during the 12-week pre-observation period of −1 to −4 points. Patients also had a Japanese ALS severity classification (25) of 1 or 2. (The Japanese ALS severity classification score ranges from 1 to 5 according to the severity classification of the Specified Disease Treatment Research Program for ALS of the Ministry of Health, Labor and Welfare of Japan. Severity Classification: 1) able to work or perform housework; 2) independent living but unable to work; 3) requiring assistance for eating, excretion or ambulation; 4) presence of respiratory insufficiency, difficulty in coughing out sputum or dysphagia; and 5) using a tracheostomy tube, tube feeding or tracheostomy positive pressure ventilation.)
Exclusion criteria were: reduced respiratory function and complaints of dyspnea; complications that may substantially influence evaluation of drug efficacy, such as Parkinson’s disease, schizophrenia and dementia; complications that require hospitalization, including liver, cardiac and renal diseases; infections that require antibiotic therapy; deteriorated general condition as judged by investigators; renal dysfunction with creatinine clearance of 50 ml/min or below within 28 days before treatment; and undergoing cancer treatment.
Patient eligibility was assessed with inclusion and exclusion criteria at the start and end of pre-observation.
Administration regimen of riluzole was required not to be changed during the study.
Study medication
Mitsubishi Tanabe Pharma Corporation provided the investigational drugs in ampoules. Only authorized personnel, independent of the sponsor and investigators, had access to the key code until unblinding. The dose of edaravone was 60 mg per day, which was indicated to show efficacy in the phase II trial (20), and placebo was chosen since no suitable comparator drug for ALS has been approved. Saline (placebo) or edaravone was administered once daily by i.v. infusion over 60 min.
Design
After the 12-week pre-observation period, eligible patients were randomized to placebo or edaravone group. Dynamic allocation was used to minimize the effects of the following three factors, which may substantially influence the evaluation of edaravone:
Factor 1: change in ALSFRS-R score during pre-observation period: two categories: −4, −3 or −2, −1.
Factor 2: initial symptom: two categories – bulbar or limb.
Factor 3: use of riluzole: two categories – yes or no.
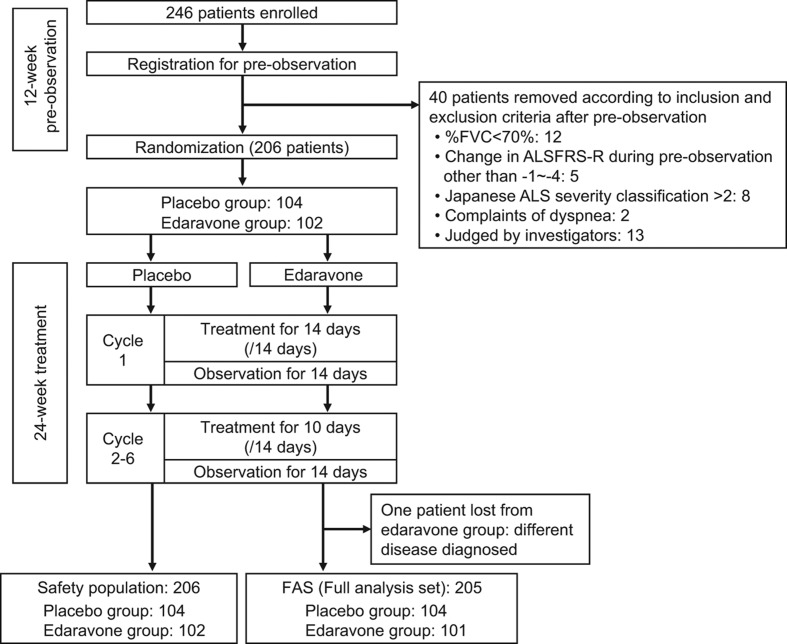
The study period was 36 weeks, consisting of a 12-week pre-observation period before the start of the first cycle, followed by a 24-week treatment period (Figure 1).
Figure 1.
Trial profile.
A single treatment cycle consisted of 14 days of study drug administration period followed by a 14-day observation period. Study drugs were administered every day for 14 days in the administration period of the first cycle, and for 10 out of 14 days in the administration periods of cycles 2 to 6. The end of the administration period in each cycle was followed by a 14-day observation period.
Efficacy evaluation
Primary efficacy endpoint was the change in ALSFRS-R score. Secondary endpoints were: changes of FVC, grip strength (left/right mean), pinch strength (left/right mean), Modified Norris Scale score (26,27), ALSAQ-40 (ALS Assessment Questionnaire) (28,29), and time to death or a specified state of disease progression (incapable of independent ambulation, loss of function in upper limbs, tracheotomy, artificial respirator with intubation, or tube feeding). The evaluations were carried out at the following times: before pre-observation, before the start of the first treatment cycle and at the end of each treatment cycle (after 14 days observation and before the first dosage of the next cycle).
Safety evaluation
Safety was assessed in terms of number and severity of adverse events (AE), adverse drug reactions and the results of clinical laboratory tests and sensory tests. Serious adverse events were identified from the adverse events according to the GCP guideline.
Statistical analysis
Based upon the experience of the phase II trial (20), we considered that it would be difficult to enroll more than 100 patients per group for the trial and the target number of patients for enrollment was set at 200. In the phase II trial, the difference of the change of ALSFRS-R between the edaravone and placebo groups was 2.2 for patients with matched severity and dose to those of the present study; and on the assumption of a standard deviation of 5.2, the statistical power of this study can be calculated as 85% when 100 patients per group were enrolled.
The primary population used for the efficacy analysis was the full analysis set (FAS). For ALSFRS-R scores, analysis of covariance (ANCOVA) was performed on the change in score during treatment, defined as the difference between the score before the start of the first treatment cycle (before treatment) and the score at two weeks after the end of the sixth treatment cycle (after treatment). Three factors were used for dynamic allocation as covariates, after which the inter-group difference was assessed. Repeated measures analysis of variance was also performed using the treatment group, period, and interaction between treatment group and period (treatment group × period) as design factors, and baseline value and the three factors used for dynamic allocation as covariates, after which the inter-group difference was assessed. Compound symmetry was assumed as a covariance structure of repeated measurement. Edaravone efficacy would be verified if a significant inter-group difference were found in at least one of the above analyses. A two-sided level of significance of 5% and a two-sided 95% confidence interval were chosen for interpretation of main effect. A two-sided level of significance of 15% was chosen for determining the existence of effect of interaction. A stratified analysis was also performed on the changes of ALSFRS-R score by diagnostic category. The level of significance for differences of patient characteristics was set at 15%.
ANCOVA and repeated measures analysis of variance were similarly performed on the secondary endpoints. Time to death or a specified state of disease progression was defined as an event and the other endpoints were followed until cut-off. A stratified, generalized Wilcoxon test and log-rank test were performed using the change in ALSFRS-R score during the pre-observation period as a stratification factor. For patients with more than one event, the onset date of the first event was defined as the survival time. In censored cases, the cut-off date was the end date of observations.
For patients with missing data at 24 weeks after starting treatment, the last observation carried forward (LOCF) method was applied to impute missing data. Patients who completed the third cycle were eligible for LOCF.
To evaluate safety, AE and adverse drug reactions were assessed in the safety population. Proportions of AE, adverse drug reactions, serious adverse events (SAE) and serious adverse drug reactions were calculated and compared between the groups using Fisher’s exact test. A two-sided level of significance of 5% and a two-sided 95% confidence interval were chosen for interpretation. Statistical analysis was performed using SAS software (version 9.1, SAS Institute, Cary, NC).
Results
Subject background
Two hundred and forty-six patients were prospectively registered. After the 12-week pre-observation period, 40 patients were excluded according to the inclusion and exclusion criteria, and the remaining 206 patients were randomized (Figure 1).
The FAS included 205 patients after exclusion of one patient who was diagnosed with a different disease. For the safety evaluation, the number of patients in the safety population was 206, which included all patients treated with the study medication. The treatment was discontinued for 23 patients (edaravone group: patients’ request 5, AE 3, tracheotomy 1; placebo group: patients’ request 5, AE 6, tracheotomy 2, protocol violation 1). There was no imbalance between the groups in either analysis set on discontinuation (FAS: p = 0.378; safety population: p = 0.377).
All patients in the safety population received at least 80% of the assigned dosages of study drug.
Patient characteristics are summarized in Table I. Among the patient characteristics, those for which inter-group differences were found at a significance level below 15% were the duration of disease (p = 0.104, paired t-test), ALSFRS-R score before pre-observation (p = 0.065, paired t-test), and ALSFRS-R score at the start of the first cycle (p = 0.146, paired t-test).
Table I.
Subject demographic characteristics.
| Item | Placebo (104) n (%) |
Edaravone (101) n (%) |
|---|---|---|
| Gender | ||
| male | 69 (66.3) | 63 (62.4) |
| Initial symptom | ||
| bulbar | 20 (19.2) | 18 (17.8) |
| limb | 84 (80.8) | 83 (82.2) |
| Diagnosis (El Escorial revisited) | ||
| definite | 21 (20.2) | 29 (28.7) |
| probable | 54 (51.9) | 52 (51.5) |
| probable laboratory-supported | 28 (26.9) | 20 (19.8) |
| possible | 1 (1.0) | 0 (0.0) |
| The Japanese severity classification | ||
| grade 1 | 40 (38.5) | 36 (35.6) |
| grade 2 | 64 (61.5) | 65 (64.4) |
| Use of riluzole | ||
| yes | 92 (88.5) | 90 (89.1) |
| Change in ALSFRS-R score during pre-observation | ||
| −4, −3 | 32 (30.8) | 29 (28.7) |
| −2, −1 | 72 (69.2) | 72 (71.3) |
| Item | Placebo (104) median (min-max) |
Edaravone (101) median (min-max) |
|---|---|---|
| Age (years old) | 58.5 (28–75) | 58.0 (29–73) |
| Body weight (kg) | 57.0 (37–109) | 57.0 (35–77) |
| Duration of disease (years) | 1.20 (0.3–3.0) | 1.30 (0.4–2.9) |
| ALSFRS-R score before pre-observation | 44.0 (35–48) | 43.0 (31–48) |
| ALSFRS-R score before treatment period | 42.0 (32–47) | 41.0 (29–47) |
ALSFRS-R: the revised amyotrophic lateral sclerosis functional rating scale.
Efficacy
The results of ANCOVA for the change of ALSFRS-R score during treatment and the results of repeated measures analysis of variance are shown in Table II. In both analyses, no significant inter-group difference was observed.
Table II.
Change in endpoints during treatment.
| Change in endpoints during treatment (ANCOVA) |
Repeated-measures analysis |
|||||||
|---|---|---|---|---|---|---|---|---|
| Adjusted mean change LS Mean ± S.E. |
Inter-group difference in adjusted mean change LS Mean ± S.E. (95% C.I.) |
Adjusted mean LS Mean ± S.E. |
Inter-group difference in adjusted mean LS Mean ± S.E. (95% C.I.) |
|||||
| Placebo | Edaravone | p value | Placebo | Edaravone | p value | |||
| Primary endpoint | ||||||||
| ALSFRS-R | −6.35 ± 0.84 (99) |
−5.70 ± 0.85 (100) |
0.65 ± 0.78 (−0.90 – 2.19) |
0.411 | 37.43 ± 0.46 | 38.08 ± 0.47 | 0.65 ± 0.44 (−0.22 – 1.52) |
0.141 |
| Secondary endpoint | ||||||||
| %FVC | −17.49 ± 2.39 (99) |
−14.57 ± 2.41 (100) |
2.92 ± 2.24 (−1.49, 7.33) |
0.193 | 87.30 ± 1.56 | 88.56 ± 1.59 | 1.26 ± 1.46 (−1.63, 4.15) |
0.390 |
| Grip strength | −5.71 ± 0.69 (99) |
−4.81 ± 0.69 (100) |
0.89 ± 0.64 (−0.37, 2.16) |
0.165 | 13.22 ± 0.42 | 13.83 ± 0.43 | 0.60 ± 0.40 (−0.18, 1.38) |
0.130 |
| Pinch strength | −1.03 ± 0.15 (99) |
−0.83 ± 0.15 (100) |
0.20 ± 0.14 −0.08, 0.48) |
0.165 | 2.62 ± 0.11 | 2.83 ± 0.11 | 0.21 ± 0.10 (0.01, 0.41) |
0.038 |
| Modified Norris scale | −16.15 ± 2.00 (97) |
−14.12 ± 2.05 (95) |
2.03 ± 1.89 (−1.69, 5.75) |
0.284 | NA | NA | NA | NA |
| ALSAQ40 | 19.13 ± 3.79 (95) |
19.60 ± 3.82 (95) |
0.48 ± 3.50 (−6.44, 7.39) |
0.892 | NA | NA | NA | NA |
ALSFRS-R: interaction between treatment group and period (p = 0.915). ALSFRS-R: the revised amyotrophic lateral sclerosis functional rating scale. NA: not applicable. For Modified Norris scale and ALSAQ40, repeated measures analysis was not conducted
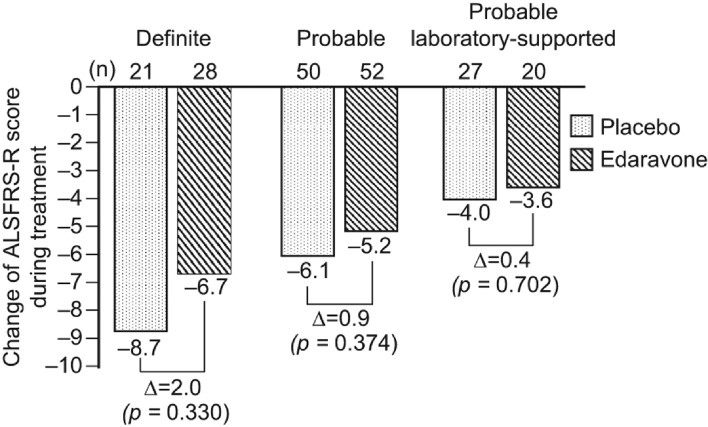
The changes in ALSFRS-R score during treatment according to diagnostic category, i.e. ‘definite’, ‘probable’ and ‘probable laboratory-supported’, are shown in Figure 2.
Figure 2.
Change of ALSFRS-R score during treatment by diagnostic category. ALSFRS-R: the revised amyotrophic lateral sclerosis functional rating scale.
The results of secondary endpoints are presented in Table II. The pinch strength analyzed by repeated measures analysis of variance showed a statistically significant difference, as there was no interaction between the treatment group and period (p = 0.292). The other endpoints did not show a significant difference.
The proportion of events of death or a particular state of disease progression was documented in 27 patients in the placebo group (14 patients with −4, −3 change in ALSFRS-R score during pre-observation, and 13 patients with −2, −1 change) and 32 in the edaravone group (12 patients with −4, −3 change and 20 patients with −2, −1 change). There was no significant inter-group difference (stratified log-rank test: p = 0.381, stratified generalized Wilcoxon test: p = 0.399).
Safety
The proportion of AE reported in the safety population was 88.5% in the placebo group and 89.2% in the edaravone group. All AE and SAE with a proportion of at least 5% in either group are listed (Table III). The inter-group difference in proportion with 95% confidence interval is 0.8% (−7.8% to 9.4%). There were no significant inter-group differences in the proportion of AE (p = 1.000), and in adverse drug reactions (p = 0.349). The proportion of SAE was 23.1% in the placebo group and 17.6% in the edaravone group. Two cases of respiratory failure in the placebo group resulted in death; in the edaravone group, there were three deaths (two cases of respiratory disorder and one case of respiratory failure). The investigators determined that the deaths were due to the primary disease and were not related to the study drug. There was no significant inter-group difference in the proportion of SAE (p = 0.389). No serious adverse drug reactions occurred in either group.
Table III.
Adverse events and serious adverse events.
| AE |
SAE |
|||||||
|---|---|---|---|---|---|---|---|---|
| Placebo (104) |
Edaravone (102) |
Placebo (104) |
Edaravone (102) |
|||||
| Treatment | n | (%) | n | (%) | n | (%) | n | (%) |
| Total | 92 | (88.5) | 91 | (89.2) | 24 | (23.1) | 18 | (17.6) |
| Constipation | 17 | (16.3) | 13 | (12.7) | ||||
| Dysphagia | 12 | (11.5) | 8 | (7.8) | 11 | (10.6) | 8 | (7.8) |
| Nasopharyngitis | 22 | (21.2) | 22 | (21.6) | ||||
| Muscular weakness | 9 | (8.7) | 7 | (6.9) | 1 | (1.0) | 1 | (1.0) |
| Contusion | 5 | (4.8) | 12 | (11.8) | ||||
| Headache | 3 | (2.9) | 8 | (7.8) | ||||
| Insomnia | 10 | (9.6) | 9 | (8.8) | ||||
| Gait disturbance | 16 | (15.4) | 20 | (19.6) | 2 | (1.9) | 3 | (2.9) |
| Eczema | 2 | (1.9) | 7 | (6.9) | ||||
| Glucose urine present | 3 | (2.9) | 6 | (5.9) | ||||
All AE with an incidence greater than 5% are tabulated by the primary term, MedDRA version 11.1.
Discussion
The results of prior clinical trials (20,30) indicated that edaravone may delay the progression of symptoms in some ALS patients. Because evaluation of edaravone would be difficult in patients in whom ALS progression was either acute or non-existent, only patients whose ALSFRS-R score changed by −1 to −4 points during the 12-week pre-observation period were eligible for the study. Since efficacy was found over the 24-week treatment period in the phase II trial, a treatment period of 24 weeks was also chosen for this study.
Based on the results of the previous phase II trial, the inter-group difference in the change of ALSFRS-R score at the end of treatment was expected to be 2 points in this trial. However, the actual inter-group difference was only 0.65 points by ANCOVA and this was not statistically significant. No significant inter-group difference was found by repeated measures analysis of variance either. The results of ANCOVA for pinch strength, a secondary endpoint, suggested a beneficial effect in the edaravone group compared to the placebo group.
Additionally, stratified analysis by diagnostic category (Figure 2) revealed that the change in ALSFRS-R score during treatment was greater in those patients fulfilling the criteria for clinically definite ALS using the Airlie House diagnostic classification. This trial enrolled patients with longer duration of disease and higher ALSFRS-R scores at the start of treatment compared to those of the patients in other trials (31–34). As shown in Table I, the mean duration of disease for the edaravone group and the placebo group was 1.3 years and 1.2 years, respectively, and the mean ALSFRS-R score at the start of treatment was 41 and 42, respectively. While the mean change in ALSFRS-R score during the treatment was −5.70 for the edaravone group and −6.35 for the placebo group, our internal analysis showed that 25% of patients in the edaravone group and 26% of patients in the placebo group showed the change of 0 or −1 point in ALSFRS-R score indicating a more slowly progressive form of the disease than had originally been anticipated when the trial was designed, and thus attenuating the power of the study. Future trials will aim to enroll patients with more rapidly progressive illness. AE occurred in nearly 90% of both groups, i.e. 88.5% of patients in the placebo group and 89.2% in the edaravone group, with no significant differences between the two groups.
In conclusion, although the elimination of free radicals to inhibit the degeneration of motor neurons appears to be a promising new strategy for the treatment of ALS, this study failed to demonstrate efficacy of edaravone to delay the progression of ALS. While the primary endpoint was not achieved, we consider that the results are helpful to identify the patient population in which edaravone could be expected to show efficacy. On the basis of this information, we have designed and are conducting a phase III study.
Acknowledgements
The authors thank all participating patients and their family members, and all the investigators and study coordinators at the 29 centers involved in the trial. Thanks are also due to Mitsubishi Tanabe Pharma Corporation for monitoring of the study, data collection and management, and statistical analysis.
The Edaravone ALS Study Group: Site Investigators
MCI-186 ALS study group investigators are as follows.
Hokkaido University Hospital, Sapporo: Hidenao Sasaki; Hokuyukai Neurological Hospital, Sapporo: Asako Takei, Isao Yamashita; Tohoku University Hospital, Sendai: Masashi Aoki; National Hospital Organization Miyagi National Hospital, Watari: Takashi Imai; Jichi Medical School Hospital, Shimotsuke: Imaharu Nakano; Gunma University Hospital, Maebashi: Koichi Okamoto; Saitama Center of Neurology and Psychiatry, Saitama: Yuichi Maruki; Kohnodai Hospital, National Center for Global Health and Medicine, Ichikawa: Shuichi Mishima, Jin Nishimiya; Toho University Omori Medical Center, Tokyo: Yasuo Iwasaki; Nippon Medical School Hospital, Tokyo: Mineo Yamazaki; The University of Tokyo Hospital, Tokyo: Yuji Takahashi; Kitasato University East Hospital, Sagamihara: Mieko Ogino, Yutaka Ogino; National Center of Neurology and Psychiatry, Kodaira: Masafumi Ogawa; Shonan Fujisawa Tokushukai Hospital, Chigasaki: Tetsumasa Kamei; Seirei Hamamatsu General Hospital, Hamamatsu: Tsuyoshi Uchiyama; Nagoya University Hospital, Nagoya: Hirohisa Watanabe; Mie University Hospital, Tsu: Yasumasa Kokubo; National Hospital Organization Utano Hospital, Kyoto: Hideyuki Sawada; Osaka General Medical Center, Osaka: Takanori Hazama; Osaka Medical College Hospital, Takatsuki: Fumiharu Kimura; National Hospital Organization Toneyama National Hospital, Toyonaka: Harutoshi Fujimura; Kansai Medical University Takii Hospital, Moriguchi: Hirofumi Kusaka; Okayama University Hospital, Okayama: Koji Abe; National Hospital Organization Ehime National Hospital, Toon: Tsukasa Hashimoto; Saiseikai Fukuoka General Hospital, Fukuoka: Takeshi Yamada, Kanamori Yuji, Yamasaki Kenji; Fukuoka Tokushukai Medical Center, Kasuga: Shizuma Kaku; Murakami Karindou Hospital, Fukuoka: Hitoshi Kikuchi; National Hospital Organization Kumamoto Saishunso National Hospital, Koshi: Shigehiro Imamura; National Hospital Organization Miyazaki Higashi Hospital, Miyazaki: Seiichiro Sugimoto, Kishi Masahiko.
Footnotes
Declaration of interest: K. Abe received funding for travel and speaker honoraria from Mitsubishi Tanabe Pharma Corp. Y. Itoyama received speaker honoraria from Mitsubishi Tanabe Pharma Corp. G. Sobue received funding for travel and speaker honoraria from Mitsubishi Tanabe Pharma Corp, and serves on the scientific advisory board for the Kanae Science Foundation for the Promotion of Medical Science, Naito Science Foundation and serves as an advisory board member of Brain, an editorial board member of Degenerative Neurological and Neuromuscular Disease, the Journal of Neurology, and Amyotrophic Lateral Sclerosis and Frontotemporal Degeneration, and received funding from the Ministry of Education, Culture, Sports, Science and Technology of Japan; the Ministry of Welfare, Health and Labor of Japan; the Japan Science and Technology Agency, Core Research for Evolutional Science and Technology. S. Tsuji received funding for travel and speaker honoraria from Mitsubishi Tanabe Pharma Corp. M. Aoki received speaker honoraria, travel expenses, and fees for conducting and consulting on pharmacological test of edaravone in a rat ALS model, from Mitsubishi Tanabe Pharma Corp, and has received research grants, Research on Nervous and Mental Disorders, Research on Measures for Intractable Diseases, Research on Psychiatric and Neurological Diseases and Mental Health from the Japanese Ministry of Health Labor and Welfare, Grants-in-Aid for Scientific Research, an Intramural Research Grant for Neurological Psychiatric Disorders from NCNP and Grants-in-Aid for Scientific Research from the Japanese Ministry of Education, Culture, Sports, Science and Technology. M. Doyu received funding for travel or speaker honoraria from Mitsubishi Tanabe Pharma Corp. C. Hamada is a consultant for Chugai Pharmaceutical Co. Ltd., Taiho Pharmaceutical Co. Ltd., Kowa Company Ltd., Sanwa Kagaku Kenkyusho Co. Ltd., Maruho Co. Ltd., Daiichi Sankyo Co. Ltd., Eisai Co. Ltd., Mochida Pharmaceutical Co. Ltd., Otsuka Pharmaceutical Co. Ltd., Nippon Shinyaku Pharmaceutical Co. Ltd. and Mitsubishi Tanabe Pharma Corp. K. Kondo is an employee of Mitsubishi Tanabe Pharma Corporation. T. Yoneoka is an employee of and co-owns a patent with Mitsubishi Tanabe Pharma Corporation. M. Akimoto is an employee of Mitsubishi Tanabe Pharma Corporation. Y.Yoshino received funding for speaker honoraria from, co-owns a patent with, and is a consultant for Mitsubishi Tanabe Pharma Corp.
The study was funded by Mitsubishi Tanabe Pharma Corporation.
The authors alone are responsible for the content and writing of the paper.
References
- 1.Rowland LP, Shneider NA. Amyotrophic lateral sclerosis. N Engl J Med. 2001;344:1688–700. doi: 10.1056/NEJM200105313442207. [DOI] [PubMed] [Google Scholar]
- 2.Chio A, Logroscino G, Hardiman O, Swingler R, Mitchell D, Beghi E, et al. Prognostic factors in ALS: a critical review. Amyotroph Lateral Scler. 2009;10:310–23. doi: 10.3109/17482960802566824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beckman JS, Carson M, Smith CD, Koppenol W. ALS, SOD and peroxynitrite. Nature. 1993;364:584. doi: 10.1038/364584a0. [DOI] [PubMed] [Google Scholar]
- 4.Ferrante RJ, Shinobu LA, Schulz JB, Matthews RT, Thomas CE, Kowall NW, et al. Increased 3-nitrotyrosine and oxidative damage in mice with a human Cu/Zn superoxide disumutase mutation. Ann Neurol. 1997;42:326–34. doi: 10.1002/ana.410420309. [DOI] [PubMed] [Google Scholar]
- 5.Beal MF, Ferrante RJ, Browne SE, Matthews RT, Kowall NW, Brown RH., Jr Increased 3-nitrotyrosine in both sporadic and familial amyotrophic lateral sclerosis. Ann Neurol. 1997;42:644–54. doi: 10.1002/ana.410420416. [DOI] [PubMed] [Google Scholar]
- 6.Abe K, Pan LH, Watanabe M, Kato T, Itoyama Y. Induction of nitrotyrosine-like immunoreactivity in the lower motor neuron of amyotrophic lateral sclerosis. Neurosci Lett. 1995;199:152–4. doi: 10.1016/0304-3940(95)12039-7. [DOI] [PubMed] [Google Scholar]
- 7.Sasaki S, Shibata N, Komori T, Iwara M. iNOS and nitrotyrosine immunoreactivity in amyotrophic lateral sclerosis. Neurosci Lett. 2000;291:44–8. doi: 10.1016/s0304-3940(00)01370-7. [DOI] [PubMed] [Google Scholar]
- 8.Tohgi H, Abe T, Yamazaki K, Murata T, Ishizaki E, Isobe C. Remarkable increase in cerebrospinal fluid 3-nitotyrosine in patients with sporadic amyotrophic lateral sclerosis. Ann Neurol. 1999;46:129–31. doi: 10.1002/1531-8249(199907)46:1<129::aid-ana21>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 9.Itoh K, Wakabayashi N, Katoh Y, Ishii T, Igarashi K, Engel JM, et al. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, et al. TDP-43 is a component of ubiquitin-posive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–11. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 11.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, et al. Ubiqutinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–3. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 12.Duan W, Li X, Shi J, Guo Y, Li Z, Li C. Mutant TAR DNA-binding protein-43 induces oxidative injury in motor neuron-like cell. Neuroscience. 2010;169:1621–9. doi: 10.1016/j.neuroscience.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 13.The Edaravone Acute Brain Infarction Study Group. Effect of a novel free radical scavenger, edaravone (MCI-186), on acute brain infarction. Cerebravascuar Dis. 2003;15:222–9. doi: 10.1159/000069318. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe T, Yuki S, Egawa M, Nishi H. Protective effects of MCI-186 on cerebral ischemia: possible involvement of free radical scavenging and antioxidant actions. J Pharmacol Exp Ther. 1994;268:1597–1604. [PubMed] [Google Scholar]
- 15.Mizuno A, Umemura K, Nakashima M. Inhibitory Effect of MCI-186, a free radical scavenger, on cerebral ischemia following the rat middle cerebral artery occlusion. Gen Pharmacol. 1998;30:575–8. doi: 10.1016/s0306-3623(97)00311-x. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto T, Yuki S, Watanabe T, Mitsuka M, Saito K, Kogure K. Delayed neuronal death prevented by inhibition of increased hydroxyl radical formation in a transient cerebral ischemia. Brain Research. 1997;762:240–2. doi: 10.1016/s0006-8993(97)00490-3. [DOI] [PubMed] [Google Scholar]
- 17.Ikeda K, Iwasaki Y, Kinoshita M. Treatment of wobbler mice with free radical scavenger. Molecular Mechanism and Therapeutics of Amyotrophic Lateral Sclerosis. Elsevier Science B.V; 2001. pp. 335–40. [Google Scholar]
- 18.Ito H, Wate R, Zhang J, Ohnishi S, Kaneko S, Ito H, et al. Treatment with edaravone, initiated at symptom onset, slows motor decline and decreases SOD1 deposition in ALS mice. Exp Neurol. 2008;213:448–55. doi: 10.1016/j.expneurol.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 19.Aoki M, Warita H, Mizuno H, Suzuki N, Yuki S, Itoyama Y. Feasibility study for functional test battery of SOD transgenic rat (H46R) and evaluation of edaravone, a free radical scavenger. Brain Res. 2011;25:321–5. doi: 10.1016/j.brainres.2011.01.058. [DOI] [PubMed] [Google Scholar]
- 20.Yoshino H, Kimura A. Investigation of the therapeutic effects of edaravone, a free radical scavenger, on amyotrophic lateral sclerosis (phase II study) Amyotroph Lateral Scler. 2006;7:241–5. doi: 10.1080/17482960600881870. [DOI] [PubMed] [Google Scholar]
- 21.Brooks BR. Introduction defining optimal management in ALS: from first symptoms to announcement. Neurology. 1999;53:S1–3. [PubMed] [Google Scholar]
- 22.Brooks BR, Miller RG, Swash M, Munsat TL. El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:293–9. doi: 10.1080/146608200300079536. [DOI] [PubMed] [Google Scholar]
- 23.Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, et al. The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. J Neurol Sci. 1999;169:13–21. doi: 10.1016/s0022-510x(99)00210-5. [DOI] [PubMed] [Google Scholar]
- 24.Ohashi Y, Tashiro K, Itoyama Y, Nakano I, Sobue G, Nakamura S, et al. Study of functional rating scale for amyotrophic lateral sclerosis: revised ALSFRS (ALSFRS-R) Japanese Version. No To Shinkei. 2001;53:346–55. [PubMed] [Google Scholar]
- 25.Japan intractable diseases information center. http://www.nanbyou.or.jp/entry/52
- 26.Lacomblez L, Bouche P, Bensimon G, Meininger V. A double-blind, placebo-controlled trial of high doses of gangliosides in amyotrophic lateral sclerosis. Neurology. 1989;39:1635–7. doi: 10.1212/wnl.39.12.1635. [DOI] [PubMed] [Google Scholar]
- 27.Oda E, Ohashi Y, Tashiro K, Mizuno Y, Kowa H, Yanagisawa N. Reliability and factorial structure of a rating scale for amyotrophic lateral sclerosis. No To Shinkei. 1996;48:999–1007. [PubMed] [Google Scholar]
- 28.Jenkinson C, Fitzpatrick R, Brennen C, Swash M. Evidence for the validity and reliability of the ALS assessment questionnaire: the ALSAQ-40. Amyotroph Lateral Scler Other Motor Neuron Disord. 1999;1:33–40. doi: 10.1080/146608299300080022. [DOI] [PubMed] [Google Scholar]
- 29.Yamaguchi T, Ohbu S, Ito Y, Moriwaka F, Tashiro K, Ohashi Y, et al. Validity and clinical applicability of the Japanese version of amyotrophic lateral sclerosis: Assessment questionnaire 40(ALSAQ-40) No To Shinkei. 2004;56:483–94. [PubMed] [Google Scholar]
- 30.Yoshino H, Kimura A. Clinical trial for amyotrophic lateral sclerosis with free radical scavenger, edaravone. Neurol Therap. 2003;20:557–64. [Google Scholar]
- 31.Dupuis L, Dengler R, Heneka MT, Meyer T, Zierz S, Kassubek J, et al. A randomized, double-blind, placebo-controlled trial of pioglitazone in combination with riluzole in amyotrophic lateral sclerosis. PlosOne. 2012:e37885. doi: 10.1371/journal.pone.0037885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cudkowicz M, Bozik ME, Ingersoll EW, Miller R, Mitsumoto H, Shefner J, et al. The effects of dexpramipexole (KNS-76704) in individuals with amyotrophic lateral sclerosis. Nat Med. 2011;17:1652–6. doi: 10.1038/nm.2579. [DOI] [PubMed] [Google Scholar]
- 33.Pascuzzi RM, Shefner J, Chappell AS, Bjerke JS, Tamura R, Chaudhry V, et al. A phase II trial of talampanel in subjects with amyotrophic lateral sclerosis. Amyotroph Lateral Scler. 2010;11:266–71. doi: 10.3109/17482960903307805. [DOI] [PubMed] [Google Scholar]
- 34.Meininger V, Drory VE, Leigh PN, Ludolph A, Robberecht W, Silani V. Glatiramer acetate has no impact on disease progression in ALS at 40 mg/day: a double- blind, randomized, multicentre, placebo-controlled trial. Amyotroph Lateral Scler. 2009;10:378–83. doi: 10.3109/17482960902803432. [DOI] [PubMed] [Google Scholar]