Abstract
Background
Babesia ovis and Theileria ovis are among the important and main etiological agents causing ovine babesiosis and ovine theileriosis, causing severe economic losses among sheep and goats. The aim of the present study was to determine the prevalence and molecular diagnosis of B. ovis and T. ovis in Lohi sheep at Livestock Experiment Station Bahadurnagar, Okara, Pakistan.
Methods
The prevalence of B. ovis and T. ovis was investigated in 200 Lohi sheep of mixed age and sex by PCR during 2011. The assay was employed using primers Bbo-F & Bbo-R, specific for a 549-bp fragment in B. ovis genomic DNA and primers TSsr 170F & TSsr 670R, specific for a 520-bp fragment in T. ovis genomic DNA. The animals were also screened for both haemoparasites through stained thin blood smears.
Results
Thirty two (16%), 48 (24%) and 26 (13%) were the number of animals found positive for B. ovis, T. ovis and for mixed infection with both parasites, respectively, through microscopy. Sixty eight (34%), 73 (37%) and 42 (21%) were the number of animals found positive for B. ovis, T. ovis and for mixed infection with both parasites, respectively, through PCR test.
Conclusion
The results indicate the high sensitivity of PCR for surveying babesiosis and theileriosis and there is noteworthy prevalence of these diseases in sheep at an experimental station where environmental conditions are relatively controlled as compared to field conditions.
Keywords: Babesia ovis, Theileria ovis, PCR, Sheep, Pakistan
Introduction
In Pakistan, livestock contributed approximately 55.1% of the agriculture value added and 11.6% to the national GDP during 2011-2012 (1). Livestock sector’s prospective role towards rural economic development can be gauged from the fact that 35–40 million rural populations are dependent on livestock, as every family has 2-3 cattle/buffaloes and 5-6 sheep/goats, thus leading to derive 30-40% of their income from these animals (2). Sheep and goats rearing carry tremendous importance in rural economy particularly for non-agricultural poor class of people (3). The population of sheep has been estimated as 28.4 million (M) in Pakistan and it yielded 0.037 M tons of milk for human consumption, 0.629 M tons of mutton and 0.043 M tons of wool during 2011-2012 (1). Punjab Province is the homeland for Lohi sheep and shares 24% of the total sheep population in the country (4).
Despite such a heavy population of livestock in Pakistan, the production by these animals is not as much as it could be. There are number of reasons for this discrepancy, e.g. poor breeding selection, management deficiencies and prevalence of blood born parasitic diseases (3). Pakistan, being a sub-tropical region of South Asia, has favorable environmental conditions for the growth of various ticks responsible for transmitting different haemoparasitic diseases in cattle and small ruminants. Small ruminant babesiosis and theileriosis develop in erythrocytes in peripheral blood and within the reticuloendothelial system.
Mostly three species of Babesia involved in causing the disease in ovine are; B. motassi, B. crassa and B. ovis, (5). The disease caused by B. motassi may be acute and/or chronic (6), whereas B. crassa appears to have little or no pathogenicity (7).
B. ovis is highly pathogenic especially in sheep and causes severe infection which is characterized by fever, anemia, icterus and hemoglobinuria with mortality rates ranging from 30 to 50% in susceptible host during field infections (8). It leads to significant losses among small ruminants due to its drastic effect on hemobiotic system (9). Babesia species are also capable of causing diseases in humans (10) and is a significant public health threat due to emerging blood borne zoonosis in developing countries like Pakistan. T. lestoquardi, T. seprata and T. ovis are mostly involved among different species of Theileria that cause theileriosis in sheep. T. lestoquardi causes malignant theileriosis in sheep and goats where as T. seprata causes low or nonpathogenic theileriosis (11). Theileria ovis is an important and main etiological agent of ovine theileriosis, causing severe economic losses among sheep and goats in tropical and subtropical areas including Pakistan (12-15). The disease is manifested by fever, progressive weight loss, decrease in production and ultimately may lead to death.
Some workers studied the prevalence of both protozoans in areas which were different from the area which was selected for present study. Durrani et al. (12) has found Theileria spp. in 35% sheep blood samples in district Lahore. Iqbal et al. (13) has detected B. ovis in 50% sheep blood samples by PCR from southern Punjab (Pakistan). Similarly Durrani et al. (16) has detected T. ovis in 6% blood samples of sheep and goats through PCR, in Punjab and Khyber Pukhtoon Khaw Provinces of Pakistan. However, before present study, little or no work was done in Pakistan on molecular diagnosis of Babesia and Theileria species especially in Lohi sheep. These types of sheep maintained at Livestock Experiment Station (LES), Bahadurnagar, Okara showed clinical signs of Babesiosis and Theileriosis such as persistent fever, loss of body weight, jaundice, anemia and sudden death in young animals during the summer season in 2011-(May to July).
Traditionally the haemoparasitic diseases were diagnosed on the basis of clinical signs and microscopy. Both methods have the limitation of their inability to detect the subclinical (e.g. trophozoite stage of parasites in blood, which is very difficult to detect microscopically) and carrier animals. The carrier animals need to be brought into limelight to know the exact status of haemoparasitic infestation in a herd so that disease control program can be implemented in a more rational way in order to enhance the productivity of the animals. The modern molecular tools of diagnosis have the ability to overwhelm the limitations of the conventional diagnostic techniques.
Keeping in view the advantage of PCR for identifying the exact species of Babesia and Theileria species and to know the prevalence of these haemoparasites, this study was initiated in Lohi sheep maintained at LES, Bahadurnagar, Okara. In Pakistan, the previous studies on haemoparasitic infestation in sheep were carried out in some other breeds of sheep. There was no such type of study conducted in Lohi sheep previously.
Materials and Methods
A total of 1360 Lohi sheep of different age groups and sex were maintained at LES, Bahadurnagar, Okara. During the summer season, from May to July 2011, clinical signs such as persistent high fever up to 105 °F, loss of body weight, jaundice, loss of appetite, anemia and sudden death were observed especially in young lambs (1-9 months old). During these four months, a total number of 200 blood samples were collected from the ear veins of 200 animals of different age groups and sex showing signs and symptoms of both haemoparasitic diseases. Thin smears were prepared, air dried, stained with Field’s stain (Hema-ColorTM, Merck), and examined under oil immersion on a microscope (Digi 3, Labomed, USA) for the presence of intracellular Babesia schizonts and/or Theileria piroplasms based on morphology (17). At least 50 microscopic areas per slide were searched carefully. Those blood smears were recorded as negative for Babesia spp. or Theileria spp. in which no schizonts or piroplasms were observed.
DNA isolation and polymerase chain reactions
A 5-ml blood sample was also collected from jugular vein of each of the above described 200 sheep and placed in 10 ml clean sterile vacutainers containing ethylenediaminetetraacetic acid. They were brought to the laboratory and stored at -20 °C until used for DNA extraction for PCR amplification. Whole blood was used for the extraction of total genomic DNA using a commercially available DNA isolation kit (PureGene®, Gentra, Minnesota, USA) according to the manufacturer’s instructions. The nucleic acid was extracted, also from known negative samples (40 healthy sheep maintained at a private Livestock farm at Okara). DNA concentration was determined spectrophotometrically at 260 ηm and 280 ηm and the number of genomic copies was determined by using Minevra Biolabs kit “VenorGem®” and the samples were stored at -20 °C till further use.
Three sets of primers (Table 1) were used for PCR amplification of different gene segments of both of the parasites. The first pair of oligo-nucleotide primer was used to amplify 549 base pair (bp) region of small sub unit rRNA (ssu rRNA) gene of B. ovis (18). The second primer pair specific for Theileria spp. was used for the amplification of small subunit ribosomal RNA (srRNA) gene fragment (1098 bp) from Theileria species genomic DNA as described by Allsopp et al. (19). The third primer pair, described by Altay et al. (20), was used to amplify SSU rRNA gene fragment of 520 bp length, which is specific for Theileria ovis genomic DNA. All PCR amplification reactions, including control negative samples, were carried out in a final volume of 25 μl containing 4 μl lysate or 2 ng purified DNA as DNA template, 12.5 μl commercially available PCR master mix (Pyro Start™ Fast PCR Master Mix- 2X, Fermentas, Ontario, Canada), 2 U Taq DNA polymerase (Thermoscientific, California, USA) and 2 mM MgCl2 (Thermo scientific, California, USA). The primers were used at a concentration of 10 pmol μl-1 for B. ovis and 80 pmol μl-1 for Theileria spp. and T. ovis.
Table 1.
The primer pair name, sequence, product, target gene and annealing temperature of Babesia ovis, Theileria spp. & Theileria ovis primers used in the present study
| PN | F.P. S. (5’-3’) | PN | R. P.S (5’-3’) | P (bp) | T.G | AT °C | Specificity | Reference |
|---|---|---|---|---|---|---|---|---|
| Bbo-F | TGGGCAGGACCTTGGTTCTTCT | Bbo-R | CCGCGTAGCGCCGGCTAAATA | 549 | ssu rRNA gene | 62 | B. ovis | (18) |
| 989 F | AGTTTCTGACCTATCAG | 990 R | TTGCCTTAAACTTCCTTG | 1098 | sr RNA gene | 60 | Theileria spp. | (19) |
| TSsr 170F | TCGAGACCTTCGGGT | TSsr 670R | TCCGGACATTGTAAAACAAA | 520 | SSU rRNA gene | 60 | T. ovis | (20) |
PN = Primer Name, FPS = Forward Primer Sequence, RPS = Reverse Primer Sequence, P = Product, TG = Target gene, AT = Annealing Temperature
PCR was carried out in a DNA thermal cycler (PEQLAB Biotechnologie, GmbH, Erlangen, Deutschland) with following cycling program for B. ovis: 94 °C for 3 min, followed by 35 cycles at 94 °C for 1 min, 62 °C for 1 min and 72 °C for 1 min, with a final extension step at 72 °C for 5 min The amplification was carried with 30 cycles for Theileria species specific primer set. After initial denaturation at 94 °C for 3 minutes., each cycle involved denaturation for 1 min at 94 °C, annealing for 1 min at 60 °C and extension for I min at 72 °C, with a final extension step at 72 °C for 5 min Cycling conditions for T. ovis were 96 °C for 3 min followed by 30 cycles at 94 °C for 30 s, 60 °C for 30 s and 72 °C for 2 min with a final extension step at 72 °C for 10 min. Ten microliters of the PCR products admixed with 6X DNA loading dye ™ (Fermentas, Ontario, Canada) were sized by electrophoresis on a 1% agarose gel (Gene choice ® Maryland, USA) (1 h at 90 V) with 1-kb ladder (Fermentas, Ontario, Canada) as size marker. The gels were stained with ethidium bromide (HP 47.1, ROTH, Karisruhe) (250 μg/ml @ one drop/100 ml of 1% agarose gel) and analyzed in a UV trans-illuminator (Dolphin -Doc, Wealtec, NV, USA) for visualization of PCR products.
Results
The stained blood films revealed schizonts as teardrop-shape in pairs in red blood cells which were identified as Babesia, while piroplasm appeared in oval and ring forms, detectable inside the red blood cells and these were identified as Theileria (5, 17). Out of the 200 blood smears examined, 32 (16%) samples were positive for Babesia, 48 (24%) for Theileria while 26 (13%) were positive for mixed infections with both parasites through microscopy. Data recorded for extracted DNA concentration by spectrophotometrie analysis showed optical density values of 1.83 and 0.83 at 260 ηm and 280 ηm wavelengths, respectively. Sixty eight (34%) out of 200 samples showed amplification of a 549-bp fragment (Fig 1a) with the primer pair Bbo-F and Bbo-R, specific for B. ovis. Similarly 1098-bp fragment (Fig. 1b) was generated with primer pair 989F and 990R specific for Theileria spp. in 78/200 (39%) samples while a 520-bp fragment (Fig. 1c) was amplified with primer pair TSsr170F and TSsr670R specific for T. ovis in 73/200 (37%) samples. Forty two (21%) samples showed mixed infection and were positive for both T. ovis and B. ovis. No such amplicon was detected in control negative samples. All samples positive with microscopic examination of blood smears, were also positive with PCR test. The performance of both tests in terms of detection of positive cases has been compared in Table 2. Statistically (Z- test statistic), there was significant difference between the performance of PCR technique and microscopy for the diagnosis of haemoparasites.
Fig. 1.
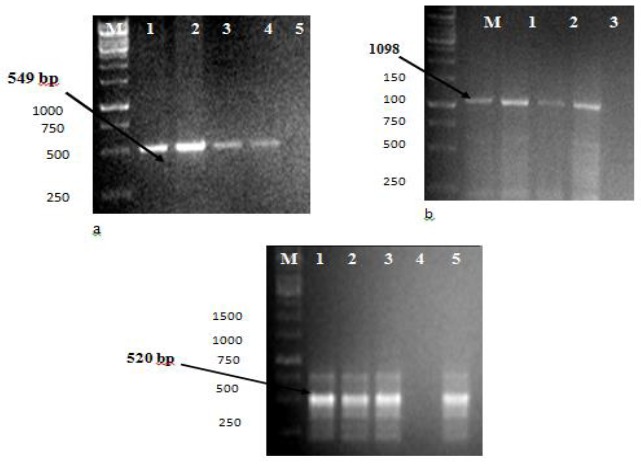
Confirmation of Babesia and Theileria species by PCR amplification from Lohi sheep (a) Confirmation of B. ovis from field samples by PCR by amplification of four field samples with primer pair BboF and BboR at 549- bp. Lane M: Molecular Ladder; Lanes 1, 2, 3, 4: test samples + ve for B. ovis; Lane 5: Negative control (b) Confirmation of Theileria spp. from field samples by PCR by amplification of four field samples with primer pair 989 F and 990 R at 1098- bp. Lane M: Molecular Ladder; Lanes 1, 2, 3, 4: test samples + ve for Theileria spp.; Lane 5: Negative control (c) Confirmation of T. ovis from field samples by PCR by amplification of four field samples with primer pair TSsr 170F and TSsr 670R at 520- bp. Lane M: Molecular Ladder; Lanes 1, 2, 3, 5: test samples + ve for T. ovis; Lane 4: Negative control
Table 2.
Comparison of prevalence of T. ovis & B. ovis in Lohi sheep by microscopic & PCR methods at LES, Bahadurnagar, Okara, Pakistan
| Technique | Total No. of samples | No. of sheep positive for B. ovis | No. of sheep positive for T. ovis | No. of sheep positive for T. ovis&B. ovis |
|---|---|---|---|---|
| Microscopic Examination | 200 | 32 | 48 | 26 |
| PCR | 200 | 68 | 73 | 42 |
Discussion
Several studies in Pakistan revealed that B. ovis and T. ovis are endemic in sheep and goats in different parts of the country (12, 13, 15, 16, 21, 22). There are very few case reports about sheep being infested with these parasites. This is the first molecular diagnostic report about the prevalence of B. ovis and T. ovis in Lohi sheep at LES, Bahadurnagar, Okara, Pakistan. Iqbal et al. (13) observed 50% prevalence of B. ovis in sheep in Southern Punjab through PCR which was higher as compared to the prevalence of B. ovis (34%) found during the present study. The difference in prevalence rate between the two studies may be attributed to the difference in geographical areas. Also the author of previous study has not mentioned the specific breed/s of sheep, which might be more susceptible to this disease as compared to Lohi breed. Durrani et al. (12) reported 27.5% (55/200) prevalence of T. ovis in sheep through PCR at district Lahore, which was less than the prevalence of the same protozoan (37%) in the present study. Although climatic conditions of the two study areas are almost the same, yet the higher prevalence of T. ovis in the present study cannot be compared with low prevalence reported in the previous study due to the facts that in present study the samples were collected in peak summer season which was ideal for tick infestation, as compared to the previous study in which the samples were collected in spring season, when the tick population was not as much as that was in summer season. Another reason for this discrepancy was the animal rearing type difference. In the present research, the samples were collected from intensive farming system at LES Bahadurnagar, Okara as compared to the collection of samples from individual animal keepings in the previous study. The prevalence for a mixed infection (21%) with T. ovis and B. ovis has also been observed during this study which is supported by a previous study conducted by Inci et al. (23) who observed 1.7% (7/421) prevalence of mixed infection for T. ovis & B. ovis in sheep in central Anatolia (Turkey).
The Lohi sheep which is an indigenous breed in Pakistan is recognized as relatively disease resistant breed as compared to contemporary sheep breeds in this country. During this study, it was noted that this type of sheep is susceptible to tick born diseases. The environment at LES is relatively controlled as compared to the field conditions and tickicidals are being used regularly especially in the summer season. The results of this study are indicative of the fact that even a very few ticks can cause the spread of Babesiosis and Theileriosis. Although the disease prevalence recording in different age groups was not the main focus of this study, but it was observed that the disease was more prevalent in animals less than one year of age as compared to the animals having more than one year of age. This finding is in accordance to the findings by Ramzi et al. (24) and Iqbal et al. (13) who were of the view that probably the animals develop immunity against these parasites with the age. The disease was diagnosed in small ruminants through symptoms and microscopy in earlier studies in Pakistan (15, 21, 22). Microscopic detection methods are still the cheapest and fastest methods used to identify Babesia and Theleria parasites, although these have limited sensitivity and specificity. These techniques can detect for up to one infected erythrocyte per ten thousand cells, requiring the analysis of 100-200 fields, the equivalent to 0.5 μl of blood. It is an inexpensive technique but requires an experienced person to differentiate species and is reliable only if the amount of parasites in the blood is high enough to be detected, which is usually only possible during acute cases (25). The observation of paired intra-erythrocytic merozoites is indicative of infection but there are other stages of the parasite like the trophozoites, which present different forms and sizes depending on the species, and these make their detection difficult and time consuming. An exact differentiation between the parasites is crucial to understand their epidemiology. Detection methods based on nucleic acid identification and their amplification are the most sensitive and reliable techniques available today; they are fast, very specific and although most of them relay on sophisticated equipment (25). Recently, molecular techniques have become the preferred methods for diagnosis of babesiosis and theileriosis, because these techniques are more sensitive and specific than other conventional methods (20, 26).
In this study a molecular technique was used to diagnose parasitic infestation in Lohi sheep. Microscopy of blood smears once again proved to be less sensitive as compared PCR. Through the latter technique the parasites were identified and confirmed up to genus level. Morphologically only a few blood smears were identified up to species level. During this study, all the samples found positive for haemo-parasites through microscopy, were also found positive for these protozoon through PCR, however the molecular technique detected some additional cases (of Theileriosis) that were found negative with microscopy. The specificity of PCR product of these samples was confirmed when compared to the amplicon size of those samples which were found positive with both of the techniques. Five samples positive for Theileria infestation detected through PCR were not typed with T. ovis specific primers. The probable reason behind this might be the genome sequence difference (due to involvement of some other strain/s or species of Theileria) that needed additional primer pairs specific for them. This hypothesis can be confirmed by gene sequencing of SSU rRNA gene which was beyond the scope of this study. The results of present study are in agreement with the findings of previous studies (12, 13, 16) who also compared microscopic examination test with PCR in small ruminants and found PCR to be a sensitive method for B. ovis and T. ovis diagnosis. Similar observations have also been made earlier (18, 20, 27-32), while comparing PCR with blood smear examination.
Conclusion
PCR for the detection of B. ovis and T. ovis is specific and sensitive. The tests is suitable for tracing carrier animals and provide a qualitative and validated measure that is useful in epidemiological surveys and follow ups for drug treatment in Lohi sheep. In addition, it would be useful while designing haemoparasitic (ovine babesiosis and ovine theileriosis) control program in endemic areas. The study also revealed that Lohi sheep is very much susceptible to babesiosis and theileriosis. The prevalence rate of haemoparasitic infestation in sheep at LES cannot be disregarded. Special attention should be paid for the control of tick infestation to the animals in order to enhance the livestock productivity.
Acknowledgments
The authors are thankful to Director, Livestock Production Research Institute, Bahadurnagar, Okara for providing necessary research facilities. Partial support for this research study was provided through the project of Government of Punjab titled “Up- gradation of research facilities at LPRI, Bahadurnagar, Okara”. The authors also gratefully acknowledge Mr Sohail Ahmad (Research Officer, Statistics) for his valuable contribution to statistical inferences.
Footnotes
The authors declare that there is no conflict of interest.
References
- 1.Anonymous. Economic Advisors Wing. Ministry of Finance. Government of Pakistan; Islamabad: 2012. Economic Survey of Pakistan. Finance Division; pp. 29–30. [Google Scholar]
- 2.Anonymous. Economic Advisors Wing. Ministry of Finance. Government of Pakistan; Islamabad: 2011. Economic Survey of Pakistan. Finance Division; pp. 28–29. [Google Scholar]
- 3.Shahzad W, Munir R, Rana MY, Ahmad R, Khan MS, Akbar G, Ijaz M, Mehmood F. Prevalence, molecular diagnosis and treatment of Mycoplasma conjunctivae isolated from infectious keratoconjunctivitis affected Lohi sheep maintained at Livestock Experiment Station, Bahadurnagar, Okara, Pakistan. Trop Ani Health Prod. 2013;45:737–742. doi: 10.1007/s11250-012-0282-2. [DOI] [PubMed] [Google Scholar]
- 4.Anonymous. Agricultural Census Organization. Government of Pakistan; 2006. Pakistan Livestock Census. Statistics Division; p. 22. [Google Scholar]
- 5.Soulsby EYL. Arthropods and Protozoa of domestic animals. Bailliere & Tindall; London: 1986. The Helminths; p. 809. [Google Scholar]
- 6.Morel P. Tick-Borne diseases of livestock in Africa. In: Fischer MS, Ralph S, editors. Manual of Tropical Veterinary Parasitology. CAB International; Wallingford: 1989. p. 473. [Google Scholar]
- 7.Friedhoff KT. Tick-borne diseases of sheep and goats caused by Babesia. Theileria or Anaplama sp Parasitologia. 1997;39:99–109. [PubMed] [Google Scholar]
- 8.Hashemi-Fesharki R. Tick-borne diseases of sheep and goats and their related vectors in Iran. Parasitologia. 1997;39:115–117. [PubMed] [Google Scholar]
- 9.Radostits OM, Gay CC, Hinchcliff KW, Constable PD. A textbook of the diseases of cattle, horses, sheep, pigs and goats. 10. WB Saunders Co. London; New York, Philadelphia: 2007. Veterinary Medicine; pp. 1482–1540. [Google Scholar]
- 10.Kim JY, Shin-Hyeong C, Hyun-Na J, Tsuji M, Sung-Ran C, Park J, Gyung-Tae C, Jung-Won J, Cheun H, Hyeong-Woo L, Young-Hee L, Tong-Soo K. First case of human Babesiosis in Korea: Detection and characterization of a novel type of Babesia sp. (KO1) similar to ovine Babesia. J Clini Microbiol. 2007;45:2084–2087. doi: 10.1128/JCM.01334-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sayin F, Serpil N, Bayram AY, Ayse C, Zafer K. Epidemiological studies on sheep and goat Theileria infection. Ankara Uni Vet Fakultesi Dergisi. 2009;56:127–129. [Google Scholar]
- 12.Durrani AZ, Younus M, Kamal N, Mehmood N, Shakoori AR. Prevalence of ovine Theileria species in district Lahore Pakistan. Pak J Zool. 2011;43:57–60. [Google Scholar]
- 13.Iqbal F, Ali M, Fatima M, Shahnawaz S, Zulifqar S, Fatima R, Shaikh RS, Shaikh AS, Aktas M, Ali M. A Study on prevalence and determination of the risk factors of infection with Babesia ovis in small ruminants from southern Punjab (Pakistan) by PCR amplification. Parasite. 2011;18:229–234. doi: 10.1051/parasite/2011183229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esmaeilnejad B, Tavassoli M, Asri-Rezaei S. Investigation of hematological and biochemical parameters in small ruminants naturally infected with Babesia ovis. Vet Res Forum. 2012;3:31–36. [PMC free article] [PubMed] [Google Scholar]
- 15.Naz S, Maqbool A, Ahmed S, Ashraf K, Ahmed N, Saeed K, Latif M, Iqbal J, Ali Z, Shafi K, Nagra IA. Prevalence of theileriosis in small ruminants Lahore -Pakistan. J Vet Ani Sci. 2012;2:16–20. [Google Scholar]
- 16.Durrani S, Khan Z, Khattak RM, Ali M, Hameed H, Taqddas A, Faryal M, Kiran S, Riaz M, Hussain S, Shiek RS, Ali M, Iqbal F. A comparison of the presence of Theileria ovis by PCR amplification of their ssu rRNA gene in small ruminants from two provinces of Pakistan. Asian Pacific J Trop Dise. 2012;2:43–47. [Google Scholar]
- 17.Zajac AM, Conboy GA. Veterinary Clinical Parasitology. 7. Blackwell Publishing Ltd; UK: 2000. pp. 172–175. [Google Scholar]
- 18.Aktas M, Altay K, Dumanli N. Development of a polymerase chain reaction method for diagnosis of Babesia ovis infection in sheep and goats. Vet Parasitol. 2005;133(277):281. doi: 10.1016/j.vetpar.2005.05.057. [DOI] [PubMed] [Google Scholar]
- 19.Allsopp BA, Baylis HA, Allsopp MT, Cavalier-Smith T, Bishop RP, Carrington DM, Sohanpal B, Spooner P. Discrimination between six species of Theileria using oligonucleotide probes which detect small subunit ribosomal RNA sequences. Parasitology. 1993;107:157–165. doi: 10.1017/s0031182000067263. [DOI] [PubMed] [Google Scholar]
- 20.Altay K, Dumanli N, Holman PJ, Aktas M. Detection of Theileria ovis in naturally infected sheep by nested PCR. Vet Parasitol. 2005;127:99–104. doi: 10.1016/j.vetpar.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 21.Shahabuddin, Nawaz Y, Nawaz J, Nawaz M. Epidemiological study on the occurrence of natural babesiosis in sheep and goats of Balochistan, Pakistan. Proceedings of Parasitol. 2006;42:47–60. [Google Scholar]
- 22.Rashid A, Khan JA, Khan MS, Rasheed K, Maqbool A, Iqbal J. Prevalence and chemotherapy of babesiosis among Lohi sheep in the Livestock Experiment Station Qadirabad, Pakistan and environs. J Venomous Ani and Tox including Trop Dise. 2010;16:587–591. [Google Scholar]
- 23.Inci A, Ica A, Yildirim A, Duzlu O. Identification of Babesia and Theileria species in small ruminants in Central Anatolia (Turkey) via reverse line blotting. Turkish J Vet Ani Sci. 2010;34:205–210. [Google Scholar]
- 24.Razmi GR, Naghibi A, Aslani MR, Dastjerdi K, Hossieni H. An epidemiological study on Babesia infection in small ruminants in Mashhad suburb Khorasan province, Iran. Small Rumin Res. 2003;50:39–44. [Google Scholar]
- 25.Mosqueda J, Olvera-Ramirez A, Aguilar-Tipacamu G, Canto GL. Current advances in detection and treatment of Babesiosis. Current Medi Chem. 2012;19:1504–1518. doi: 10.2174/092986712799828355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagore D, García-Sanmartín J, García-Pírez AL, Juste RA, Hurtado Identification, genetic diversity and prevalence of Theileria and Babesia species in a sheep population from Nortern Spain. Int J Parasitol. 2004;34:1059–1067. doi: 10.1016/j.ijpara.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Altay K, Dumanli N, Aktas M. Molecular identification, genetic diversity and distribution of Theileria and Babesia species infecting small ruminants. Vet Parasitol. 2007;147:161–165. doi: 10.1016/j.vetpar.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Altay K, Aktas M, Dumanli N. Detection of Babesia ovis by PCR in Rhipicephalus bursa collected from naturally infested sheep and goats. Res Vet Sci. 2008;85:116–119. doi: 10.1016/j.rvsc.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Shayan P, Rahbari S. Differentiation of sheep Theileria sp and Babesia sp by polymerase chain reaction. J Vet Res. 2007;62:15–20. [Google Scholar]
- 30.Shayan P, Hooshmand E, Nabian S, Rahbari S. Biometrical and genetically characterization of large Babesia ovis in Iran. J Parasitol Res. 2008;103:217–221. doi: 10.1007/s00436-008-0960-1. [DOI] [PubMed] [Google Scholar]
- 31.Bami MH, Khazraiinia P, Haddadzadeh HR, Kazemi B. Identification of theileria species in sheep in the eastern half of Iran using nested PCR-RFLP and microscopic techniques. Iranian J Vet Res. 2010;11:262–266. [Google Scholar]
- 32.Dehkordi ZS, Zakeri S, Nabian S, Bahonar A, Ghasem,i F, Noorollahi F, Rahbari S. Molecular and bio-morphometrical identification of ovine babesiosis in Iran. Iranian J Parasitol. 2010;4:21–30. [PMC free article] [PubMed] [Google Scholar]


