Abstract
Background
Early diagnosis of HIV is important for the prognosis of individual patients, because antiretroviral treatment can be started at the appropriate time, and for public health, because transmission can be prevented.
Methods
Data were collected from 767 HIV patients who were diagnosed in Sweden during 2003–2010 and were infected in Sweden or born in Sweden and infected abroad. A recent infection testing algorithm (RITA) was applied to BED-EIA test results (OD-n < 0.8), CD4 counts (≥ 200 cells/μl), and clinical information. A recent infection classification was used as indicator for early diagnosis. Time trends in early diagnosis were investigated to detect population changes in HIV testing behavior. Patients with early diagnosis were compared to patients with delayed diagnosis with respect to age, gender, transmission route, and country of infection (Sweden or abroad).
Results
Early diagnosis was observed in 271 patients (35%). There was no statistically significant time trend in the yearly percentage of patients with early diagnosis in the entire study group (p = 0.836) or in subgroups. Early diagnosis was significantly more common in men who have sex men (MSM) (45%) than in heterosexuals (21%) and injecting drug users (27%) (p < 0.001 and p = 0.001, respectively) in both univariate and multivariable analyses. The only other factor that remained associated with early diagnosis in multivariable analysis was young age group.
Conclusion
Approximately one-third of the study patients were diagnosed early with no significant change over time. Delayed HIV diagnosis is a considerable problem in Sweden, which does not appear to diminish.
Keywords: HIV, delayed diagnosis, immunoassay, trends, risk factors
Introduction
Early diagnosis of HIV is important for the prognosis of individual patients, because antiretroviral treatment (ART) can be started at the appropriate time, as well as for public health, since transmission can be prevented [1]. In the early years of the 21st century, several studies were published in Europe investigating the trend in late diagnosis [2]. The objective of these studies was to investigate whether the introduction of more effective ART had decreased the time from infection to diagnosis. Surprisingly, most studies found a stable or increasing trend of late diagnosis. The ‘late presenters’ were found mainly among immigrants or in population groups considered to have a low risk of HIV infection or who were not covered by routine screening, such as people above 40 years of age, heterosexuals, men (compared to women), and those having children [2]. Newer studies on late presentation or diagnosis are scarce and the reported percentages of late presenters among newly diagnosed HIV patients in Europe vary considerably (from 12% to 60%) [1].
The definitions for late diagnosis have varied widely between studies. Some studies used laboratory-based definitions, such as CD4 + T-lymphocyte (CD4) counts below 200 or 350 cells/μl at the time of diagnosis, whereas other studies used clinical definitions based on time to development of AIDS [2,3]. Unfortunately, none of these definitions are linearly related to time from infection, but rather a measure of disease progression at time of diagnosis in relation to the optimal time for start of ART [4,5]. A related approach to explore the epidemiology of the disease is to estimate HIV incidence in populations using serological assays [6], such as the BED assay (HIV-1 IgG capture BED enzyme-linked immunoassay). These assays detect recent infections among all HIV-positive cases; where recent infection means seroconversion within approximately the previous 6 months. A major advantage of this method is that it provides information regarding current HIV transmission and testing, rather than late presentation. The BED assay was developed by the Centers for Disease Control and Prevention (CDC) in Atlanta, USA and measures the levels of HIV-1-specific antibodies out of total IgG, which increases with time since infection [7]. However, the BED assay can give false-positive recent results, e.g. for patients with low CD4 counts [8,9]. Thus, removing patients with low CD4 count from those classified as having recent infections was recommended by UNAIDS [10]. To further increase the specificity of the result from the BED assay a recent infection testing algorithm (RITA), taking CD4 counts as well as clinical information into account, was developed by the European Centre for Disease Prevention and Control (ECDC) [11] and used in testing algorithms in, for example, the United Kingdom [12].
To allow for international comparability, we used the algorithm proposed by the ECDC in the present surveillance study on HIV patients diagnosed in Sweden between 2003 and 2010. Patients classified as having recent infection were compared to patients with delayed diagnosis to investigate whether there were trends in early HIV diagnosis over recent years and to identify factors associated with early diagnosis. In order to detect changes over time in HIV testing behavior due to prevention efforts within the national strategy for HIV prevention in Sweden [13], the study was restricted to people who should have been reached by these efforts, i.e. we did not include immigrants infected abroad.
Materials and methods
Data collection and inclusion criteria
We used clinical, epidemiological, and laboratory data as well as plasma samples from a previously published study, which investigated transmitted drug resistance among 1463 HIV patients who were diagnosed in Sweden during 2003–2010 [14]. The previous study included approximately 44% of all newly diagnosed patients who had been reported as HIV-1-infected in the national Swedish surveillance system (www.sminet.se) during the study period and was reasonably well matched to them, except for a slight over-representation of men who have sex with men (MSM).
Informed written or oral consent was obtained from all adult participants and from the next of kin, caregivers or guardians on behalf of minors and children and was documented in the patient’s records. The research was conducted according to the Declaration of Helsinki and was approved by the Regional Medical Ethics Board in Stockholm, Sweden (Dnr 02–367, 04–797, 2007/1533, and 2011/1854) which permitted use of either written or oral consent to minimize the risk of selection biases due to patient drop-out due to willingness to take part in the study, but reluctance to provide written consent. All data were anonymized [14]. The study protocol, questionnaire, and database, as well as a system for data verification, were developed as part of a pan- European study of transmitted drug resistance [15].
In the present study we included study subjects from the previous study if they: (i) were reported to have been infected in Sweden or were born in Sweden and reported to have been infected abroad; (ii) had a successful BED result from a sample collected within 120 days from the date of diagnosis; (iii) had a CD4 count available from 120 days before to 120 days after the date of sampling for BED testing.
Data on country of origin, probable country of infection, and transmission route were collected in standardized questionnaires filled out by the patient’s doctor or nurse and were obtained as part of mandatory contact tracing [14].
Laboratory methods
The BED assay (Calypte Biomedical Corp, Lake Oswega, OR, USA) was performed according to the manufacturer’s instructions with the recommended cut-off for recent infection of OD-n < 0.8. As mentioned, this cut-off corresponds to approximately 6 months between infection and sampling for BED testing [7]. Data on CD4 counts at diagnosis were available from the previous study [14] and most data on BED test results were available from a separate recent study [16].
Definition of early diagnosis and statistical methods
Patients were classified as having early or delayed diagnosis using the ECDC recent infection testing algorithm [11] with one minor modification. Thus, patients were classified as having an early diagnosis if they had a BED OD value < 0.8 in a blood sample drawn within 4 months from diagnosis provided that they did not have low CD4 count (< 200 cells/μl), low viral load (< 400 copies/ml), ongoing ART, and/or an AIDS-defining illness at diagnosis. The last criterion differs from the ECDC RITA, which specifies that patients with AIDS within 1 year of diagnosis should not be classified as recently infected. This modification was used because we had information about AIDS at diagnosis, but not during follow-up. Patients fulfilling the criteria for early diagnosis were compared with remaining patients, who were defined as having delayed diagnosis.
We performed a trend analysis using a logistic regression model with early diagnosis as dependent variable and year as the only independent variable. Trend analyses were also done separately for each major transmission group and by country of infection (Sweden vs abroad). We investigated the following factors for association with early diagnosis: age, gender, transmission route, country of infection, and country of birth. Factors identified in univariate logistic regression analysis at a significance level of 5% were included in a multivariable model. All factors were treated as categorical variables. Patients with missing data on country of infection (n = 37) and country of birth (n = 3) were included in the abroad category in the logistic regression.
Results
The present study included 767 HIV-1-infected patients. Of 1463 patients in the previous study, 609 were excluded as they were reported as both born and infected abroad. An additional 87 patients were excluded due to lack of BED or CD4 test results fulfilling the inclusion criteria (n = 78 and n = 9, respectively).
Table I shows the main characteristics of the study subjects. The three main transmission groups were: men who have sex with men (MSM) (n = 402), heterosexuals (n = 191), and injecting drug users (IDUs) (n = 121). A majority of the study subjects were infected in Sweden (n = 576, 75%). However, it should be stressed that we did not investigate immigrants infected abroad, who constituted around half of all diagnosed patients during the study period.
Table I.
Factors associated with obtaining an early diagnosis among 767 patients diagnosed with HIV infection in Sweden, 2003–2010.
| Patients, n (%) |
Univariate analysis |
Multivariable analysis |
||||
|---|---|---|---|---|---|---|
| Factor | Total | With early diagnosis | OR | p value | OR | p value |
| Transmission route | ||||||
| MSM | 402 | 182 (45) | (ref) | (ref) | ||
| Heterosexual | 191 | 41 (21) | 0.330 | < 0.001 | 0.356 | < 0.001 |
| Injecting drug use | 121 | 33 (27) | 0.453 | 0.001 | 0.459 | 0.001 |
| Other | 53 | 15 (28) | 0.477 | 0.021 | 0.490 | 0.044 |
| Country of infection | ||||||
| Sweden | 576 | 218 (38) | (ref) | (ref) | ||
| Abroad or unknown | 191 | 53 (28) | 0.631 | 0.012 | 0.857 | 0.449 |
| Gender | ||||||
| Men | 652 | 240 (37) | (ref) | (ref) | ||
| Women | 115 | 31 (27) | 0.634 | 0.043 | 0.963 | 0.893 |
| Age group | ||||||
| < 25 years | 40 | 22 (55) | (ref) | (ref) | ||
| 25–34 years | 180 | 83 (46) | 0.700 | 0.310 | 0.552 | 0.113 |
| 35–44 years | 263 | 87 (33) | 0.404 | 0.008 | 0.325 | 0.002 |
| 45–54 years | 180 | 53 (29) | 0.341 | 0.003 | 0.313 | 0.002 |
| 55–64 years | 82 | 21 (26) | 0.282 | 0.002 | 0.280 | 0.003 |
| ≥ 65 years | 22 | 5 (23) | 0.241 | 0.018 | 0.242 | 0.024 |
| Country of birth | ||||||
| Sweden | 593 | 211 (36) | (ref) | |||
| Abroad or unknown | 174 | 60 (34) | 0.953 | 0.790 | ||
MSM, men who have sex with men; OR, odds ratio.
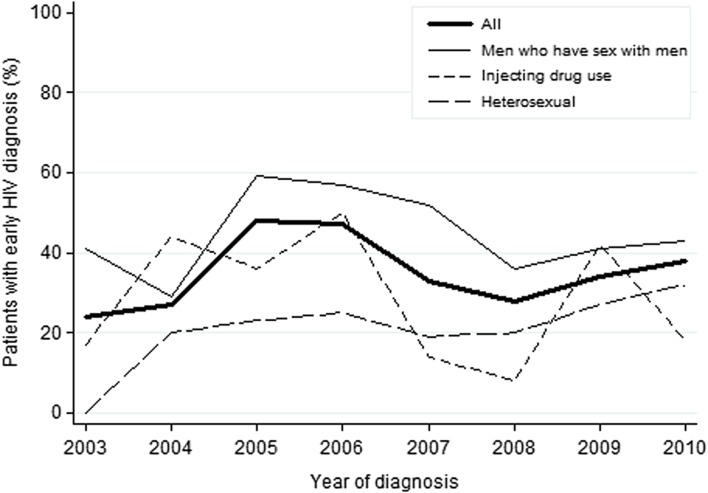
A total of 271 patients (35%) were diagnosed early according to our definition. The yearly percentage of patients with early diagnosis varied between 24% (year 2003) and 48% (year 2005), but there was no significant time trend (p = 0.836) (Figure 1). Similarly, no significant time trend was found in any of three transmission groups (heterosexuals, p = 0.064; MSM, p = 0.536; or IDUs, p = 0.230) (Figure 1) or by country of infection (Sweden, p = 0.665 or abroad, p = 0.132) (not shown). Early diagnosis was significantly more common among MSM (45%) than among heterosexuals (21%) and IDUs (27%) (p < 0.001 and p = 0.001, respectively). This difference remained in the multivariable analysis (Table I). In the univariate analysis we also found that women were less likely than men to have an early diagnosis (odds ratio 0.63, p = 0.043). This association was no longer significant in the multivariable analysis that adjusted for transmission route and was entirely explained by the difference in time to diagnosis between the heterosexually infected group and MSM (Table I). Similarly, there was a difference in the univariate analysis between persons infected in Sweden or abroad, which disappeared in the multivariable analysis. This was explained by a higher proportion of heterosexual transmission in the group infected abroad compared with the predominance of MSM transmission in Sweden. The factors that remained significantly associated with early diagnosis in the multivariable analysis were young age group and being MSM (Table I).
Figure 1.
Percentage of patients with an early HIV diagnosis. The early diagnosis among 767 patients diagnosed with HIV infection in Sweden, 2003–2010, and included in the study, by transmission route.
Discussion
In this surveillance study we investigated trends in early diagnosis among HIV patients diagnosed in Sweden over the time period 2003–2010 to detect possible changes in HIV testing behavior due to national prevention efforts. The study was restricted to patients reported as infected in Sweden and patients born in Sweden and infected abroad. Immigrants infected abroad were not included, as further discussed below. Our results indicated that about one-third of study patients received their diagnosis within 6 months of infection. Thus, a majority of HIV patients were diagnosed at an undefined time later in the course of the disease. This underlines the fact that delayed HIV diagnosis is a considerable problem in Sweden, as it is internationally [1]. Furthermore, this problem does not appear to diminish over time. For some patients the delayed HIV diagnosis will affect the prognosis because treatment cannot be started at an optimal time [1–19]. Delayed diagnosis is also likely to affect public health because the risk of onward transmission is higher from persons who are unaware of their infection as compared with persons who have been diagnosed [20]. In addition, a majority of diagnosed HIV-1 patients in Sweden receive successful ART [21,22], which greatly reduces infectivity [23].
The number of patients in each group and each year was small, leading to estimates fluctuating between years and limited statistical power to detect small changes in the trends over the time period. However, this is due to the low incidence of HIV in Sweden. Our study included approximately half of all eligible patients in Sweden during the study period. The total number of notified newly diagnosed HIV cases in Sweden who would have been eligible for the study fluctuated over the years of the study period, with a slight upward trend (4% per year, p < 0.001). There was an HIV outbreak among IDUs in Stockholm in the autumn of 2006, which resulted in an increased number of reported cases in 2007 [24]. However, a majority were not diagnosed early according to our definition and thus were reflected in the dip in early diagnosis among IDUs in 2007.
A strength of our study is that patients were defined as having an early diagnosis according to the algorithm proposed by the ECDC, which is based on a composite measure of two laboratory tests, i.e. the BED test and CD4 count, as well as clinical information. This should reduce, albeit not eliminate, the risk of misclassification of early infection when the BED assay is used alone. However, we cannot exclude the possibility that individual patients might still have been misclassified.
Studies in other European countries, including the UK, France, and Germany, have also documented that many patients have delayed diagnosis [12,25,26]. In our study the percentage of patients with early diagnosis was slightly higher than in these latter might be due to the exclusion of persons born and infected abroad. Our rationale for excluding this group in the study was to avoid trends in immigration affecting our results. To investigate trends in early and delayed diagnosis in this group, data on date of immigration and knowledge of whether the diagnosis was known at the time of immigration are needed, which was not the case for our study population. In an ongoing study in Sweden, immigrants have been identified as having the highest proportion of late presenters (CD4 < 350) [27].
In the present study the MSM group had the highest percentage of early diagnosis, concordant with studies which show that testing coverage and frequency is higher in this group than in other groups [22]. Nonetheless, a majority of MSM and IDUs were estimated to have been diagnosed more than 6 months after infection, despite both groups being recognized as having an increased risk of HIV infection. Therefore, the present study shows that there is room for improvements in secondary prevention efforts in these, as well as in other, transmission groups. As we did not investigate reasons behind delayed diagnosis, additional in-depth studies are needed to guide improvements in prevention strategies, including investigation of whether there is mainly patient’s or doctor’s delay.
Although delayed diagnosis is of concern, recent infection implies ongoing HIV transmission. Our results indicate that this occurred among MSM, IDUs, and other groups in Sweden, emphasizing that primary prevention strategies also need to be strengthened and directed to groups and individuals at high risk of contracting HIV.
Acknowledgments
Thanks to Rigmor Thorstensson, Hans Gaines, Monica Ideström, Kajsa Aperia, Frida Hansdotter, Maria Axelsson, Gunilla Rådö, and Susanne Karregård at the Public Health Agency of Sweden and Eva C. Eriksson at Karolinska University Hospital. The research leading to these results has received funding from the Swedish Research Council (grant nos K2008-56X-09935-17-3 and K2001-35 56X-0095-20-6); from the Swedish International Development Cooperation Agency (grant no. SWE-2006-018); and the EU projects: SPREAD (QLK2-CT-2001-37 01344); EHR (LSHP-CT-2006–518211); CHAIN (FP7/2007–2013) ‘Collaborative HIV and Anti-HIV Drug Resistance Network,’ and FP7 grant agreement no. 223131 under EuroCoord grant agreement no. 260694. H.S. was supported by a postdoctoral fellowship from the Swedish Research Council (623-2011-1100, 623-2013-8905).
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Stockholm: European Centre for Disease Prevention and Control (ECDC); 2010. HIV testing: increasing uptake and effectiveness in the European Union.http://www.ecdc.europa.eu/en/publications/publications/101129_ter_hiv_testing_evidence.pdf [Google Scholar]
- 2.Adler A, Mounier-Jack S, Coker RJ. Late diagnosis of HIV in Europe: definitional and public health challenges. AIDS Care. 2009;21:284–93. doi: 10.1080/09540120802183537. [DOI] [PubMed] [Google Scholar]
- 3.Antinori A, Coenen T, Costagiola D, Dedes N, Ellefson M, Gatell J, et al. Late presentation of HIV infection: a consensus definition. HIV Med. 2011;12:61–4. doi: 10.1111/j.1468-1293.2010.00857.x. [DOI] [PubMed] [Google Scholar]
- 4.Lodi S, Phillips A, Touloumi G, Pantazis N, Bucher HC, Babiker A, et al. CD4 decline in seroconverter and seroprevalent individuals in the precombination of antiretroviral therapy era. AIDS. 2010;24:2697–704. doi: 10.1097/QAD.0b013e32833ef6c4. [DOI] [PubMed] [Google Scholar]
- 5.Antiretroviral treatment of HIV infection 2013, updated recommendations. [Antiretroviral behandling av HIV-infektion 2013, uppdaterad rekommendation.] Swedish reference group for antiretroviral therapy (RAV) 2013 http://www.folkhalsomyndigheten.se/documents/projektwebbar/rav/rekommendationer/rav-behandling-antiviral-hivinfektion-2013-uppdaterad-version.pdf
- 6.B rnighausen T, McWalter TA, Rosner Z, Newell ML, Welte A. HIV incidence estimation using the BED capture enzyme immunoassay: systematic review and sensitivity analysis. Epidemiology. 2010;21:685–97. doi: 10.1097/EDE.0b013e3181e9e978. [DOI] [PubMed] [Google Scholar]
- 7.Parekh BS, Hanson DL, Hargrove J, Branson B, Green T, Dobbs T, et al. Determination of mean recency period for estimation of HIV type 1 Incidence with the BED-capture EIA in persons infected with diverse subtypes. AIDS Res Hum Retroviruses. 2011;27:265–73. doi: 10.1089/aid.2010.0159. [DOI] [PubMed] [Google Scholar]
- 8.Busch MP, Pilcher CD, Mastro TD, Kaldor J, Vercauteren G, Rodriguez W, et al. Beyond detuning: 10 years of progress and new challenges in the development and application of assays for HIV incidence estimation. AIDS. 2010;24:2763–71. doi: 10.1097/QAD.0b013e32833f1142. [DOI] [PubMed] [Google Scholar]
- 9.Guy R, Gold J, Calleja JM, Kim AA, Parekh B, Busch M, et al. Accuracy of serological assays for detection of recent infection with HIV and estimation of population incidence: a systematic review. Lancet Infect Dis. 2009;9:747–59. doi: 10.1016/S1473-3099(09)70300-7. [DOI] [PubMed] [Google Scholar]
- 10.Methods for estimating HIV incidence. UNAIDS. 2010 http://www.unaids.org/en/media/unaids/contentassets/dataimport/pub/basedocument/2010/epi_alert_1stqtr2010_en.pdf
- 11.Stockholm: European Centre for Disease Prevention and Control (ECDC); 2013. Monitoring recently acquired HIV infections in the European context.http://www.ecdc.europa.eu/en/publications/Publications/monitoring-recently-acquired-HIV-infections-european-context.pdf [Google Scholar]
- 12.HIV in the United Kingdom: 2013 report. Health Protection Agency. 2013 http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1317140300680
- 13.National Strategy to Combat HIV/AIDS and Certain Other Communicable Diseases. Government Bill 2005/06:60. [Nationell strategi mot hiv/aids och vissa andra smittsamma sjukdomar. Regeringens proposition 2005/06:60.] y
- 14.Karlsson A, Björkman P, Bratt G, Ekvall H, Gisslén M, Sönnerborg A, et al. Low prevalence of transmitted drug resistance in patients newly diagnosed with HIV-1 infection in Sweden 2003-2010. PloS One. 2012;7:e33484. doi: 10.1371/journal.pone.0033484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.SPREADprogramme. Transmission of drug-resistant HIV-1 in Europe remains limited to single classes. AIDS. 2008;22:625–35. doi: 10.1097/QAD.0b013e3282f5e062. [DOI] [PubMed] [Google Scholar]
- 16.Skar H, Albert J, Leitner T. Towards estimation of HIV-1 date of infection: a time-continuous IgG-model shows that seroconversion does not occur at the midpoint between negative and positive tests. PloS One. 2013;8:e60906. doi: 10.1371/journal.pone.0060906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.d’Arminio Monforte A, Cozzi-Lepri A, Girardi E, Castagna A, Mussini C, Di Giambenedetto S, et al. Late presenters in new HIV diagnoses from an Italian cohort of HIV-infected patients: prevalence and clinical outcome. Antivir Ther. 2011;16:1103–12. doi: 10.3851/IMP1883. [DOI] [PubMed] [Google Scholar]
- 18.Chadborn TR, Delpech VC, Sabin CA, Sinka K, Evans BG. The late diagnosis and consequent short-term mortality of HIV-infected heterosexuals (England and Wales, 2000–2004) AIDS. 2006;20:2371–9. doi: 10.1097/QAD.0b013e32801138f7. [DOI] [PubMed] [Google Scholar]
- 19.Chadborn TR, Baster K, Delpech VC, Sabin CA, Sinka K, Rice BD, et al. No time to wait: how many HIV-infected homosexual men are diagnosed late and consequently die? (England and Wales, 1993–2002) AIDS. 2005;19:513–20. doi: 10.1097/01.aids.0000162340.43310.08. [DOI] [PubMed] [Google Scholar]
- 20.Marks G, Crepaz N, Senterfitt JW, Janssen RS. Meta- analysis of high-risk sexual behavior in persons aware and unaware they are infected with HIV in the United States: implications for HIV prevention programs. J Acquir Immune Defic Syndr. 2005;39:446–53. doi: 10.1097/01.qai.0000151079.33935.79. [DOI] [PubMed] [Google Scholar]
- 21.Bontell I, Haggblom A, Bratt G, Albert J, Sonnerborg A. Trends in antiretroviral therapy and prevalence of HIV drug resistance mutations in Sweden 1997–2011. PloS One. 2013;8:e59337. doi: 10.1371/journal.pone.0059337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Global AIDS Response Progress Report 2012, Sweden. Swedish Institute for Communicable Disease Control. 2012 http://www.folkhalsomyndigheten.se/pagefiles/12846/global-aids-response-progress-report.pdf
- 23.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365:493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Skar H, Axelsson M, Berggren I, Thalme A, Gyllensten K, Liitsola K, et al. Dynamics of two separate but linked HIV-1 CRF01_AE outbreaks among injection drug users in Stockholm, Sweden, and Helsinki, Finland. J Virol. 2011;85:510–18. doi: 10.1128/JVI.01413-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Vu S, Le Strat Y, Barin F, Pillonel J, Cazein F, Bousquet V, et al. Population-based HIV-1 incidence in France, 2003-08: a modelling analysis. Lancet Infect Dis. 2010;10:682–7. doi: 10.1016/S1473-3099(10)70167-5. [DOI] [PubMed] [Google Scholar]
- 26.Bätzing-Feigenbaum J, Loschen S, Gohlke-Micknis S, Zimmermann R, Herrmann A, Kamga Wambo O, et al. Country-wide HIV incidence study complementing HIV surveillance in Germany. Euro Surveill. 2008;13(36) pii:18971. [PubMed] [Google Scholar]
- 27.Brännström J, Svedhem V, Yilmaz A, Blaxhult A, Wendahl S, Sönnerborg A. A high occurrence of late presenters and missed HIV diagnosis in clinical care in Sweden. Journal of the International AIDS Society. 2010;13((Suppl 4)):169. [Google Scholar]