Abstract
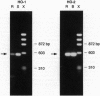
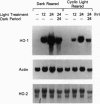
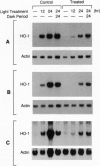
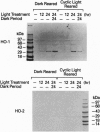
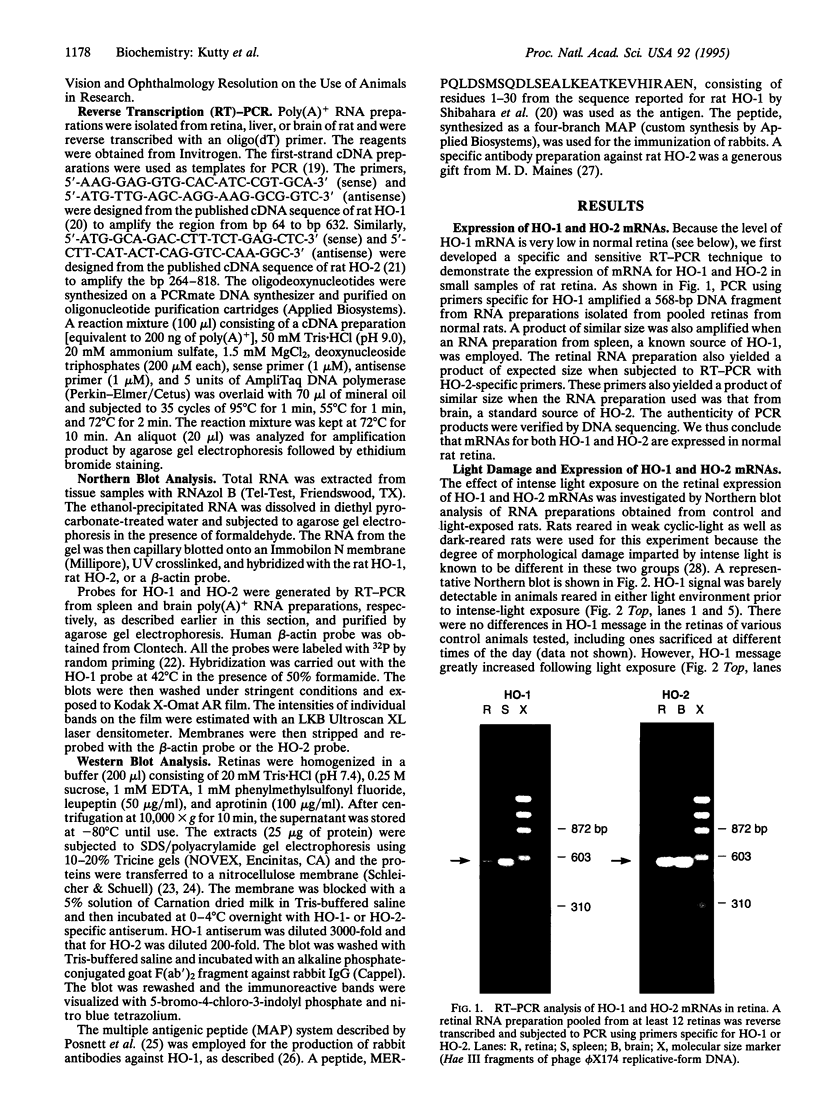
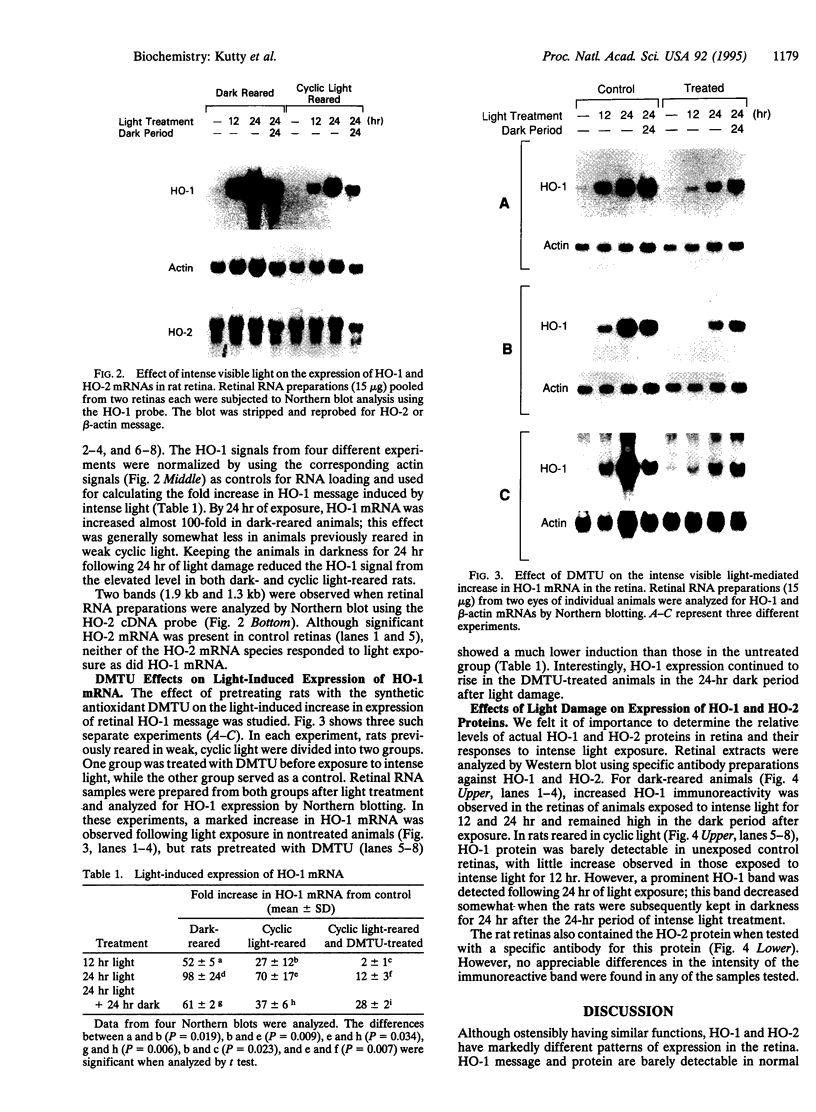
The effect of intense visible light (light damage) on the expression of heme oxygenase 1 (HO-1), a protein induced by oxidative stress, was investigated in the rat retina. A sensitive reverse transcription-PCR assay demonstrated the expression of mRNA for HO-1 as well as HO-2, the noninducible HO form, in the normal retina. As analyzed by Northern blotting, however, HO-1 mRNA was barely detectable under normal circumstances. After exposure to intense visible light, retinas had markedly higher HO-1 mRNA levels than unexposed controls, with increases up to 52- and 98-fold at 12 and 24 hr of exposure, respectively. Intense light exposure also resulted in an increase in HO-1 protein. In contrast, no appreciable change in HO-2 mRNA or protein was observed. The increase in HO-1 message was more pronounced in rats previously reared in the dark than in those reared in a weak cyclic-light environment. A marked decrease from the high level of HO-1 mRNA induced by light insult was observed when the animals were allowed to recover in the dark for 24 hr after light exposure. Most important, treatment of animals with 1,3-dimethylthiourea, a synthetic antioxidant, prior to light exposure effectively blocked the increase in HO-1 mRNA. Thus, HO-1 is a sensitive marker for assessing light-induced insult in the retina. Since increased expression of HO-1 is thought to be a cellular defense against oxidative damage, its expression may play an important role in protecting the retina against light damage.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Shigenaga M. K., Hagen T. M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc Natl Acad Sci U S A. 1993 Sep 1;90(17):7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booher R. N., Deshaies R. J., Kirschner M. W. Properties of Saccharomyces cerevisiae wee1 and its differential regulation of p34CDC28 in response to G1 and G2 cyclins. EMBO J. 1993 Sep;12(9):3417–3426. doi: 10.1002/j.1460-2075.1993.tb06016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross F. R., Blake C. M. The yeast Cln3 protein is an unstable activator of Cdc28. Mol Cell Biol. 1993 Jun;13(6):3266–3271. doi: 10.1128/mcb.13.6.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross F. R. Cell cycle arrest caused by CLN gene deficiency in Saccharomyces cerevisiae resembles START-I arrest and is independent of the mating-pheromone signalling pathway. Mol Cell Biol. 1990 Dec;10(12):6482–6490. doi: 10.1128/mcb.10.12.6482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross F. R., Tinkelenberg A. H. A potential positive feedback loop controlling CLN1 and CLN2 gene expression at the start of the yeast cell cycle. Cell. 1991 May 31;65(5):875–883. doi: 10.1016/0092-8674(91)90394-e. [DOI] [PubMed] [Google Scholar]
- De Bondt H. L., Rosenblatt J., Jancarik J., Jones H. D., Morgan D. O., Kim S. H. Crystal structure of cyclin-dependent kinase 2. Nature. 1993 Jun 17;363(6430):595–602. doi: 10.1038/363595a0. [DOI] [PubMed] [Google Scholar]
- Dirick L., Nasmyth K. Positive feedback in the activation of G1 cyclins in yeast. Nature. 1991 Jun 27;351(6329):754–757. doi: 10.1038/351754a0. [DOI] [PubMed] [Google Scholar]
- Eisenstein R. S., Garcia-Mayol D., Pettingell W., Munro H. N. Regulation of ferritin and heme oxygenase synthesis in rat fibroblasts by different forms of iron. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):688–692. doi: 10.1073/pnas.88.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber D. B., Lolley R. N. Enzymic basis for cyclic GMP accumulation in degenerative photoreceptor cells of mouse retina. J Cyclic Nucleotide Res. 1976;2(3):139–148. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fernandez-Sarabia M. J., Sutton A., Zhong T., Arndt K. T. SIT4 protein phosphatase is required for the normal accumulation of SWI4, CLN1, CLN2, and HCS26 RNAs during late G1. Genes Dev. 1992 Dec;6(12A):2417–2428. doi: 10.1101/gad.6.12a.2417. [DOI] [PubMed] [Google Scholar]
- Fesquet D., Labbé J. C., Derancourt J., Capony J. P., Galas S., Girard F., Lorca T., Shuttleworth J., Dorée M., Cavadore J. C. The MO15 gene encodes the catalytic subunit of a protein kinase that activates cdc2 and other cyclin-dependent kinases (CDKs) through phosphorylation of Thr161 and its homologues. EMBO J. 1993 Aug;12(8):3111–3121. doi: 10.1002/j.1460-2075.1993.tb05980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field J., Nikawa J., Broek D., MacDonald B., Rodgers L., Wilson I. A., Lerner R. A., Wigler M. Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition method. Mol Cell Biol. 1988 May;8(5):2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadwiger J. A., Wittenberg C., Richardson H. E., de Barros Lopes M., Reed S. I. A family of cyclin homologs that control the G1 phase in yeast. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6255–6259. doi: 10.1073/pnas.86.16.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt D. F., Organisciak D. T., Wang H. M., Wu R. L. alpha-Tocopherol in the developing rat retina: a high pressure liquid chromatographic analysis. Curr Eye Res. 1984 Nov;3(11):1281–1288. doi: 10.3109/02713688409007414. [DOI] [PubMed] [Google Scholar]
- Keyse S. M., Tyrrell R. M. Heme oxygenase is the major 32-kDa stress protein induced in human skin fibroblasts by UVA radiation, hydrogen peroxide, and sodium arsenite. Proc Natl Acad Sci U S A. 1989 Jan;86(1):99–103. doi: 10.1073/pnas.86.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutty G., Hayden B., Osawa Y., Wiggert B., Chader G. J., Kutty R. K. Heme oxygenase: expression in human retina and modulation by stress agents in a human retinoblastoma cell model system. Curr Eye Res. 1992 Feb;11(2):153–160. doi: 10.3109/02713689209000066. [DOI] [PubMed] [Google Scholar]
- Kutty R. K., Maines M. D. Purification and characterization of biliverdin reductase from rat liver. J Biol Chem. 1981 Apr 25;256(8):3956–3962. [PubMed] [Google Scholar]
- Kutty R. K., Maines M. D. Selective induction of heme oxygenase-1 isozyme in rat testis by human chorionic gonadotropin. Arch Biochem Biophys. 1989 Jan;268(1):100–107. doi: 10.1016/0003-9861(89)90569-9. [DOI] [PubMed] [Google Scholar]
- Kutty R. K., Nagineni C. N., Kutty G., Hooks J. J., Chader G. J., Wiggert B. Increased expression of heme oxygenase-1 in human retinal pigment epithelial cells by transforming growth factor-beta. J Cell Physiol. 1994 May;159(2):371–378. doi: 10.1002/jcp.1041590221. [DOI] [PubMed] [Google Scholar]
- Lam S., Tso M. O., Gurne D. H. Amelioration of retinal photic injury in albino rats by dimethylthiourea. Arch Ophthalmol. 1990 Dec;108(12):1751–1757. doi: 10.1001/archopht.1990.01070140105039. [DOI] [PubMed] [Google Scholar]
- Lautier D., Luscher P., Tyrrell R. M. Endogenous glutathione levels modulate both constitutive and UVA radiation/hydrogen peroxide inducible expression of the human heme oxygenase gene. Carcinogenesis. 1992 Feb;13(2):227–232. doi: 10.1093/carcin/13.2.227. [DOI] [PubMed] [Google Scholar]
- Lörincz A. T., Reed S. I. Primary structure homology between the product of yeast cell division control gene CDC28 and vertebrate oncogenes. Nature. 1984 Jan 12;307(5947):183–185. doi: 10.1038/307183a0. [DOI] [PubMed] [Google Scholar]
- Maines M. D., Trakshel G. M., Kutty R. K. Characterization of two constitutive forms of rat liver microsomal heme oxygenase. Only one molecular species of the enzyme is inducible. J Biol Chem. 1986 Jan 5;261(1):411–419. [PubMed] [Google Scholar]
- Nath K. A., Balla G., Vercellotti G. M., Balla J., Jacob H. S., Levitt M. D., Rosenberg M. E. Induction of heme oxygenase is a rapid, protective response in rhabdomyolysis in the rat. J Clin Invest. 1992 Jul;90(1):267–270. doi: 10.1172/JCI115847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noell W. K., Walker V. S., Kang B. S., Berman S. Retinal damage by light in rats. Invest Ophthalmol. 1966 Oct;5(5):450–473. [PubMed] [Google Scholar]
- Ogas J., Andrews B. J., Herskowitz I. Transcriptional activation of CLN1, CLN2, and a putative new G1 cyclin (HCS26) by SWI4, a positive regulator of G1-specific transcription. Cell. 1991 Sep 6;66(5):1015–1026. doi: 10.1016/0092-8674(91)90445-5. [DOI] [PubMed] [Google Scholar]
- Organisciak D. T., Darrow R. M., Jiang Y. I., Marak G. E., Blanks J. C. Protection by dimethylthiourea against retinal light damage in rats. Invest Ophthalmol Vis Sci. 1992 Apr;33(5):1599–1609. [PubMed] [Google Scholar]
- Organisciak D. T., Jiang Y. L., Wang H. M., Bicknell I. The protective effect of ascorbic acid in retinal light damage of rats exposed to intermittent light. Invest Ophthalmol Vis Sci. 1990 Jul;31(7):1195–1202. [PubMed] [Google Scholar]
- Organisciak D. T., Wang H. M., Kou A. L. Ascorbate and glutathione levels in the developing normal and dystrophic rat retina: effect of intense light exposure. Curr Eye Res. 1984 Jan;3(1):257–267. doi: 10.3109/02713688408997208. [DOI] [PubMed] [Google Scholar]
- Organisciak D. T., Wang H. M., Li Z. Y., Tso M. O. The protective effect of ascorbate in retinal light damage of rats. Invest Ophthalmol Vis Sci. 1985 Nov;26(11):1580–1588. [PubMed] [Google Scholar]
- Penn J. S., Anderson R. E. Effect of light history on rod outer-segment membrane composition in the rat. Exp Eye Res. 1987 Jun;44(6):767–778. doi: 10.1016/s0014-4835(87)80040-4. [DOI] [PubMed] [Google Scholar]
- Penn J. S., Naash M. I., Anderson R. E. Effect of light history on retinal antioxidants and light damage susceptibility in the rat. Exp Eye Res. 1987 Jun;44(6):779–788. doi: 10.1016/s0014-4835(87)80041-6. [DOI] [PubMed] [Google Scholar]
- Poon R. Y., Yamashita K., Adamczewski J. P., Hunt T., Shuttleworth J. The cdc2-related protein p40MO15 is the catalytic subunit of a protein kinase that can activate p33cdk2 and p34cdc2. EMBO J. 1993 Aug;12(8):3123–3132. doi: 10.1002/j.1460-2075.1993.tb05981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posnett D. N., McGrath H., Tam J. P. A novel method for producing anti-peptide antibodies. Production of site-specific antibodies to the T cell antigen receptor beta-chain. J Biol Chem. 1988 Feb 5;263(4):1719–1725. [PubMed] [Google Scholar]
- Reed S. I., Wittenberg C. Mitotic role for the Cdc28 protein kinase of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5697–5701. doi: 10.1073/pnas.87.15.5697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotenberg M. O., Maines M. D. Isolation, characterization, and expression in Escherichia coli of a cDNA encoding rat heme oxygenase-2. J Biol Chem. 1990 May 5;265(13):7501–7506. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schwartzman M. L., Masferrer J., Dunn M. W., McGiff J. C., Abraham N. G. Cytochrome P450, drug metabolizing enzymes and arachidonic acid metabolism in bovine ocular tissues. Curr Eye Res. 1987 Apr;6(4):623–630. doi: 10.3109/02713688709025223. [DOI] [PubMed] [Google Scholar]
- Shibahara S., Müller R., Taguchi H., Yoshida T. Cloning and expression of cDNA for rat heme oxygenase. Proc Natl Acad Sci U S A. 1985 Dec;82(23):7865–7869. doi: 10.1073/pnas.82.23.7865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Solomon M. J., Glotzer M., Lee T. H., Philippe M., Kirschner M. W. Cyclin activation of p34cdc2. Cell. 1990 Nov 30;63(5):1013–1024. doi: 10.1016/0092-8674(90)90504-8. [DOI] [PubMed] [Google Scholar]
- Solomon M. J., Harper J. W., Shuttleworth J. CAK, the p34cdc2 activating kinase, contains a protein identical or closely related to p40MO15. EMBO J. 1993 Aug;12(8):3133–3142. doi: 10.1002/j.1460-2075.1993.tb05982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker R., Yamamoto Y., McDonagh A. F., Glazer A. N., Ames B. N. Bilirubin is an antioxidant of possible physiological importance. Science. 1987 Feb 27;235(4792):1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Rosenberg A. H., Dunn J. J., Dubendorff J. W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- Sun Y., Rotenberg M. O., Maines M. D. Developmental expression of heme oxygenase isozymes in rat brain. Two HO-2 mRNAs are detected. J Biol Chem. 1990 May 15;265(14):8212–8217. [PubMed] [Google Scholar]
- Surana U., Robitsch H., Price C., Schuster T., Fitch I., Futcher A. B., Nasmyth K. The role of CDC28 and cyclins during mitosis in the budding yeast S. cerevisiae. Cell. 1991 Apr 5;65(1):145–161. doi: 10.1016/0092-8674(91)90416-v. [DOI] [PubMed] [Google Scholar]
- Tenhunen R., Marver H. S., Schmid R. Microsomal heme oxygenase. Characterization of the enzyme. J Biol Chem. 1969 Dec 10;244(23):6388–6394. [PubMed] [Google Scholar]
- Tenhunen R., Ross M. E., Marver H. S., Schmid R. Reduced nicotinamide-adenine dinucleotide phosphate dependent biliverdin reductase: partial purification and characterization. Biochemistry. 1970 Jan 20;9(2):298–303. doi: 10.1021/bi00804a016. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyers M., Tokiwa G., Nash R., Futcher B. The Cln3-Cdc28 kinase complex of S. cerevisiae is regulated by proteolysis and phosphorylation. EMBO J. 1992 May;11(5):1773–1784. doi: 10.1002/j.1460-2075.1992.tb05229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma A., Hirsch D. J., Glatt C. E., Ronnett G. V., Snyder S. H. Carbon monoxide: a putative neural messenger. Science. 1993 Jan 15;259(5093):381–384. doi: 10.1126/science.7678352. [DOI] [PubMed] [Google Scholar]
- Vile G. F., Basu-Modak S., Waltner C., Tyrrell R. M. Heme oxygenase 1 mediates an adaptive response to oxidative stress in human skin fibroblasts. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2607–2610. doi: 10.1073/pnas.91.7.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vile G. F., Tyrrell R. M. Oxidative stress resulting from ultraviolet A irradiation of human skin fibroblasts leads to a heme oxygenase-dependent increase in ferritin. J Biol Chem. 1993 Jul 15;268(20):14678–14681. [PubMed] [Google Scholar]
- Wiegand R. D., Giusto N. M., Rapp L. M., Anderson R. E. Evidence for rod outer segment lipid peroxidation following constant illumination of the rat retina. Invest Ophthalmol Vis Sci. 1983 Oct;24(10):1433–1435. [PubMed] [Google Scholar]
- Williams T. P., Howell W. L. Action spectrum of retinal light-damage in albino rats. Invest Ophthalmol Vis Sci. 1983 Mar;24(3):285–287. [PubMed] [Google Scholar]
- Wittenberg C., Sugimoto K., Reed S. I. G1-specific cyclins of S. cerevisiae: cell cycle periodicity, regulation by mating pheromone, and association with the p34CDC28 protein kinase. Cell. 1990 Jul 27;62(2):225–237. doi: 10.1016/0092-8674(90)90361-h. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Connolly T., Futcher B., Beach D. Human D-type cyclin. Cell. 1991 May 17;65(4):691–699. doi: 10.1016/0092-8674(91)90100-d. [DOI] [PubMed] [Google Scholar]