Abstract
Background
Toxocara is a common nematode of cats in different parts of Iran. Despite the close association of cats with human, no attempt has been done so far for molecular identification of this nematode in the country. Therefore, current study was performed on identification of some isolates of Toxocara from stray cats in Shiraz, Fars Province, Southern Iran, based on morphological and molecular approaches, and also determination of intensity of infection.
Methods
This cross-sectional study was carried out on 30 stray cats trapped from different geographical areas of Shiraz in 2011. Adult male and female worms were recovered from digestive tract after dissection of cats. Morphological features using existing keys and PCR-sequencing of ITS-rDNA region and pcox1 mitochondrial l gene were applied for the delineating the species of the parasites.
Results
Eight out of 30 cats (26.7%) were found infected with Toxocara nematodes. All the isolates were confirmed as Toxocara cati based on morphological features and the sequence of ribosomal and mitochondrial targets. Intensity of infection ranged from one to a maximum of 39 worms per cat, with a mean of 10.25±12.36, and higher abundance of female nematodes.
Conclusion
The most prevalent ascaridoid nematode of stray cats in the study area was T. cati and female nematodes were more abundant than that of males. This issue has important role in spreading of eggs in the environment and impact on human toxocariasis.
Keywords: Intensity, Toxocara cati, Cat, Molecular, Iran
Introduction
Toxocara species are common ascaridoid nematodes of cats and dogs throughout the world. They are causative agents of toxocariasis, a zoonotic parasitic disease in human with worldwide distribution. The most widespread species of Toxocara in dogs and cats are Toxocara canis and Toxocara cati, respectively (1). Humans are infected by the ingestion of Toxocara eggs from contaminated soil, unwashed hands or raw vegetables. The larvae emerge in the intestine and migrate to muscle and neurological tissues, where they can remain for many years without growth, differentiation or reproduction (2). Some peoples may be infected by eating the larvae present in undercooked meat of infected paratenic hosts such as chickens, sheep and cattle or earthworm (3-6). The clinical symptoms of toxocariasis depend on where in the body infected. There are several forms of toxocariasis, namely visceral larva migrans, ocular larva migrans, covert toxocariasis, and neurotoxocariasis (7-10).
Recent epidemiological studies demonstrated the widespread prevalence of human infection with Toxocara in the world (7, 11-16). Toxocara in cats has important role on human health. Prevalence of T. cati in cats has been estimated to vary from 0.8 to 59.3% in different parts of the world (17-26). In Iran, cats live freely in urban and rural areas, discharging Toxocara eggs in the environment which are transmittable to human. There are some reports of contamination of the soil in public areas with Toxocara eggs in Iran (27-30). The prevalence of T. cati in cats ranges from 8% to 52.8% in different parts of Iran (31-38). Shiraz, Fars Province, Southern Iran is a city with high prevalence of Toxocara infection in cats (31, 32), as well as seroepidemiological evidence of high prevalence of toxocariasis in school children (39). Nevertheless, no attempt has been done so far for molecular identification of this nematode from cats in this city or any other endemic areas in the country.
Therefore, current study was performed on identification of some isolates of Toxocara from stray cats in Shiraz, based on morphological and molecular approaches, and also determination of intensity of infection.
Materials and Methods
Sample collection
This cross-sectional study was carried out in Shiraz, capital city of Fars Province, situated in southern Iran. Cats were captured from different geographic areas of the city, from February to November 2011. Pregnant females were excluded from this study. Overall 30 cats were anaesthetized by intra muscular injection of high doses of anesthetic drug (Ketamine, 15-40 mg/kg) and then were humanely euthanatized by chloroform. Their digestive systems were removed and the intestinal contents were examined for the presence of Toxocara nematodes. Recovered helminthes were washed extensively in physiological saline and kept in ethanol alcohol 70% for further examinations. This research project was reviewed and approved by the Ethics Committee of Tehran University of Medical Sciences, Iran.
Morphological studies
The mature male and female worms were identified according to the morphological features using existing keys and descriptions (40,41). The female worms were distinguished by their eggs, that are brown and pitted, and males by their posterior end which is curved with paired spicules, showing a prominent point at the tail-end.
Molecular studies
Total genomic DNA was extracted using Qiamp DNA Mini Kit (Qiagen, Hilden, Germany) according to manufacturer’s instructions. The mitochondrial and ribosomal DNA regions were subjected to PCR amplification by forward (JB3:5′-TTT TTT GGG CAT CCT GAG GTT TAT-3′) and reverse (JB4.5:5′-TAA AGA AAG AAC ATA ATG AAA ATG-3′) primers for amplification of mitochondrial gene (pcox1) (42); and forward (NC5:5′- GTA GGT GAA CCT GCG GAA GGA TCA TT-3′) and reverse (NC2:5′- TTA GTT TCT TTT CCT CCG CT-3′) primers for amplification of internal transcribed spacer (ITS) region in ribosomal DNA (43). The PCR products were electrophoresed on agarose gel and the DNA bands were visualized using a UV transilluminator and photographed. All PCR products of pcox1 gene and four PCR products of ITS fragment were purified with PCR purification Kit (BIONEER, Korea) according to manufacturer’s instructions. Purified products were submitted to sequencing in two directions using the same forward and reverse primers employed in the PCR. Nucleotide sequences were compared with GenBank sequences using the BLAST system (http://www.ncbi.nlm.nih.gov/).
Statistical analysis
Statistical analyses were performed using SPSS 20.0 (Statistical Package for Social Science). All statistical tests were expressed as significant at 95% confidence interval.
Results
The collected Toxocara worms were identified in the laboratory based on morphological features. All samples had very broad cephalic alae (Fig.1, A). The male nematodes had a curved posterior end with paired spicules, showing a prominent point at the tail-end which was distinguishable from the straight-tailed female nematodes (Fig. 1, B and C). In the female worms, the egg shell of Toxocara was pitted (Fig.1, D).
Fig 1.
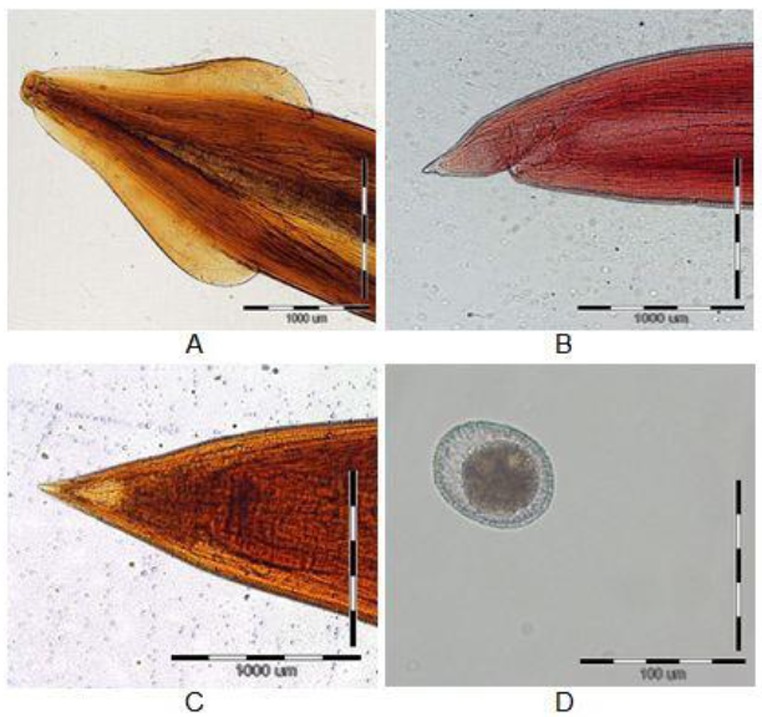
The cephalic alae of Toxocara cati (A), the tail-end of Toxocara cati male (B), the tail-end of Toxocara cati female (C), Egg of Toxocara cati (D) A, B, C: Bars indicate 1000 μm. / D: Bars indicate 100 μm.
Morphologically, the genus of all these Toxocara isolates was identified as T. cati. Then, the isolates were characterized by amplification of the pcox1 gene and ITS fragment. For all of them, amplicons of about 450 bp for the pcox1 gene and 1100 bp for ITS fragment were successfully produced by PCR (Fig. 2).The sequences were achieved and compared with other available sequences in GenBank. By using the BLAST system either, all above mentioned isolates were identified as T. cati. The ITS sequences of four isolates obtained have been deposited in GenBank database (Accession numbers: JX53-6258-JX53-6261).
Fig 2.
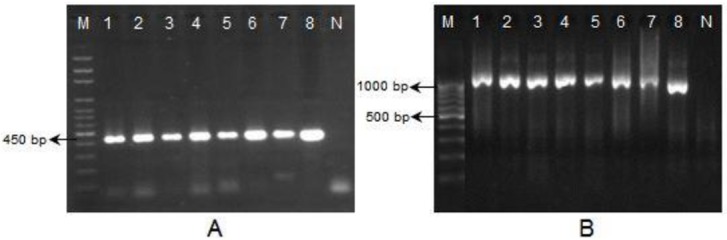
Agarose gel electrophoresis of pcox1-PCR (A) and ITS-PCR (B) products from Toxocara isolates, M: 100 bp DNA Marker, N: negative control
Overall, 30 cats were examined in this study. As the relationships of age or sex of the animals and their infectivity with Toxocara was not the aim of this study, pregnant females were not enrolled in the study; therefore, 22 cats were male and the rest females. In general, 8 out of 30 stray cats (26.7%) were found infected with T. cati (Table 1). The intensity of infection ranged from one to a maximum of 39 worms per cat, with a mean of 10.25±12.36 (Table 2). The mean intensity of infection with female and male nematodes was 7.125 and 3.125, respectively. No infectivity with Toxascaris leonina was found in these cats at all.
Table 1.
Infection rate of Toxocara cati in stray cats in Shiraz, Iran
| Number | Percent | 95% Confidence Interval | ||
|---|---|---|---|---|
| Lower | Upper | |||
| Infected cats | 8 | 26.7 | 12.3 | 45.9 |
| Non-infected cats | 22 | 73.3 | 54.1 | 87.8 |
| Total | 30 | 100 | ||
Table 2.
Intensity of Toxocara cati infection in stray cats in Shiraz, Iran
| Statistic | 95% Confidence Interval | |||
|---|---|---|---|---|
| Lower | Upper | |||
| Numbers of infected cats | 8 | |||
| Numbers of Toxocara in each cat | Minimum | 1 | ||
| Maximum | 39 | |||
| Mean | 10.25 | 4 | 19.87 | |
| Std. Deviation | 12.36 | |||
Discussion
In association with the infection rate of T. cati in stray cats collected from different parts of Shiraz City, this study showed that 8 out of 30 cats (26.7%) were infected. Sadjjadi et al. reported the prevalence of T. cati on 108 stray cats in Shiraz 52.8% (31). Another study showed that the infection rate of T. cati on 114 stray cats in Shiraz was 42.6% (32). In other studies from North (33, 34), Northwest (36) and Northeast of Iran (37), the prevalence of infection with T. cati was 8-44%, 8% and 28.8%, respectively. Similar studies have been done on prevalence of T. cati in central parts of Iran; for example in Kashan, 113 stray cats showed a prevalence of 13.3% (35). The prevalence of T. cati in Tehran was 9.4% (38). Although, investigation on the relationship between the prevalence of Toxocara and age or sex of the cats was not the aim of this study, however, previous studies showed that there was no significant difference in the prevalence of infection between male and female cats (31, 33, 34, 36, 37); and cats with less than 6 months old being more likely to be infected with T. cati than older cats (33, 37). Sadjjadi et al. reported the prevalence of infection was higher in younger cats compared to older animals; however, the difference was not significant (31).
In this study, the intensity of infection ranged from one to a maximum of 39 worms per cat, with a mean of 10.25. In a report from Shiraz, the mean intensity of infection with T. cati was 6.52 with a range of 1 to 50 worms per cat (31). Sharif et al. indicated that the intensity of infection ranged from 1 to 32 worms per cat, with a mean of 7.3 (33). In other study in stray cats from north of Iran, the mean intensity of infection with T. cati in cats was an average of 3 T. cati in each cat (34). Considering the sex of the T. cati recovered from the cats in the current study, female nematodes were more abundant than male ones with a mean of 7.125 and 3.125 nematodes per cat, respectively. This issue is important respect to the distribution of T. cati eggs in environment, because every female Toxocara shed about 200000 eggs per day (44) that will become capable of transmission to human and paratenic hosts after development in the soil.
Since the morphological identification of some ascaridoid species or their larval and egg stages is difficult; therefore some molecular methods using ribosomal and mitochondrial markers have been developed and used for accurate identification and diagnosis of such nematodes. As with the ascaridoid nematode of cats from Kuala Lumpur, Malaysia which based on morphology identified as T. canis (45), and characterized by the ribosomal DNA (rDNA) sequences as Toxocara sp. cf canis (46). Detailed morphological study of this nematode was done by Gibbons et al. and the parasite was described and named Toxocara malaysiensis (47). The first report of T. malaysiensis in cats outside Malaysia reported from China. The nematodes collected from cats in China were morphologically and genetically consistent with T. malaysiensis (48). These studies showed that this new species has a broader geographical distribution. According to these reports, molecular methods using ribosomal and mitochondrial sequences as genetic markers have been shown to provide reliable alternatives to more traditional methods for the specific identification of nematodes. In this study all ascaridoid nematodes isolated from cats based on morphological features identified as T. cati; and for confirmation using molecular approach, PCR amplification of the mitochondrial and ribosomal DNA and sequence analysis of the amplicons were applied on the same samples. Accordingly, all of the isolates were characterized by amplification of the mitochondrial (pcox1) and ribosomal (ITS) genes as T. cati.
The result of this study is coincident with the previous studies in Shiraz (31, 32) indicating that the infection of cats with Toxocara nematodes in this city is considerable. The high infection rate of T. cati, high intensity of infection and twice more abundance of female T. cati in cats in the present study emphasize that stray cats have important role in distribution of Toxocara eggs into the environment and their transmission to humans. Current diagnostic techniques cannot discriminate which species of Toxocara is responsible as causative agent of toxocariasis in human; therefore identification of parasite species in animals using molecular methods can be foundation for planning of prevention and control programs in human and animal communities.
Conclusion
The result of this study implies that T. cati, as the most prevalent acaridoid nematode of cats in the study area, might have the most important role in human toxocariasis in that area, but further studies on human cases will better clarify this issue. Similar studies on isolates from canid hosts and also from other geographical areas of Iran in recommended.
Acknowledgments
The authors would like to thank Dr Tanideh and Mr O. Kohi from animal lab of Shiraz University of Medical Sciences, and Mr M. Sharifdini, Mr A. Rahimi and Mrs. N. Jalaizand from Tehran University of Medical Sciences for their kind helps. This study has been supported by the Tehran University of Medical Sciences; Project No. 90-02-27-14163.
Footnotes
The authors declare that there is no conflict of interests.
References
- 1.Despommier D. Toxocariasis: Clinical Aspects. Epidemiology, Medical Ecology, and Molecular Aspects. Clin Microbiol Rev. 2003;16:265–272. doi: 10.1128/CMR.16.2.265-272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holland CV, Smith HV. Toxocara: The Enigmatic Parasite. CAB International. 2006 [Google Scholar]
- 3.Ito K, Sakaei K, Okajima T, Ouchi K, Funakoshi A, Nishimura J, Ibayashi H, Tsuji M. Three cases of visceral larva migrans due to ingestion of raw chicken or cow liver. J Jpn Soc Intern Med. 1986;75:759–766. doi: 10.2169/naika.75.759. [DOI] [PubMed] [Google Scholar]
- 4.Taira N, Fujita J. Morphological observation of Toxocara vitulorum found in Japanese calves. J Vet Med Sci. 1991;53(3):409–413. doi: 10.1292/jvms.53.409. [DOI] [PubMed] [Google Scholar]
- 5.Cianferoni A, Schneider L, Schantz PM, Brown D, Fox LM. Visceral larva migrans associated with earthworm ingestion: clinical evolution in an adolescent patient. Pediatrics. 2006;117(2):e336–339. doi: 10.1542/peds.2005-1596. [DOI] [PubMed] [Google Scholar]
- 6.Choi D, Lim JH, Choi DC, Lee KS, Paik SW, Kim SH, Choi YH, Huh S. Transmission of Toxocara canis via ingestion of raw cow liver: a cross-sectional study in healthy adults. Korean J Parasitol. 2012;50(1):23–27. doi: 10.3347/kjp.2012.50.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magnaval JF, Glickman LT, Dorchies P, Morassin B. Highlights of human toxocariasis. Korean J Parasitol. 2001;39:1–11. doi: 10.3347/kjp.2001.39.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher M. Toxocara cati: an underestimated zoonotic agent. Trends Parasitol. 2003;19(4):167–170. doi: 10.1016/s1471-4922(03)00027-8. [DOI] [PubMed] [Google Scholar]
- 9.Smith H, Holland C, Taylor M, Magnaval JF, Schantz P, Maizels R. How common is human toxocariasis? Towards standardizing our knowledge. Trends Parasitol. 2009;25(4):182–188. doi: 10.1016/j.pt.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 10.Rubinsky-Elefant G, Hirata CE, Yamamoto JH, Ferreira MU. Human toxocariasis: diagnosis, worldwide seroprevalences and clinical expression of the systemic and ocular forms. Ann Trop Med Parasitol. 2010;104(1):3–23. doi: 10.1179/136485910X12607012373957. [DOI] [PubMed] [Google Scholar]
- 11.Auer H, Aspöck H. Nosology and epidemiology of human toxocarosis-the recent situation in Austria. Wien Klin Wochenschr. 2004;116(4):7–18. [PubMed] [Google Scholar]
- 12.De Andrade Lima Coelho R, De Carvalho LB, Jr, Perez EP, Araki K, Takeuchi T, Ito A, Aoki T, Yamasaki H. Prevalence of toxocariasis in northeastern Brazil based on serology using recombinant Toxocara canis antigen. Am J Trop Med Hyg. 2005;72(1):103–107. [PubMed] [Google Scholar]
- 13.Cojocariu IE, Bahnea R, Luca C, Leca D, Luca M. Adult toxocariasis. Rev Med Chir Soc Med Nat Iasi. 2012;116(2):432–435. [PubMed] [Google Scholar]
- 14.Mendonca LR, Veiga RV, Dattoli VC, Figueiredo CA, Fiaccone R, Santos J, Cruz AA, Rodrigues LC, Cooper PJ, Pontes-de-Carvalho LC, Barreto ML, Alcantara-Neves NM. Toxocara seropositivity, atopy and wheezing in children living in poor neighbourhoods in urban latin american. PLoS Negl Trop Dis. 2012;6(11):e1886. doi: 10.1371/journal.pntd.0001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou M, Chang Q, Gonzales JA, Chen Q, Zhang Y, Huang X, Xu G, Wang W, Jiang R. Clinical characteristics of ocular toxocariasis in Eastern China. Graefes Arch Clin Exp Ophthalmol. 2012;250(9):1373–1378. doi: 10.1007/s00417-012-1971-2. [DOI] [PubMed] [Google Scholar]
- 16.Caldera F, Burlone ME, Genchi C, Pirisi M, Bartoli E. Toxocara encephalitis presenting with autonomous nervous system involvement. Infection. 2012 doi: 10.1007/s15010-012-0342-6. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto N, Kon M, Saito T, Maeno N, Koyama M, Sunaoshi K, Yamaguchi M, Morishima Y, Kawanaka M. Prevalence of intestinal canine and feline parasites in Saitama Prefecture. Japan. Kansenshogaku Zasshi. 2009;83(3):223–228. doi: 10.11150/kansenshogakuzasshi.83.223. [DOI] [PubMed] [Google Scholar]
- 18.Becker AC, Rohen M, Epe C, Schnieder T. Prevalence of endoparasites in stray and fostered dogs and cats in Northern Germany. Parasitol Res. 2012;111(2):849–857. doi: 10.1007/s00436-012-2909-7. [DOI] [PubMed] [Google Scholar]
- 19.Millán J, Casanova JC. High prevalence of helminth parasites in feral cats in Majorca Island (Spain) Parasitol Res. 2009;106(1):183–188. doi: 10.1007/s00436-009-1647-y. [DOI] [PubMed] [Google Scholar]
- 20.Abu-Madi MA, Behnke JM, Prabhaker KS, Al-Ibrahim R, Lewis JW. Intestinal helminths of feral cat populations from urban and suburban districts of Qatar. Vet Parasitol. 2010;168(3-4):284–292. doi: 10.1016/j.vetpar.2009.11.027. [DOI] [PubMed] [Google Scholar]
- 21.Mircean V, Titilincu A, Vasile C. Prevalence of endoparasites in household cat (Felis catus) populations from Transylvania (Romania) and association with risk factors. Vet Parasitol. 2010;171(1-2):163–166. doi: 10.1016/j.vetpar.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 22.Khalafalla RE. A survey study on gastrointestinal parasites of stray cats in northern region of Nile delta. Egypt. Plos One. 2011;6(7):e20283. doi: 10.1371/journal.pone.0020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borthakur SK, Mukharjee SN. Gastrointestinal helminthes in stray cats (Felis catus) from Aizawl. Mizoram, India. Southeast Asian J Trop Med Public Health. 2011;42(2):255–258. [PubMed] [Google Scholar]
- 24.Barutzki D, Schaper R. Results of parasitological examinations of faecal samples from cats and dogs in Germany between 2003 and 2010. Parasitol Res. 2011;109(1):S45–60. doi: 10.1007/s00436-011-2402-8. [DOI] [PubMed] [Google Scholar]
- 25.Lucio-Forster A, Bowman DD. Prevalence of fecal-borne parasites detected by centrifugal flotation in feline samples from two shelters in upstate New York. J Feline Med Surg. 2011;13(4):300–303. doi: 10.1016/j.jfms.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nareaho A, Puomio J, Saarinen K, Jokelainen P, Juselius T, Sukura A. Feline intestinal parasites in Finland: prevalence, risk factors and anthelmintic treatment practices. J Feline Med Surg. 2012;14(6):378–383. doi: 10.1177/1098612X12439257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Motazedian H, Mehrabani D, Tabatabaee SH, Pakniat A, Tavalali M. Prevalence of helminth ova in soil samples from public places in Shiraz. East Mediterr Health J. 2006;12(5):562–565. [PubMed] [Google Scholar]
- 28.Zibaei M, Sadjjadi SM. Prevalence of helminth ova in soil samples from public places in Shiraz. East Mediterr Health J. 2010;16(5):580. [PubMed] [Google Scholar]
- 29.Zibaei M, Abdollahpour F, Birjandi M, Firoozeh F. Soil contamination with Toxocara spp. eggs in the public parks from three areas of Khorram Abad. Iran. Nepal Med Coll J. 2010;12(2):63–65. [PubMed] [Google Scholar]
- 30.Khazan H, Khazaei M, Tabaee SS, Mehrabi A. Prevalence of Toxocara Spp. eggs in Public Parks in Tehran City Iran. Iran J Parasitol. 2012;7(3):38–42. [PMC free article] [PubMed] [Google Scholar]
- 31.Sadjjadi SM, Oryan A, Jalali AR, Mehrabani D. Prevalence and intensity of infestation with Toxocara cati stray cats in Shiraz Iran. Veterinarski Archiv. 2001;71(3):149–157. [Google Scholar]
- 32.Zibaei M, Sadjjadi SM, Sarkari B. Prevalence of Toxocara cati and other intestinal helminths in stray cats in Shiraz. Iran. Trop Biomed. 2007;24(2):39–43. [PubMed] [Google Scholar]
- 33.Sharif M, Nasrolahei M, Ziapour SP, Gholami S, Ziaei H, Daryani A, et al. Toxocara cati infections in stray cats in Northern Iran. J Helminthol. 2007;81:63–66. doi: 10.1017/S0022149X07214117. [DOI] [PubMed] [Google Scholar]
- 34.Changizi E, Mobedi I, Salimi-Bejestani MR, Rezaei-Doust A. Gastrointestinal Helminthic Parasites in Stray Cats (from North of Iran) Iranian J Parasitol. 2007;2(4):25–29. [Google Scholar]
- 35.Arbabi M, Hooshyar H. Gastrointestinal parasites of stray cats in Kashan Iran. Trop Biomed. 2009;26(1):16–22. [PubMed] [Google Scholar]
- 36.Esmaeilzadeh M, Shamsfard M, Kazemi A, Khalafi SA, Altome SA. Prevalence of Protozoa and Gastrointestinal Helminthes in Stray Cats in Zanjan Province, North-West of Iran. Iranian J Parasitol. 2009;4(3):71–75. [Google Scholar]
- 37.Borji H, Razmi GR, Karami HR, Yaghfoori S, Ahmadi A, Abedi V. A survey on endoparasites and ectoparasites of stray cats from Mashhad (Iran) and association with risk factors. J Parasit Dis. 2011;35(2):202–206. doi: 10.1007/s12639-011-0057-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pezeshki A, Zarebavani M, Rezaeian M. Toxocara cati infection in cats in Tehran and their importance in medicine. Asian Pacific J Trop Biomed. 2012:1–2. [Google Scholar]
- 39.Sadjjadi SM, Khosravi M, Mehrabani D, Orya A. Seroprevalence of Toxocara infection in school children in Shiraz, southern Iran. J Trop Pediatr. 2000;46(6):327–330. doi: 10.1093/tropej/46.6.327. [DOI] [PubMed] [Google Scholar]
- 40.Soulsby EJL. Helminths, Arthropods and Protozoa of Domesticated Animals. Bailliere Tindall; London, UK: 1982. [Google Scholar]
- 41.Yamaguti S. Systema Helminthum: Nematodes of vertebrates. Inter Science publisher Inc; New York, USA: 1961. [Google Scholar]
- 42.Li MW, Lin RQ, Song HQ, Sani RA, Wu XY, Zhu XQ. Electrophoretic analysis of sequence variability in three mitochondrial DNA regions for ascaridoid parasites of human and animal health significance. Electrophoresis. 2008;29:2912–2917. doi: 10.1002/elps.200700752. [DOI] [PubMed] [Google Scholar]
- 43.Li MW, Lin RQ, Chen HH, Sani RA, Song HQ, Zhu XQ. PCR tools for the verification of the specific identity of ascaridoid nematodes from dogs and cats. Mol Cell Probes. 2007;21:349–354. doi: 10.1016/j.mcp.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 44.Glickman LT, Schantz PM. Epidemiology and pathogenesis of zoonotic toxocariasis. Epidemiol Rev. 1981;3:230–250. doi: 10.1093/oxfordjournals.epirev.a036235. [DOI] [PubMed] [Google Scholar]
- 45.Lee CC, Cheng NABY, Bohari Y. Toxocara canis from domestic cats in Kuala Lumpur. Trop Biomed. 1993;10:79–80. [Google Scholar]
- 46.Zhu XQ, Jacobs DE, Chilton NB, Sani RA, Cheng NA, Gasser RB. Molecular characterization of a Toxocara variant from cats in Kuala Lumpur, Malaysia. Parasitol. 1998;117(Pt 2):155–164. doi: 10.1017/s0031182098002856. [DOI] [PubMed] [Google Scholar]
- 47.Gibbons LM, Jacobs DE, Sani RA. Toxocara malaysiensis n. sp. Nematoda: Ascaridoidea) from the domestic cat (Feliscatus Linnaeus, 1758) J Parasitol. 2001;87:660–665. doi: 10.1645/0022-3395(2001)087[0660:TMNSNA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 48.Li MW, Zhu XQ, Gasser RB, Lin RQ, Sani RA, Lun ZR, et al. The occurrence of Toxocara malaysiensis in cats in China, confirmed by sequence based analyses of ribosomal DNA. Parasitol Res. 2006;99:554–557. doi: 10.1007/s00436-006-0194-z. [DOI] [PubMed] [Google Scholar]


