Abstract
IMPORTANCE
We developed a novel strategy for treatment of Leber hereditary optic neuropathy (LHON) caused by a mutation in the nicotinamide adenine dinucleotide dehydrogenase subunit IV (ND4) mitochondrial gene.
OBJECTIVE
To demonstrate the safety and effects of the gene therapy vector to be used in a proposed gene therapy clinical trial.
DESIGN AND SETTING
In a series of laboratory experiments, we modified the mitochondrial ND4 subunit of complex I in the nuclear genetic code for import into mitochondria. The protein was targeted into the organelle by agency of a targeting sequence (allotopic expression). The gene was packaged into adeno-associated viral vectors and then vitreally injected into rodent, nonhuman primate, and ex vivo human eyes that underwent testing for expression and integration by immunohistochemical analysis and blue native polyacrylamide gel electrophoresis. During serial follow-up, the animal eyes underwent fundus photography, optical coherence tomography, and multifocal or pattern electroretinography. We tested for rescue of visual loss in rodent eyes also injected with a mutant G11778A ND4 homologue responsible for most cases of LHON.
EXPOSURE
Ocular infection with recombinant adeno-associated viral vectors containing a wild-type allotopic human ND4 gene.
MAIN OUTCOMES AND MEASURES
Expression of human ND4 and rescue of optic neuropathy induced by mutant human ND4.
RESULTS
We found human ND4 expressed in almost all mouse retinal ganglion cells by 1 week after injection and ND4 integrated into the mouse complex I. In rodent eyes also injected with a mutant allotopic ND4, wild-type allotopic ND4 prevented defective adenosine triphosphate synthesis, suppressed visual loss, reduced apoptosis of retinal ganglion cells, and prevented demise of axons in the optic nerve. Injection of ND4 in the ex vivo human eye resulted in expression in most retinal ganglion cells. Primates undergoing vitreal injection with the ND4 test article and followed up for 3 months had no serious adverse reactions.
CONCLUSIONS AND RELEVANCE
Expression of our allotopic ND4 vector in the ex vivo human eye, safety of the test article, rescue of the LHON mouse model, and the severe irreversible loss of visual function in LHON support clinical testing with mutated G11778A mitochondrial DNA in our patients.
The clinical features of Leber hereditary optic neuropathy (LHON), a degenerative visual disorder, were first described in 1871.1 Most patients with LHON have the G11778A mutation, which affects the ND4 gene (NCBI Entrez Gene 4538). The remaining patients have the G3460A mutation, which affects the ND1 gene (NCBI Entrez Gene 4535), or the T14484C mutation, which affects the ND6 gene (NCBI Entrez Gene 4541).2 These 3 mutations are considered the primary causes of LHON, and each presents a significant risk for severe visual loss.3 All are associated with focal degeneration of retinal ganglion cells (RGCs).
Because most mitochondrial proteins are expressed in the nucleus and imported into the organelle, we adapted the approach termed allotopic expression,4 in which a nuclear version of the mitochondrial gene (ND4 in this case) was expressed in the nucleus, translated on cytoplasmic polyribosomes, and imported into the mitochondria with the addition of an N-terminal mitochondrial targeting sequence.5–8 In studies more than a decade ago, expression of the allotopic ND4 gene in G11778A LHON cells corrected defective adenosine triphosphate (ATP) synthesis.9 Next, this technology was used to generate a mouse model of LHON by delivering a nuclear-encoded version of the mutant human ND4 that induced swelling of the optic nerve head, followed by a progressive demise of RGCs and their axons.10 In contrast, expression of the wild-type (WT) allotopic ND4 produced no abnormalities in visual function or pathologic changes in the retina or optic nerve.11 Herein we describe the efficacy and safety of the vector proposed for the treatment of LHON caused by mutated G11778A mitochondrial DNA.
Methods
Construction of the Viral Vector and Intraocular Injections
The WT and mutant (MT) allotopic ND4FLAG fusion genes containing the mitochondrial targeting sequences and epitope tag were constructed and packaged into adeno-associated viral (AAV) virions as previously described.12,13 This process is described in detail in the Supplement (eMethods). All animal procedures were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. Fifteen mice received 1 µL of self-complementary AAV type 2 (scAAV2)–WT-ND4FLAG (triple Y-F capsid) (3.98 × 1012 vector genomes[vg]/mL) vitreally injected into the right eyes and 1 µL of scAAV–green fluorescent protein (GFP) (NCBI Entrez Gene 7011691) (1.09 × 1012 vg/mL) into the left eyes. Seventy-two hours after these injections, single-stranded AAV2 (ssAAV2)–MT-ND4FLAG (G11778A) (1.02 × 1012 vg/mL) was injected into both eyes. In another group of mice (n = 10), 1 µL of ssAAV2-WT-ND4FLAG (6.80 × 1012 vg/mL) was injected into the right eye and 1 µL of ssAAV2-GFP was injected into the left eye. Seventy-two hours after these injections, 1µL of ssAAV2-MT-ND4FLAG (G11778A) was injected into both eyes. A summary of the use of animals in our experiments is provided in the Supplement (eTable).
To test the safety of the vector for a phase 1 clinical trial, 3 rhesus macaques (1 male and 2 female, aged 3–7 years) were injected with the test article scAAV2 (Y444,500,730F)-P1ND4v2 that lacked the FLAG epitope. Two additional animals were injected with scAAV2-GFP (triple Y-F capsid), driven by the same promoter (small cytomegalovirus chicken β-actin [smCBA]) as the P1ND4v2 test article.
Electrophysiological Examinations
Pattern electroretinograms (PERGs) were obtained in mice at 1, 6, and 12 months after the viral injection as previously described.14 Rhesus macaques underwent assessment by multifocal electroretinography before intravitreal injection and at 1 and 3 months after vector injection.
Imaging
The mouse retina was imaged at 1, 3, 6, and 12 months after AAV injection using optical coherence tomography (OCT) (Bioptigen, Inc) as described previously.15 Rhesus macaques underwent assessment by ophthalmologic examination, color retinal fundus photography (FF450; Zeiss), and OCT (Heidelberg Spectralis; Heidelberg Engineering, Inc) before intravitreal injection and at 5 days, 1 and 2weeks, and 1 and 3 months after vector injection. For 1 animal, retinal imaging was also done at 4 months.
Expression Analyses
For expression studies, mice were humanely killed 1, 3, and 7 days after the viral injections. The eyes were removed and underwent immunohistochemical processing as previously described.16 Because our goal is to treat patients with LHON, we also tested for transgene expression in 2 normal human eyes that were surgically removed for control of periocular spread of cancers.
In rhesus macaque eyes, retinal longitudinal sections (8 µm)were obtained. The FLAG-positive cells (right eyes), GFP-positive cells (left eyes), and Thy1.2-positive RGCs (both eyes) were counted. Images of the optic nerves from the right and left eyes were analyzed for diameters.
Mice receiving the double viral injections were killed humanely 1 year (scAAV) or 6months (ssAAV) after injections. The eyes and optic nerves underwent immunohistochemical processing and electron microscopic examination as previously described.16 Primates injected with scAAV-P1ND4v2 (triple Y-F capsid) were killed humanely 3months after scAAV injections.
Total RNA was isolated from the retinas and optic nerves of injected eyes and uninjected controls. Sequencing of reverse transcription polymerase chain reaction products was performed using a commercially available cycle sequencing kit (ABI Prism BigDye Terminator kit; Applied Biosciences).
One year after intravitreal viral injections, 5 mice were killed humanely and the optic nerves were immediately dissected for measurements of the rate of ATP synthesis as previously described.9 Fragmentation of DNA characteristic of apoptosis in mice retinal whole mounts 1 year after AAV injections (n = 3) was monitored by terminal deoxynucleotidyl transferase–mediated deoxyuridine triphosphate nick-end labeling.
Blue native–polyacrylamide gel electrophoresis of rats intravitreally injected with scAAV2-WT-ND4FLAG (triple-Y-F capsid) (n = 5) or uninjected rats (n = 5) was performed based on the method of Calvaruso et al.17
Statistical Analysis
We used the 2-tailed, unpaired t test to compare the transduction efficiency of right eyes inoculated with scAAV2-WT-ND4 (triple Y-F capsid) and RGCs identified by Thy1.2 positivity. We also used the 2-tailed, unpaired t test in rescue studies to compare the right eyes inoculated with scAAV2-WT-ND4FLAG (triple Y-F capsid) plus ssAAV2-MT-ND4FLAG and the control (left) eyes receiving scAAV2-GFP plus ssAAV2-MT-ND4FLAG. P ≤ .05 was considered significant.
Results
Expression of WT Allotopic Human ND4 in Mice
Intravitreal injection of WT allotopic ND4FLAG packaged in scAAV with triple Y-F capsid modifications had a perinuclear pattern of immunolabeling consistent with mitochondrial localization in RGCs (Supplement [eFigure 1A]). In contrast, the contralateral eyes injected with scAAV2-GFP had nuclear and cytoplasmic expression in cells of the RGC layer (Supplement [eFigure 1B]). Quantitative analysis revealed that, relative to the number of Thy1.2-positive RGCs, ND4FLAG-expressing RGCs increased from 21% at 1 day after injection to 50% by 3 days and 85%by 7 days (Supplement [eFigure 1C]). Thus, WT allotopic ND4 was rapidly expressed in almost all RGCs.
Expression of WT Allotopic ND4 in Ex Vivo Human Eyes
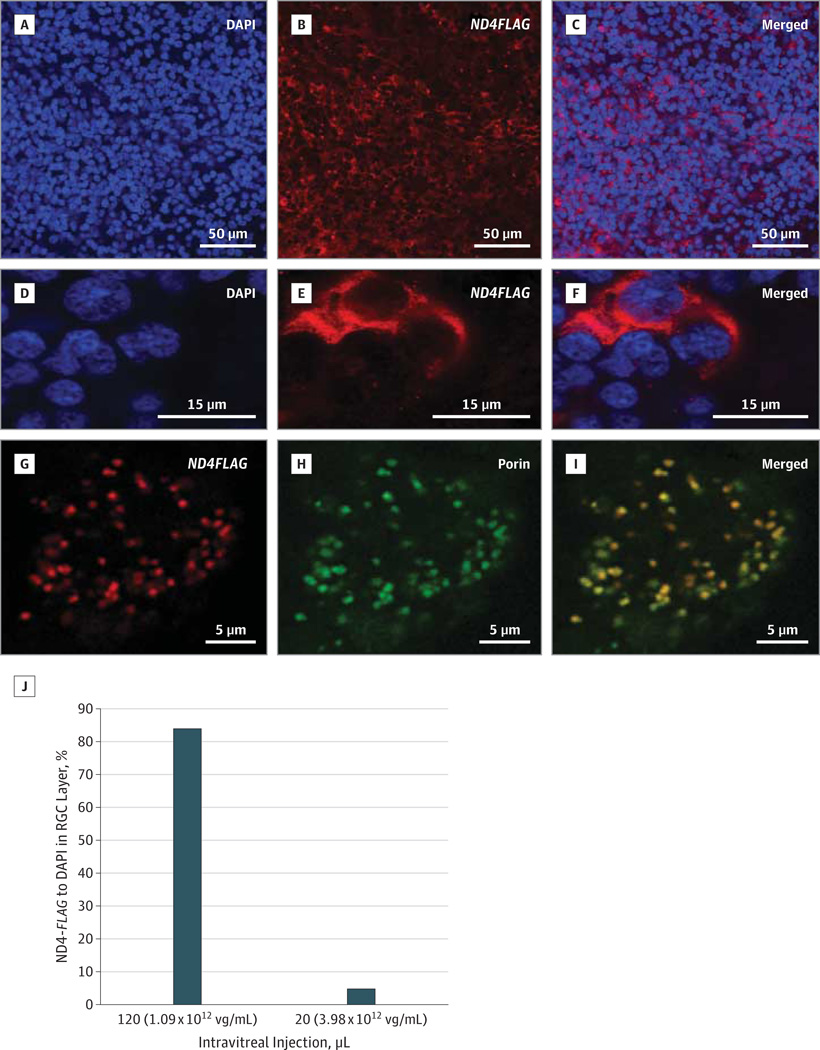
Confocal microscopy of the ex vivo human retinal flat mounts focused on the RGC layer revealed 4',6-diamidino-2-phenylindole (DAPI)–labeled cell nuclei (Figure 1A) with ND4FLAG expression (Figure 1B) surrounding the nucleus of almost all RGCs (Figure 1C). At higher magnification, human RGC (Figure 1D) expression of ND4FLAG (Figure 1E) was peri-nuclear (Figure 1F). At high power, ND4FLAG expression was punctate (Figure 1G), as were mitochondria labeled with a bona fide mitochondrial marker voltage-dependent anion channel/porin (Figure 1H) that colocalized with ND4FLAG (Figure 1I). Quantitative analysis revealed that, with intravitreal injection of more than 1011 vg, 84% of cells in the RGC layer expressed ND4FLAG (Figure 1J).
Figure 1. Expression of Wild-Type (WT) Human ND4.
Confocal microscopy of a retinal flat mount focused on the retinal ganglion cell (RGC) layer of the ex vivo human eye infected with WT ND4FLAG delivered by a triple Y-F capsid–modified self-complementary adeno-associated viral (scAAV) vector shows cell nuclei labeled by 4′,6-diamidino-2-phenylindole (DAPI) (A) and the anti-FLAG antibody (B) around the nuclei (C). At higher magnification, many DAPI nuclei are seen (D), with 1 cell filled with FLAG (E) surrounding the nucleus (F). High magnification of a FLAG-positive cell in the RGC layer (G) reacted with the mitochondrial membrane protein antibody against porin (H) reveals colocalization of ND4FLAG with the mitochondrial marker (I). A bar plot (J) shows counts of ND4FLAG-labeled RGCs in the ex vivo human eye. Vg indicates vector genome.
Transcription of MT and WT Allotopic Human ND4 in Mice
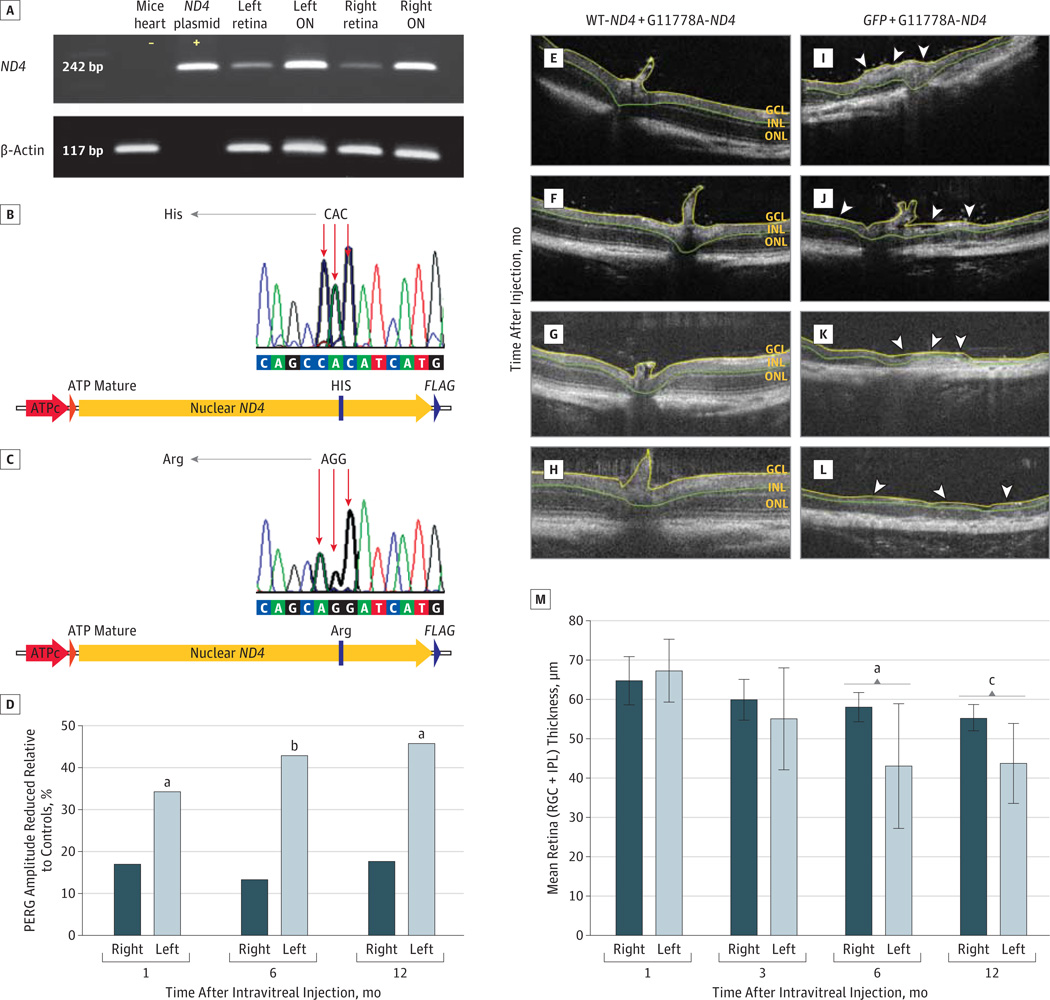
Next, we tested for rescue of optic neuropathy in a mouse model of LHON induced by coinjection of AAV virions containing the MT allotopic ND4 allele.18 To demonstrate that MT and WT human ND4 are expressed in mouse eyes coinjected with ssAAVs containing the disease-inducing MT R340H ND4 and with the rescue WT human ND4, we performed reverse transcription polymerase chain reaction of RNA isolated from 10 mice that received injections of the MT and WT alleles into both eyes. We found human ND4 messenger RNAs in the retina and optic nerves, but not in the heart (Figure 2A). Gene sequencing of the amplicons showed the presence of MT R340H ND4 (CAC-histidine) (Figure 2B) and WT R340R (AGG-arginine) complementary DNA (Figure 2C), thus confirming transcription of human MT and WT ND4 alleles in double-injected eyes. Previous studies11,12 have demonstrated that the MT ND4 allele induces optic neuropathy in mice and that WT human ND4 is safe and does not cause visual loss in mice. Our next step was to determine whether the WT human ND4 would provide rescue of visual dysfunction induced by the MT human ND4 allele.
Figure 2. Transcription and In Vivo Rescue Effects of Human ND4.
Reverse transcription polymerase chain reaction (A) of RNA isolated from the retinas and optic nerves (ONs) of 10 mice that received injections of mutant and wild-type (WT) allotopic ND4 into the right and left eyes showed the expected 242–base pair (bp) bands. The ND4 plasmid served as a positive control. Heart tissue isolated from injected animals was negative for human ND4. Gene sequencing of the amplicons (B and C) showed R340H mutant (MT) allotopic ND4 (CAC-histidine) and R340RWT allotopic ND4 (AGG-arginine) DNA to be present. ATP indicates adenosine triphosphate. For rescue of visual dysfunction, a bar plot (D) shows the percentage of worsening pattern electroretinogram (PERG) amplitudes of mock-treated left eyes relative to those of normal (no injection) controls was statistically significant, but no differences were detected between the treated right eyes and controls. Results of in vivo imaging (E-L) indicate that eyes protected against the effects of R340H MT ND4 by WT allotopic ND4 packaged in triple Y-F capsid–modified self-complementary adeno-associated viral (scAAV) vector showed no evidence of optic nerve head swelling or loss of the inner retinal layers at 1 (E), 3 (F), 6 (G), and 12 (H) months after injection. In contrast, the mock-treated left eyes developed swelling of the optic nerve head (arrowheads) (I), with progressive thinning of the inner retina at 3 (arrowheads) (J) and 6 months (arrowheads) (K) after injection and almost complete loss 12 months after injection (L). GCL indicates ganglion cell layer; INL, inner nuclear layer; and ONL, outer nuclear layer. A bar plot (M) shows that differences in thickness of the retinal ganglion cell and inner plexiform layers (RGC + IPL) between the rescued right eyes and mock-treated left eyes were statistically significant by 6 and 12 months after injection. Whiskers indicate standard error.
aP = .02.
bP = .006.
cP = .005
Rescue of Visual Loss by WT Allotopic Human ND4
Using the PERG, a sensitive measure of visual potential and RGC function,19 we recorded signals in mice 1, 6, and 12months after intraocular injections of rescue vector scAAV2-WT-ND4FLAG (packaged with triple Y-F VP3 capsid modifications) and the disease-inducing ssAAV2-MT-ND4FLAG into the right eyes. The left eyes received the disease-inducing AAV-MT-ND4FLAG followed by scAAV containing only GFP (ie, mock rescue) that had been demonstrated to have no adverse effects on the optic nerve.16 Preservation of visual function was assessed by comparisons between treated and mock-treated eyes and comparison with PERG recordings of healthy mice receiving no injection (controls) belonging to the same age groups as AAV-injected mice. Seventeen experimental mice and 14 controls contributed data to this analysis.
One month after intraocular injections, the mean (SE) PERG amplitudes in the mock-rescued left eyes receiving scAAV2-GFP along with the disease-inducing ssAAV2-MT-ND4 diminished (18.1 [2.5] µV) relative to age-matched controls (27.4 [2.8] µV; P = .02). The mean (SE) PERG amplitudes in these left eyes significantly worsened at6months (16.8[1.95]µV; P = .006) and 1 year (12.9 [3.2] µV; P = .02) after injection. However, rescued right eyes compared with the age-matched controls showed no significant differences at any point examined (Figure 2D). For PERG amplitudes, analysis of variance with random effects to account for correlated measurements of the same animals over time found a statistically significant difference between right eyes rescued with WT allotopic ND4 (mean [SE], 18.668 [0.979] µV) and left eyes mock-treated with GFP (15.254 [1.052] µV; P = .02). The mean (SE) of left and right eyes together in the controls was 23.703 (1.687) µV. Amplitudes for WT ND4–injected right eyes were reduced relative to those of the controls (P = .01). Values of the GFP-treated left eyes were also reduced relative to those of controls (P < .001). In addition, we found a statistically significant difference in PERG latency between the rescued right eyes (mean [SE], 108.343 [2.983] milliseconds) and mock-treated left eyes (126.753 [3.204] milliseconds; P < .001), but not between the right eyes and controls (110.692 [5.138] milliseconds; P = .69). These findings indicate that WT allotopic ND4 gene therapy prevents visual loss and RGC dysfunction with gene delivery using the highly efficient expression achieved by the triple Y-F capsid scAAV2.
Prevention of RGC Demise by Allotopic Human ND4: Serial OCT Imaging
Next, we looked for structural preservation of RGCs with treatment. Intraocular injections of the rescue WT allotopic ND4 packaged with triple Y-F VP3 scAAV capsids prevented loss of the RGC layer and the adjacent inner plexiform layer. Serial OCT imaging of the rescued right eyes (n = 14) at 1 (Figure 2E), 3 (Figure 2F), 6 (Figure 2G), and 12 (Figure 2H) months after injection revealed no significant differences of the RGC and inner plexiform layers. In contrast, the contralateral mock-treated eyes exhibited initial swelling of the optic nerve head, also seen in patients with LHON and acute visual loss, and of the adjacent RGC and inner plexiform layers at 1 month (Figure 2I), followed by progressive thinning of these layers at 3 (Figure 2J) and 6 (Figure 2K) months after injection and almost complete loss (Figure 2L) in some eyes examined 1 year after injection. Differences between the right eyes undergoing WT ND4 rescue and left eyes undergoing mock GFP treatment were statistically significant at 6 (P = .02) and 12 (P = .005) months after injection (Figure 2M). Thus, WT allotopic ND4 ameliorated progressive loss of cells in the RGC layer.
Prevention of RGC Loss by WT Allotopic ND4: Histopathologic Studies
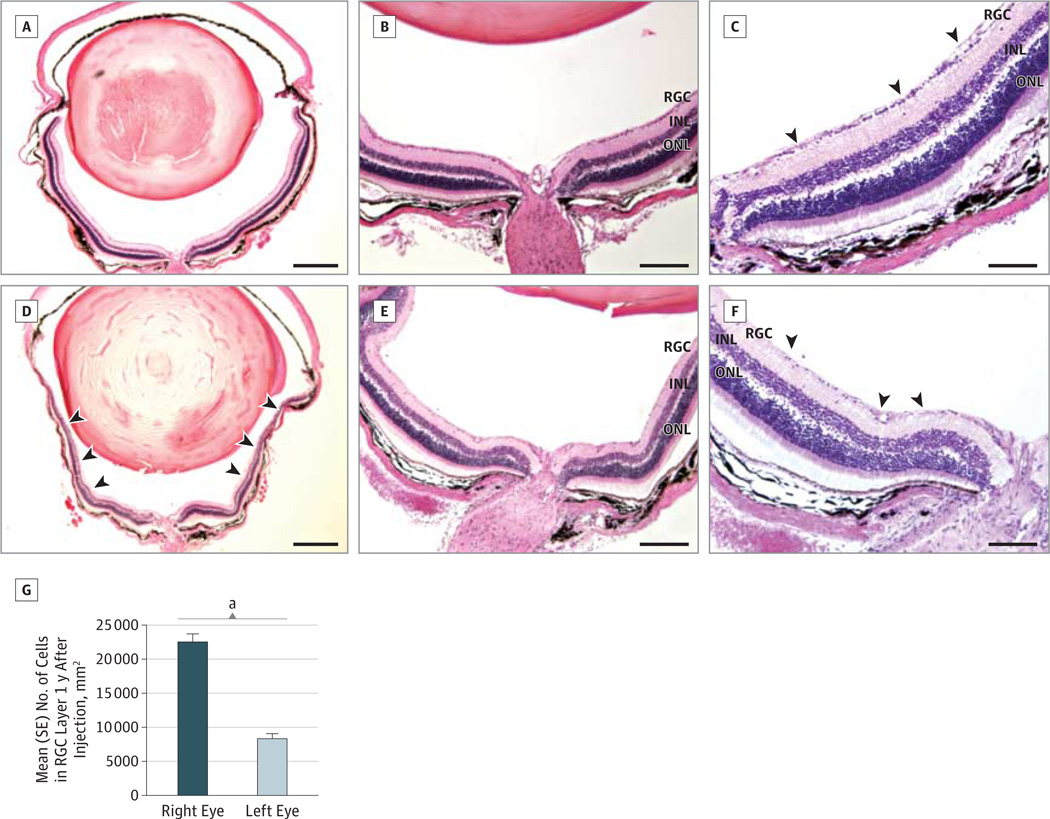
One year after intraocular injections, light microscopy showed no thinning of the inner retina in right eyes rescued from the disease-inducing R340H MT allotopic ND4 by coinjection of the WT allotopic ND4 delivered by scAAV (triple Y-F capsid) (Figure 3A). Higher magnification revealed no cell loss in the RGC layer (Figure 3B and C). In contrast, left eyes mock rescued with GFP from the adverse effects of the R340H MT allotopic ND4 had marked loss of the inner retina (Figure 3D) with substantial demise of RGCs (Figure 3E and F). Quantitative analysis revealed an almost 3-fold rescue of RGCs (mean [SE], 22 547 [1149] cells/mm2) for rescued right eyes relative to the mock-treated left eyes (8390 [695.6] cells/mm2), with differences being highly significant (P = 6.3 × 10−16) (Figure 3G).Wild-type ND4 delivered by standard ssAAV2 did not prevent RGC loss (Supplement [eFigure 2A–C]) or apoptosis induced by the MT ND4 allele (Supplement [eFigure 2D]). Mock-treated eyes showed similar ultrastructural damage (Supplement [eFigure 2E]). Thus, rescue with WT ND4 packaged with triple Y-F capsid–modified scAAV was highly effective against RGC injury induced by R340H MT ND4.
Figure 3. Histopathologic Findings of the Retina.
One year after intravitreal injections, low- (A), medium- (B), and high- (C) magnification hematoxylin-eosin–stained transverse sections of mice eyes that received double injections with self-complementary adeno-associated viral (scAAV2) vector wild-type (WT) allotopic ND4FLAG and single-stranded AAV2 (ssAAV2) mutant allotopic R340H ND4FLAG had an intact retinal ganglion cell (RGC) layer (arrowheads). In contrast, the mock-treated left eyes showed loss of the inner retina (arrowheads) (D) and loss of RGCs (arrowheads) (E and F). A bar plot (G) shows a significant decrease in the mean (SE) cell count of the RGC layer between rescued right eyes (WT-ND4 [triple Y-F capsid modifications] + G11778A) and mock-treated left eyes (green fluorescent protein + G11778A-ND4). INL indicates inner nuclear layer; ONL, outer nuclear layer. Scale bar in parts A and D indicates 200 µm; B and E, 100 µm; and C and F, 50 µm.
aP = 6.3 × 10−16.
Prevention of Optic Nerve Atrophy by WT Allotopic ND4
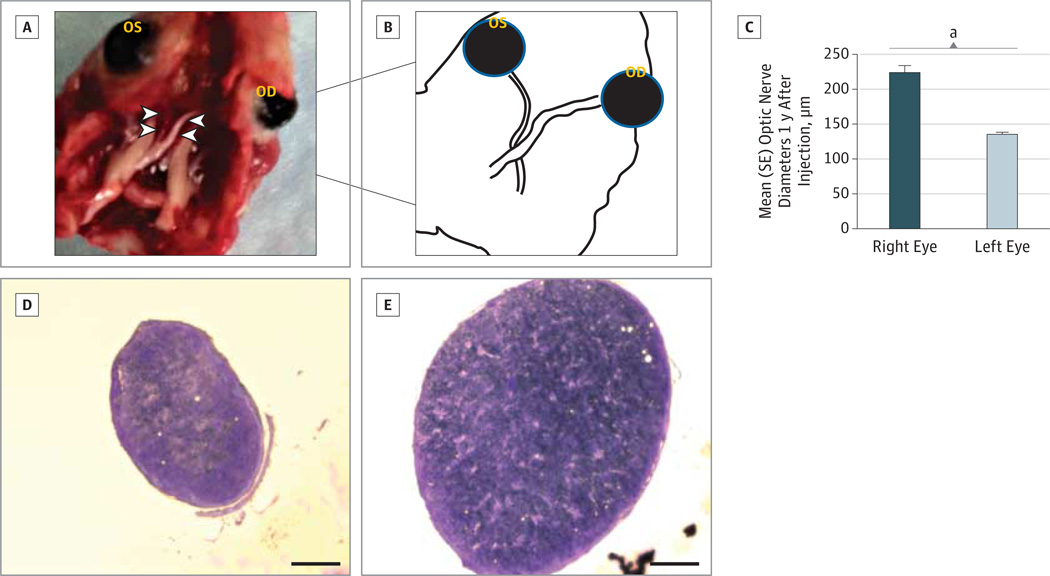
Gross dissection specimens of animals killed humanely 1 year after rescue with WT ND4 packaged with the triple Y-F capsid–modified scAAV showed striking differences between eyes. Treated right optic nerves were of normal caliber along their entire length from the back of the eye to the optic chiasm (Figure 4A and B). In contrast, the left optic nerves of these animals were markedly atrophic, with a 40% loss in diameter (Figure 4C–E) that was highly significant relative to the treated right optic nerves (P = 1.3 × 10−12). Transmission electron micrographs showed few remaining fibers, some with degenerating axon profiles in mock-treated optic nerves even 1 year after intraocular injections (Figure 5A), when axonal loss and fibers with thin myelin lamellae were prominent ultrastructural findings (Figure 5B). In contrast, axons of rescued optic nerves were relatively preserved and numerous (Figure 5C). When delivered by ssAAV, WT ND4 did not prevent axonal loss (Supplement [eFigure 2F]). Thus, WT allotopic ND4 gene delivery prevented optic neuropathy induced by the MT ND4 when delivered by the triple Y-F capsid–modified scAAV2.
Figure 4. Histopathologic Findings of the Optic Nerve.
A gross dissection specimen (A) revealed marked atrophy of the mock-treated left optic nerve (left arrowheads) along its entire length from the back of the left eye (OS) to the optic chiasm. Wild-type (WT) ND4 packaged with the triple Y-F capsid–modified (TM) self-complementary adeno-associated viral vector prevented atrophy of the right optic nerve (right arrowheads) in the right eye (OD) that was of normal caliber along its entire length. Schematic illustration (B) of part A. A bar plot (C) shows differences in optic nerve diameters between the right (WT-ND4[TM] + G11778A) and left (green fluorescent protein + G11778A-ND4) eyes were highly significant. Cross sections through the optic nerve taken approximately 1mmbehind the globe confirmed the marked atrophy of the left optic nerve (D). Cross section of the rescued right optic nerve shows no marked atrophy (E). Scale bar in parts D and E indicates 100 µm.
aP = 1.3 × 10−12.
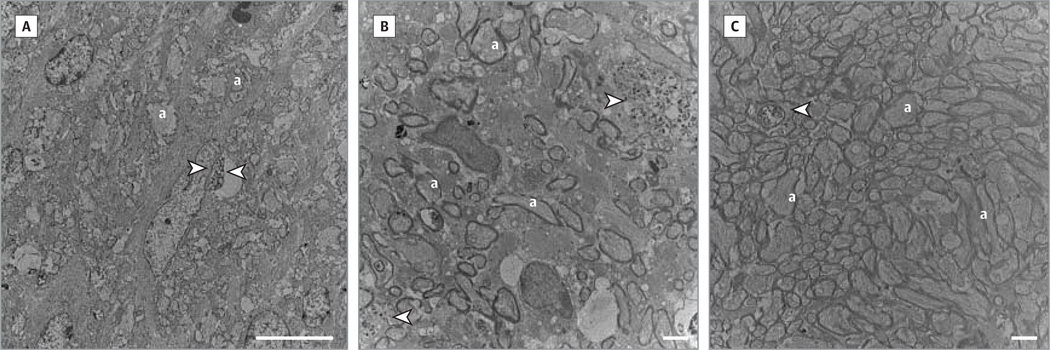
Figure 5. Ultrastructural Findings in the Optic Nerve.
Transmission electronmicrographs counterstained with uranyl acetate showed axonal loss with degenerating axon profiles (arrowheads) in mock-treated optic nerves even 1 year after intraocular injections (A); loss of axons and fibers with thin myelin lamellae and degenerating axons (arrowheads) were evident in mock-treated left eyes (B); and axons were numerous in rescued optic nerves, but some degenerating fibers were evident (arrowhead) (C). Scale bar in part A indicates 10 µm; B and C, 2 µm; and a indicates axon.
Prevention of Loss of Oxidative Phosphorylation by WT Allotopic ND4
The rate of complex I–dependent ATP synthesis was reduced almost 90% in mock-rescued mouse optic nerves (ATP level, 12 nmol/min per milligram of optic nerve tissue) relative to the uninjected optic nerves of controls (112 nmol/min per milligram) (Supplement [eFigure 3A]). This difference was significant (P = .003). The optic nerves of eyes injected with the R340H MT ND4 and rescued with the R340R WT ND4 (ATP level, 42 nmol/min per milligram of optic nerve tissue) had complex I–dependent ATP synthesis rates that were not significantly different from those of controls receiving no injection. Thus, the degree of oxidative phosphorylation loss induced by the R340H MT ND4 allele was suppressed by WT allotopic ND4.
Suppression of RGC Apoptosis by WT Allotopic ND4
Because defective respiration is linked to cell death, we tested for apoptosis next. Retinas from eyes rescued with WT allotopic ND4 had approximately 85% fewer apoptotic cells (mean [SE], 114 [15] cells/mm2) compared with mock-rescued eyes (744[105.6] cells/mm2; P < .001) (Supplement [eFigure 3B]). Thus, allotopic expression of WT ND4 provided long-term rescue against neurodegeneration when delivered by the scAAV vector.
Incorporation of Allotopic ND4 Into Respiratory Complexes
Next, we looked for incorporation of WT allotopic ND4 into respiratory complexes. Antibodies against NDUFA9 (hydroxyl-amine reductase dehydrogenase [ubiquinone] 1 alpha subcomplex 9), NDUFS3 (NDUF Fe-S protein 3), NDUFS4 (NDUF Fe-S protein 4), NDUFB8 (NDUF 1 beta subcomplex 8), or NDUFA6 (NDUF 1 alpha subcomplex 6) detected these complex I subunits in retinal mitochondrial extracts of uninfected eyes (Supplement [eFigure 4A]), whereas an anti-FLAG antibody revealed the absence of FLAG (Supplement [eFigure 4B]). Two-dimensional blue native polyacrylamide gel electrophoresis with the anti-FLAG antibody reacted against retinal mitochondrial extracts from rats 1 month after WT allotopic ND4 injection showed complex I subunits NDUFA9, NDUFS3, and NDUFB8 migrating below the approximately 1000-kDa complex I (Supplement [eFigure 4C]). The band for ND4FLAG migrated with the complex I subunits, indicating incorporation of human ND4 into the mouse complex I (Supplement [eFigure 4D]).Hydrophobic mitochondrial proteins, such as ND4, have been shown to migrate at masses ranging from 35 to 50 kDa, thus faster than its molecular mass of 52 kDa,20–22 as we also see herein. These findings further support earlier studies using immunoprecipitation of complex I subunits10,11 identified by mass spectroscopy that allotopic ND4 forms respiratory complexes.2
Safety in Nonhuman Primates
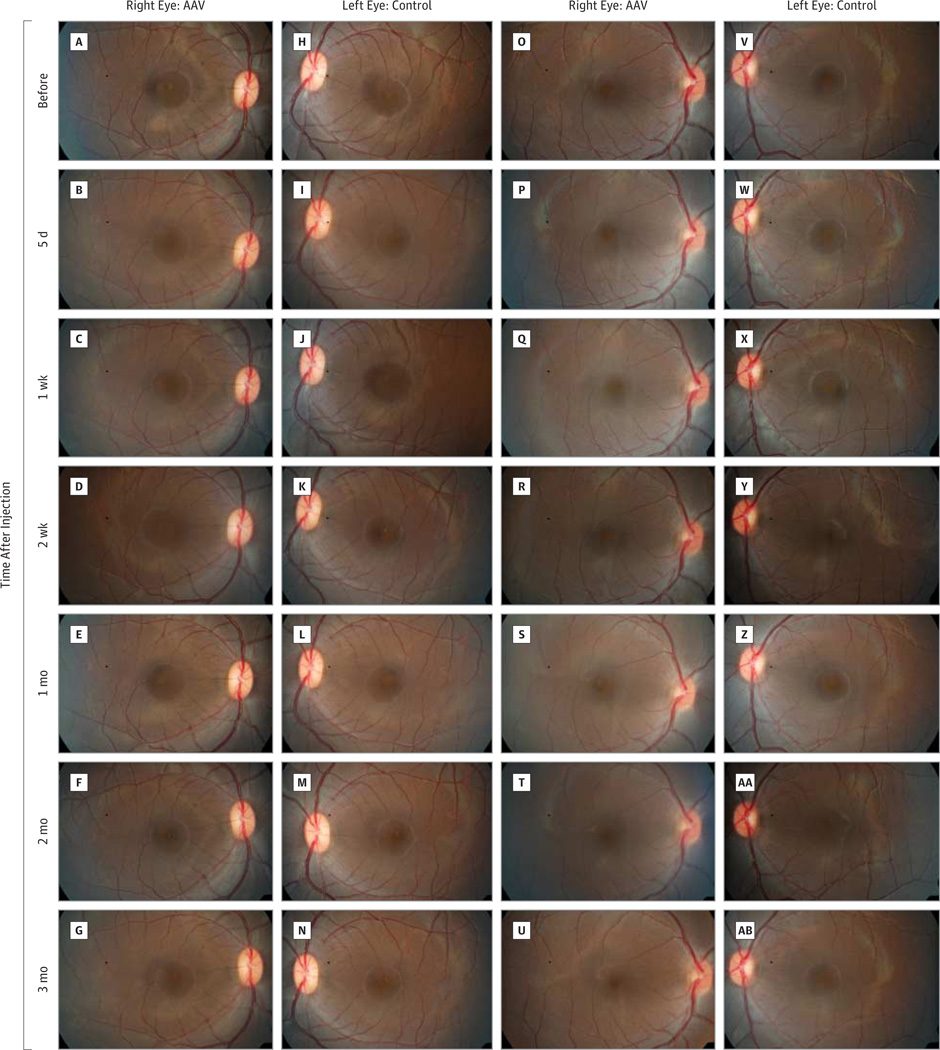
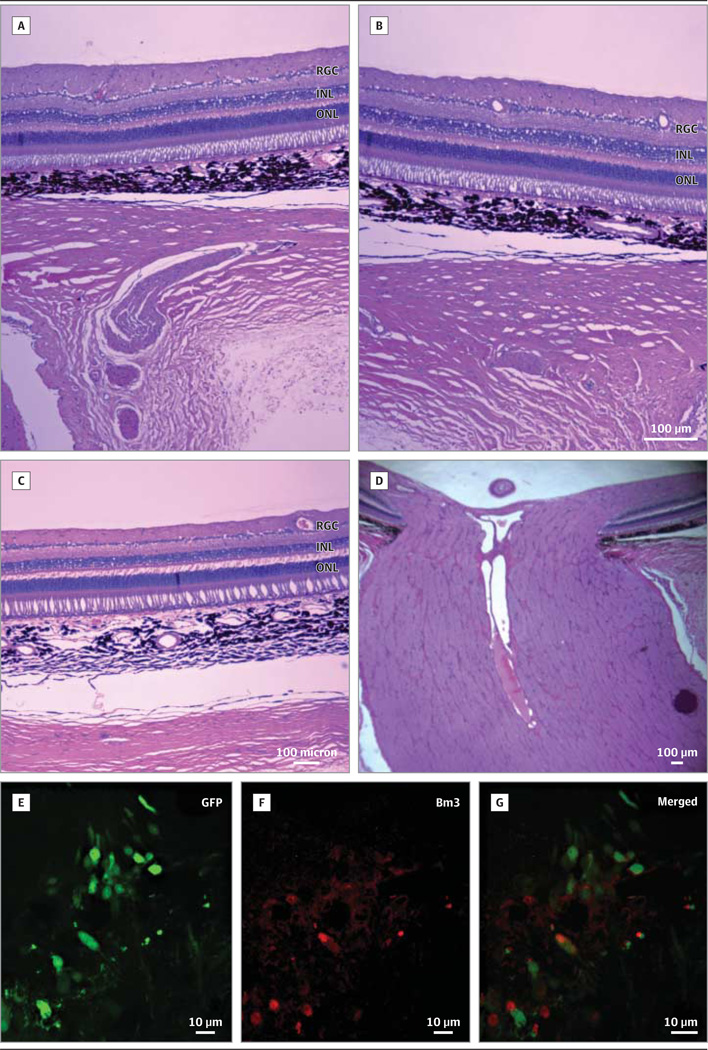
Next, we tested the safety of WT allotopic ND4 for human gene therapy using rhesus macaques. In 1 animal, fundus photographs obtained before injection (Figure 6A) and 5 days (Figure 6B), 1 week (Figure 6C), 2 weeks (Figure 6D), 1 month (Figure 6E), 2 months (Figure 6F), and 3 months (Figure 6G) after injection of the right eye remained normal and similar to the uninjected left eye photographed at the same intervals (Figure 6H–N). The scAAV-injected right eye of a second animal was normal at baseline (Figure 6O) and 5 days (Figure 6P), 1 week (Figure 6Q), and 2 weeks (Figure 6R) after injection, but developed a mild vitritis at 1 month after injection (Figure 6S) that cleared at 2 months (Figure 6T) to 3 months (Figure 6U) after injection. The uninjected left eye of this animal remained normal throughout the study (Figure 6V-AB). The third animal (not shown) had no significant adverse ocular reactions to AAV injections. The OCT measurements of macular thickness and volume showed no changes from baseline to 3 months after injection (Supplement [eFigure 5]), and multifocal electroretinography showed no loss of retinal function (Supplement [eFigure 6]) in any of the eyes. Ocular histologic studies confirmed the in-life evaluations showing that allotopic ND4 injected at a dose of 2.46 × 1010 vg had no adverse effects on the nonhuman primate eye (Figure 7A–D), nor did the scAAV2-GFP (triple Y-F capsid) that resulted in GFP expression in macular RGCs (Figure 7E–G).
Figure 6. Retinal Photographs of Rhesus Macaques.
Retinal fundus images of the right eye of a rhesus monkey before injection (A) of self-complementary adeno-associated viral vector–P1ND4v2 (AAV) and 5 days (B), 1 week (C), 2 weeks (D), 1 month (E), 2 months (F), and 3 months (G) after injection show that the macula and optic disc remained normal and similar to the uninjected left eye (H-N). The AAV-injected right eye of a second animal is normal at baseline (O), and at 5 days (P), 1 week (Q), and 2 weeks (R) after injection, but developed mild haze from vitritis at 1 month postinjection (S) that cleared at 2 months (T) to 3 months (U) after injection. The uninjected left eye of the same animal (control) was normal before injection of the opposite eye (V) and at all time points after injection (V through AB).
Figure 7. Ocular Histopathologic Findings in Rhesus Macaques.
Hematoxylin-eosin–stained sections of the right eye that developed transient vitritis shows no inflammation in the vitreous or retina 3 months after AAV injection (A–B). The retina of the uninjected left eye is shown (C). The injected right optic nerve shows no evidence of inflammation or swelling (D). Green fluorescent protein (GFP) expression in a primate injected with the triple Y-F capsid–modified self-complementary adeno-associated viral vector–GFP. The GFP (E) is seen in Bm 3a–labeled (F) macular retinal ganglion cells (G).
Discussion
An effective and safe delivery system is essential for human mitochondrial ND4 gene therapy. This delivery system can be achieved by using AAV vectors. Recombinant AAV vectors have been used safely in clinical trials for a number of ocular diseases such as Leber congenital amaurosis23–25 and nonocular diseases that include hemophilia B,26,27 cystic fibrosis,28 α1-antitrypsin deficiency,29 Parkinson disease,30 Batten disease,31 and muscular dystrophy.32 The RGC layer exclusively affected in LHON can be targeted by optimizing the vector serotype, AAV2, and by choosing the route of vector administration, intravitreal injection, as we did here.33,34 Self-complementary vectors that contain positive and negative strands35–37 and tyrosine-to-phenylalanine modifications in the capsid proteins increase the speed and efficiency of transgene expression, which is crucial to intervention in LHON with its characteristic apoplectic onset of permanent visual loss.38–42
To develop a clinically relevant vector for gene therapy for LHON, we first tested the standard ssAAV vector. Previous studies9,16 had demonstrated that this delivery system carrying the WT allotopic ND4 rescued defective ATP synthesis of LHON cells. In most cases of human LHON, the pathogenic G11778A mitochondrial DNA mutation is homoplasmic, that is, no WT ND4 is present except in 14% of cases in which the mutation exists in a heteroplasmic condition.43,44 In our mouse model generated by intravitreal injection of an allotopicR340H MT ND4 with ssAAV, expression of MT human ND4 initially induced swelling of the optic nerve head, followed by progressive demise of RGCs and their axons. Unlike the human condition, these hallmarks of human LHON occurred even in the presence of endogenous mouse ND4.10 As such, we found this dominant model system much more difficult to rescue than human LHON cells. A highly efficient triple Y444F-Y500F-Y730F–modified scAAV vector was needed to rescue visual loss and optic neuropathy caused by the allotopic R340H MT ND4 in the mouse model.
Conclusions
Previous studies11,12 have shown that expression of the WT allotopic ND4 produced no abnormalities in visual function or pathologic changes in the vitreous, retina, or optic nerve of rodents. Our allotopic WT human ND4 vector produced only mild transient inflammation in a single monkey that was also seen in another study.45 Taken with the absence of structural or functional abnormalities on OCT and multifocal electroretinograms in eyes that most closely resemble those of humans, these findings suggest that our good manufacturing practice gene therapy vector is safe for testing in a phase 1 clinical trial for G11778A LHON. Furthermore, expression of WT allotopic ND4 of the normal homologue of the defective R340H ND4 gene into the mitochondria of the mouse retina preserved vision, restored defective ATP synthesis, and prevented apoptosis of RGCs and demise of optic nerve axons for almost the entire lifespan of the laboratory mouse. Because the human vector to be used for the clinical trial could not contain an epitope tag for immunodetection, we tested expression of GFP delivered by the triple Y-F capsid–modified scAAV in 2 nonhuman primates, in which it expressed in RGCs of the macula, which are affected in patients with LHON. Our findings complement previous studies demonstrating that the tagged version of WT allotopic ND4 expresses in ex vivo primate eyes46 and enucleated human eyes, suggesting that it will also do so when injected into the eyes of patients with LHON.
A masked randomized clinical trial47 of idebenone failed to show efficacy in the primary outcome measure of improving vision in patients with LHON. The absence of other proven effective treatment modalities for mitochondrial diseases,48,49 expression of allotopic ND4 in the ex vivo human and nonhuman primate eyes, the safety of our test article in the rodent and primate visual systems, and the severe irreversible loss of vision and RGCs in human LHON43 suggest the time for translation of allotopic ND4 gene therapy in a phase 1 trial of LHON may be close at hand.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by grants R24EY018600, R01EY01714, and R01EY012355 from the National Eye Institute (Dr Guy); grants P30EY014801 (Dr Porciatti) and P51OD011092 (ONPRC core grant) from the National Institutes of Health; and unrestricted grants to Bascom Palmer Eye Institute and the University of Florida from Research to Prevent Blindness.
Role of the Sponsors: The funding sources had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Guy had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Hauswirth, Boye, Guy.
Acquisition of data: Koilkonda, Yu, Chou, Porciatti, Tse, Hauswirth, Chiodo, Neuringer, Renner.
Analysis and interpretation of data: Koilkonda, Chou, Feuer, Ruggeri, Porciatti, Hauswirth, Lewin, Neuringer, Renner, Guy.
Drafting of the manuscript: Koilkonda, Guy.
Critical revision of the manuscript for important intellectual content: Yu, Chou, Feuer, Ruggeri, Tse, Hauswirth, Chiodo, Boye, Lewin, Neuringer, Renner, Guy.
Statistical analysis: Koilkonda, Feuer.
Obtained funding: Guy.
Administrative, technical, or material support: Chou, Ruggeri, Porciatti, Tse, Hauswirth, Boye, Neuringer, Renner, Guy.
Study supervision: Chou, Neuringer, Guy.
Conflict of Interest Disclosures: Dr Hauswirth owns equity in AGTC, which makes AAV vectors. Dr Hauswirth and Mr Boye are listed as inventors on a patent that applies to the AAV technology used in this article. No other disclosures were reported.
Additional Contributions: Mabel Wilson edited the manuscript.
REFERENCES
- 1.Leber T. Über hereditare und congenital-angelegete Sehnerverleiden. Graefes Archiv Klin Experimentelle Opthalmol. 1871;7:249–271. [Google Scholar]
- 2.Koilkonda RD, Guy J. Leber’s hereditary optic neuropathy-gene therapy: from benchtop to bedside. J Ophthalmol. 2011;2011:179412. doi: 10.1155/2011/179412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu-Wai-Man P, Griffiths PG, Chinnery PF. Mitochondrial optic neuropathies: disease mechanisms and therapeutic strategies. Prog Retin Eye Res. 2011;30(2):81–114. doi: 10.1016/j.preteyeres.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gray RE, Law RH, Devenish RJ, Nagley P. Allotopic expression of mitochondrial ATP synthase genes in nucleus of Saccharomyces cerevisiae. Methods Enzymol. 1996;264:369–389. doi: 10.1016/s0076-6879(96)64035-x. [DOI] [PubMed] [Google Scholar]
- 5.Neupert W. Protein import into mitochondria. Annu Rev Biochem. 1997;66:863–917. doi: 10.1146/annurev.biochem.66.1.863. [DOI] [PubMed] [Google Scholar]
- 6.Manfredi G, Fu J, Ojaimi J, et al. Rescue of a deficiency in ATP synthesis by transfer of MTATP6, a mitochondrial DNA-encoded gene, to the nucleus. Nat Genet. 2002;30(4):394–399. doi: 10.1038/ng851. [DOI] [PubMed] [Google Scholar]
- 7.Glick B, Schatz G. Import of proteins into mitochondria. Annu Rev Genet. 1991;25:21–44. doi: 10.1146/annurev.ge.25.120191.000321. [DOI] [PubMed] [Google Scholar]
- 8.Nagley P, Farrell LB, Gearing DP, Nero D, Meltzer S, Devenish RJ. Assembly of functional proton-translocating ATPase complex in yeast mitochondria with cytoplasmically synthesized subunit 8, a polypeptide normally encoded within the organelle. Proc Natl Acad Sci U S A. 1988;85(7):2091–2095. doi: 10.1073/pnas.85.7.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guy J, Qi X, Pallotti F, et al. Rescue of a mitochondrial deficiency causing Leber hereditary optic neuropathy. Ann Neurol. 2002;52(5):534–542. doi: 10.1002/ana.10354. [DOI] [PubMed] [Google Scholar]
- 10.Qi X, Sun L, Lewin AS, Hauswirth WW, Guy J. The mutant human ND4 subunit of complex I induces optic neuropathy in the mouse. Invest Ophthalmol Vis Sci. 2007;48(1):1–10. doi: 10.1167/iovs.06-0789. [DOI] [PubMed] [Google Scholar]
- 11.Guy J, Qi X, Koilkonda RD, et al. Efficiency and safety of AAV-mediated gene delivery of the human ND4 complex I subunit in the mouse visual system. Invest Ophthalmol Vis Sci. 2009;50(9):4205–4214. doi: 10.1167/iovs.08-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koilkonda RD, Chou TH, Porciatti V, Hauswirth WW, Guy J. Induction of rapid and highly efficient expression of the human ND4 complex I subunit in the mouse visual system by self-complementary adeno-associated virus. Arch Ophthalmol. 2010;128(7):876–883. doi: 10.1001/archophthalmol.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hauswirth WW, Lewin AS, Zolotukhin S, Muzyczka N. Production and purification of recombinant adeno-associated virus. Methods Enzymol. 2000;316:743–761. doi: 10.1016/s0076-6879(00)16760-6. [DOI] [PubMed] [Google Scholar]
- 14.Porciatti V. The mouse pattern electroretinogram. Doc Ophthalmol. 2007;115(3):145–153. doi: 10.1007/s10633-007-9059-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruggeri M, Wehbe H, Jiao S, et al. In vivo three-dimensional high-resolution imaging of rodent retina with spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2007;48(4):1808–1814. doi: 10.1167/iovs.06-0815. [DOI] [PubMed] [Google Scholar]
- 16.Yu H, Koilkonda RD, Chou TH, et al. Gene delivery to mitochondria by targeting modified adenoassociated virus suppresses Leber’s hereditary optic neuropathy in a mouse model. Proc Natl Acad Sci U S A. 2012;109(20):1238–1247. doi: 10.1073/pnas.1119577109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calvaruso MA, Smeitink J, Nijtmans L. Electrophoresis techniques to investigate defects in oxidative phosphorylation. Methods. 2008;46(4):281–287. doi: 10.1016/j.ymeth.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 18.Bonnet C, Augustin S, Ellouze S, et al. The optimized allotopic expression of ND1 or ND4 genes restores respiratory chain complex I activity in fibroblasts harboring mutations in these genes. Biochim Biophys Acta. 2008;1783(10):1707–1717. doi: 10.1016/j.bbamcr.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Porciatti V, Saleh M, Nagaraju M. The pattern electroretinogram as a tool to monitor progressive retinal ganglion cell dysfunction in the DBA/2J mouse model of glaucoma. Invest Ophthalmol Vis Sci. 2007;48(2):745–751. doi: 10.1167/iovs.06-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rath A, Glibowicka M, Nadeau VG, Chen G, Deber CM. Detergent binding explains anomalous SDS-PAGE migration of membrane proteins. Proc Natl Acad Sci U S A. 2009;106(6):1760–1765. doi: 10.1073/pnas.0813167106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schilling B, Bharath MMS, Row RH, et al. Rapid purification and mass spectrometric characterization of mitochondrial NADH dehydrogenase (complex I) from rodent brain and a dopaminergic neuronal cell line. Mol Cell Proteomics. 2005;4(1):84–96. doi: 10.1074/mcp.M400143-MCP200. [DOI] [PubMed] [Google Scholar]
- 22.Sazanov LA, Peak-Chew SY, Fearnley IM, Walker JE. Resolution of the membrane domain of bovine complex I into subcomplexes: implications for the structural organization of the enzyme. Biochemistry. 2000;39(24):7229–7235. doi: 10.1021/bi000335t. [DOI] [PubMed] [Google Scholar]
- 23.Bainbridge JW, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358(21):2231–2239. doi: 10.1056/NEJMoa0802268. [DOI] [PubMed] [Google Scholar]
- 24.Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358(21):2240–2248. doi: 10.1056/NEJMoa0802315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hauswirth WW, Aleman TS, Kaushal S, et al. Treatment of Leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial. Hum Gene Ther. 2008;19(10):979–990. doi: 10.1089/hum.2008.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manno CS, Chew AJ, Hutchison S, et al. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101(8):2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- 27.Manno CS, Pierce GF, Arruda VR, et al. Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat Med. 2006;12(3):342–347. doi: 10.1038/nm1358. [DOI] [PubMed] [Google Scholar]
- 28.Flotte TR, Laube BL. Gene therapy in cystic fibrosis. Chest. 2001;120(3) suppl:124S–131S. doi: 10.1378/chest.120.3_suppl.124s. [DOI] [PubMed] [Google Scholar]
- 29.Flotte TR, Brantly ML, Spencer LT, et al. Phase I trial of intramuscular injection of a recombinant adeno-associated virus alpha 1-antitrypsin (rAAV2-CB-hAAT) gene vector to AAT-deficient adults. Hum Gene Ther. 2004;15(1):93–128. doi: 10.1089/10430340460732490. [DOI] [PubMed] [Google Scholar]
- 30.Kaplitt MG, Feigin A, Tang C, et al. Safety and tolerability of gene therapy with an adeno-associated virus (AAV) borne GAD gene for Parkinson’s disease: an open label, phase I trial. Lancet. 2007;369(9579):2097–2105. doi: 10.1016/S0140-6736(07)60982-9. [DOI] [PubMed] [Google Scholar]
- 31.Worgall S, Sondhi D, Hackett NR, et al. Treatment of late infantile neuronal ceroid lipofuscinosis by CNS administration of a serotype 2 adeno-associated virus expressing CLN2 cDNA. Hum Gene Ther. 2008;19(5):463–474. doi: 10.1089/hum.2008.022. [DOI] [PubMed] [Google Scholar]
- 32.Mandel RJ, Manfredsson FP, Foust KD, et al. Recombinant adeno-associated viral vectors as therapeutic agents to treat neurological disorders. Mol Ther. 2006;13(3):463–483. doi: 10.1016/j.ymthe.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Zaiss AK, Muruve DA. Immunity to adeno-associated virus vectors in animals and humans: a continued challenge. Gene Ther. 2008;15(11):808–816. doi: 10.1038/gt.2008.54. [DOI] [PubMed] [Google Scholar]
- 34.Peden CS, Burger C, Muzyczka N, Mandel RJ. Circulating anti-wild-type adeno-associated virus type 2 (AAV2) antibodies inhibit recombinant AAV2 (rAAV2)-mediated, but not rAAV5-mediated, gene transfer in the brain. J Virol. 2004;78(12):6344–6359. doi: 10.1128/JVI.78.12.6344-6359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCarty DM. Self-complementary AAV vectors; advances and applications. Mol Ther. 2008;16(10):1648–1656. doi: 10.1038/mt.2008.171. [DOI] [PubMed] [Google Scholar]
- 36.McCarty DM, Monahan PE, Samulski RJ. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001;8(16):1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]
- 37.McCarty DM, Fu H, Monahan PE, Toulson CE, Naik P, Samulski RJ. Adeno-associated virus terminal repeat (TR) mutant generates self-complementary vectors to overcome the rate-limiting step to transduction in vivo. Gene Ther. 2003;10(26):2112–2118. doi: 10.1038/sj.gt.3302134. [DOI] [PubMed] [Google Scholar]
- 38.Zhong L, Li B, Mah CS, et al. Next generation of adeno-associated virus 2 vectors: point mutations in tyrosines lead to high-efficiency transduction at lower doses. Proc Natl Acad Sci U S A. 2008;105(22):7827–7832. doi: 10.1073/pnas.0802866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mingozzi F, Maus MV, Hui DJ, et al. CD8+ T-cell responses to adeno-associated virus capsid in humans. Nat Med. 2007;13(4):419–422. doi: 10.1038/nm1549. [DOI] [PubMed] [Google Scholar]
- 40.Zhong L, Li B, Jayandharan G, et al. Tyrosine-phosphorylation of AAV2 vectors and its consequences on viral intracellular trafficking and transgene expression. Virology. 2008;381(2):194–202. doi: 10.1016/j.virol.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pien GC, Basner-Tschakarjan E, Hui DJ, et al. Capsid antigen presentation flags human hepatocytes for destruction after transduction by adeno-associated viral vectors. J Clin Invest. 2009;119(6):1688–1695. doi: 10.1172/JCI36891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Markusic DM, Herzog RW, Aslanidi GV, et al. High-efficiency transduction and correction of murine hemophilia B using AAV2 vectors devoid of multiple surface-exposed tyrosines. Mol Ther. 2010;18(12):2048–2056. doi: 10.1038/mt.2010.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lam BL, Feuer WJ, Abukhalil F, Porciatti V, Hauswirth WW, Guy J. Leber hereditary optic neuropathy gene therapy clinical trial recruitment: year 1. Arch Ophthalmol. 2010;128(9):1129–1135. doi: 10.1001/archophthalmol.2010.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith KH, Johns DR, Heher KL, Miller NR. Heteroplasmy in Leber’s hereditary optic neuropathy. Arch Ophthalmol. 1993;111(11):1486–1490. doi: 10.1001/archopht.1993.01090110052022. [DOI] [PubMed] [Google Scholar]
- 45.Maclachlan TK, Lukason M, Collins M, et al. Preclinical safety evaluation of AAV2-sFLT01: a gene therapy for age-related macular degeneration. Mol Ther. 2011;19(2):326–334. doi: 10.1038/mt.2010.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koilkonda RD, Hauswirth WW, Guy J. Efficient expression of self-complementary AAV in ganglion cells of the ex vivo primate retina. Mol Vis. 2009;15:2796–2802. [PMC free article] [PubMed] [Google Scholar]
- 47.Klopstock T, Yu-Wai-Man P, Dimitriadis K, et al. A randomized placebo-controlled trial of idebenone in Leber’s hereditary optic neuropathy. Brain. 2011;134(pt 9):2677–2686. doi: 10.1093/brain/awr170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sadun AA, Chicani CF, Ross-Cisneros FN, et al. Effect of EPI-743 on the clinical course of the mitochondrial disease Leber hereditary optic neuropathy. Arch Neurol. 2012;69(3):331–338. doi: 10.1001/archneurol.2011.2972. [DOI] [PubMed] [Google Scholar]
- 49.Pfeffer G, Majamaa K, Turnbull DM, Thorburn D, Chinnery PF. Treatment formitochondrial disorders. Cochrane Database Syst Rev. 2012;4:CD004426. doi: 10.1002/14651858.CD004426.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.