Abstract
IMPORTANCE
Establishing the natural history of G11778A Leber hereditary optic neuropathy (LHON) is important to determine the optimal end points to assess the safety and efficacy of a planned gene therapy trial.
OBJECTIVE
To use the results of the present natural history study of patients with G11778A LHON to plan a gene therapy clinical trial that will use allotopic expression by delivering a normal nuclear-encoded ND4 gene into the nuclei of retinal ganglion cells via an adeno-associated virus vector injected into the vitreous.
DESIGN, SETTING, AND PARTICIPANTS
A prospective observational study initiated in 2008 was conducted in primary and referral institutional practice settings. Participants included 44 individuals with G11778A LHON, recruited between September 2008 and March 2012, who were evaluated every 6 months and returned for 1 or more follow-up visits (6–36 months) as of August 2012.
EXPOSURES
Complete neuro-ophthalmic examination and main measures.
MAIN OUTCOMES AND MEASURES
Visual acuity, automated visual field testing, pattern electroretinogram, and spectral-domain optical coherence tomography.
RESULTS
Clinical measures were stable during the follow-up period, and visual acuity was as good as or better than the other visual factors used for monitoring patients. Based on a criterion of 15 or more letters from the Early Treatment Diabetic Retinopathy Study chart, 13 eyes of 8 patients (18%) improved, but 24 months after the onset of symptoms, any further improvements were to no better than 20/100. Acuity recovery occurred in some patients despite continued marked retinal nerve fiber layer thinning indistinguishable from that in patients who did not recover visual acuity.
CONCLUSIONS AND RELEVANCE
Spontaneous improvement of visual acuity in patients with G11778A LHON is not common and is partial and limited when it occurs, so improvements in vision with adeno-associated virus–mediated gene therapy of a synthetic wild-type ND4 subunit gene should be possible to detect with a reasonable sample size. Visual acuity appears to be the most suitable primary end point for the planned clinical trial.
Leber hereditary optic neuropathy (LHON) is a maternally inherited mitochondrial DNA genetic disease characterized by severe visual loss. The onset occurs typically in early adulthood, with little or no propensity for recovery.1,2 Approximately 95% of LHON is caused by 3 pathogenic point mitochondrial DNA mutations coding for the respiratory chain subunits of the nicotinamide adenine dinucleotide ubiquinone-oxidoreductase (complex I) genes: 3460G>A ND1, 11778G>A ND4, and 14484T>C ND6.3,4 The 11778G>A mutation results in the arginine to histidine substitution at amino acid position 340. Males are far more likely to develop LHON than are females. Affected individuals are usually asymptomatic until they develop central visual loss in one eye. Similar symptoms appear in the other eye an average of 8 weeks later.
No established treatment is available for LHON. A prospective randomized clinical trial5 included 55 patients (all 3 LHON subtypes included). These patients received 900 mg/d of oral idebenone, which is a synthetic short-chain benzoquinone and coenzyme Q10 derivative. Thirty other patients with LHON received placebo. No statistically significant visual acuity recovery, the primary end point of the trial, was found at the end of the 24-week study period, although post hoc interaction analysis suggested that patients with discordant visual acuities are the most likely to benefit from idebenone. A review6 of 103 patients (all 3 LHON subtypes included) described 44 individuals who were given idebenone, ranging from 270 to 675 mg/d, either within 1 year after the onset of visual loss in the second eye or before involvement of the second eye. Patients with G11778A LHON who received the earliest treatment had the best chance of recovery; however, the study was limited by its retrospective design and outcome measures different from those of other trials. In a phase 1 open-label trial,7 5 patients with LHON (all 3 LHON subtypes included) received oral EPI-743, a digital biochemical information transfer and sensing compound, within 90 days of conversion to the affected LHON state. One of the 3 patients with G11778A demonstrated reversal of visual loss; a larger-scale study is planned.
Our future goal will be to test the effectiveness of gene therapy in preventing visual loss and restoring vision in patients with G11778A LHON and maternally related family members at risk to lose vision. We chose G11778A LHON because it accounts for half of the LHON cases and causes one of the most severe forms of the disease. We have overcome the deficiency in oxidative phosphorylation in LHON by using a technique termed allotopic expression.8 A nuclear version of the mitochondrial gene ND4 (OMIM 516003.0001) subunit gene is recoded for expression and import into mitochondria.9–11 Allotopic expression normalized the defective adenosine triphosphatase synthesis of LHON cells and improved their survival.12 We have created a bona fide animal model for LHON. Using site-directed mutagenesis of the nuclear version of ND4, we replaced the codon for arginine with that for histidine at amino acid 340. Injection of this construct into the mouse visual system disrupted mitochondrial cytoarchitecture, elevated reactive oxygen species, induced swelling of the optic nerve head, and induced apoptosis, with progressive demise of retinal ganglion cells (RGCs). In contrast, ocular expression of the wild-type human ND4 subunit appeared safe,13,14 and its injection prevented blindness in rats induced by injection of the mutant ND4 allele, providing proof of principle of the LHON gene therapy approach.15
The natural history phase of the project was initiated in September 1, 2008. The baseline entry data of the first year of recruitment (25 patients) showed that mean retinal nerve fiber layer (RNFL) thickness was 78.3 µm up to 32 months after visual loss, suggesting that RGC survival with dysfunction many months after visual loss may provide a long window of opportunity for LHON gene therapy.8
The present report focuses on the serial pretreatment evaluation of affected patients who had at least 1 follow-up visit before August 31, 2012, including follow-up data through year 3. The aim of the present analysis was to assess the natural history of improvement or worsening of visual measures.
Methods
Clinical Examination
Since 2008, patients with G11778A LHON were recruited regardless of the time since visual loss and reexamined every 6 months at our eye institute. The study was approved by the University of Miami Institutional Review Board, and written informed consent was obtained. The participants received no financial compensation. Best-corrected visual acuity was measured using the Early Treatment Diabetic Retinopathy Study (ETDRS) chart. Neuro-ophthalmic examination included pupil, retina, and optic nerve assessments. Humphrey 30-2 white-on-white standard automated visual fields were obtained.
Molecular Analysis
A polymerase chain reaction–based test using the amplification refractory mutation system detected the presence or absence of G3460A, G11778A, and T14484C LHON genotypes.8 The genetic analysis was performed by The University of Iowa Carver Laboratory or by Athena Laboratories.16
Optical Coherence Tomography
Peripapillary RNFL thickness was measured by an optic disc cube 200 × 200 scan of the spectral domain (Cirrus OCT, 3.0; Carl Zeiss Meditec). The algorithms identify a circle of 3.46-mm diameter around the optic disc center and calculate the RNFL thickness at each point on the circle.17 Three consecutive scans were acquired and analyzed for each eye.
Pattern Electroretinogram
The pattern electroretinogram (PERG) was simultaneously recorded from both eyes according to a paradigm optimized for glaucoma detection.18 The method is highly reproducible,18,19 and normative data are available.20 The PERG waveforms were automatically analyzed in the frequency domain by discrete Fourier transform to isolate the frequency component at the contrast-reversal rate (16.28 Hz) to compute its amplitude in microvolts and phase in pi radians.18
Statistical Analysis
Values were expressed as mean (SD). Data were analyzed with fixed effects and linear mixed models. P < .05 was considered significant. The ETDRS visual acuity was analyzed as number of letters read. Comparisons of follow-up with baseline measurements in which both eyes were averaged at each visit (Table 1) were performed with the 2-tailed paired t test. Cumulative proportions and SEs of patients with acuity improved by 15 letters in either eye, stratified by months since the onset of visual acuity loss, were calculated with Kaplan-Meier methods. Because of the strong age dependency of PERG, these measurements were expressed as percentage of age-specific normal.21
Table 1.
Distribution of Clinical Factors Over Time
| Characteristic | Mean(SD)a | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | Month 12 | P Value | Month 24 | P Value | Month 36 | P Value | |
| Total No. of patients | 44 | 40 | 31 | 18 | |||
| Visual acuity, ETDRS score | 14.9 (18.3) | 14.4 (19.3) | .65 | 14.1 (17.3) | .57 | 15.9 (19.6) | .20 |
| Visual field, mean deviation | −24.4 (8.9) | −25.5 (6.6) | .33 | −24.9 (7.1) | .82 | −23.9 (7.0) | .23 |
| RNFL thickness on OCT, µm | 66.2 (23.9) | 55.7 (5.9) | .006 | 55.3 (4.7) | .03 | 54.8 (4.3) | .23 |
| PERG amplitude, % of normal | 40.3 (18.3) | 38.8 (19.2) | .99 | 42.2 (24.7) | .59 | 33.3 (13.5) | .13 |
| PERG phase, % of normal | 106.2 (7.2) | 106.6 (8.0) | .64 | 107.2 (9.4) | .66 | 103.5 (9.1) | .07 |
| No. of patients with onset ≤12 mo | 13 | 12 | 9 | 4 | |||
| Visual acuity, ETDRS score | 23.3 (21.4) | 23.2 (28.3) | .78 | 18.6 (24.6) | .93 | 21.9 (28.4) | .41 |
| Visual field, mean deviation | −20.6 (10.1) | −24.1 (7.9) | .33 | −24.6 (6.0) | .79 | −22.9 (5.1) | .26 |
| RNFL thickness on OCT, µm | 93.7 (24.2) | 60.3 (4.6) | .001 | 57.9 (4.8) | .003 | 56.9 (3.9) | .06 |
| PERG amplitude, % of normal | 41.3 (17.0) | 37.9 (16.6) | .98 | 37.8 (21.8) | .72 | 32.8 (18.3) | .79 |
| PERG phase, % of normal | 105.5 (5.4) | 105.4 (5.2) | .67 | 106.2 (8.0) | .99 | 109.1 (7.5) | .32 |
| No. of patients with onset >12 mo | 31 | 28 | 22 | 14 | |||
| Visual acuity, ETDRS score | 11.3 (15.9) | 10.6 (12.7) | .35 | 12.2 (13.5) | .17 | 14.1 (17.4) | .18 |
| Visual field, mean deviation | −26.0 (7.9) | −26.2 (6.0) | .83 | −25 (7.6) | .99 | −24.3 (7.6) | .62 |
| RNFL thickness on OCT, µm | 53.9 (8.8) | 53.6 (5.3) | .52 | 54.1 (4.3) | .25 | 54.1 (4.4) | .07 |
| PERG amplitude, % of normal | 39.9 (19.1) | 39.2 (20.5) | .99 | 43.8 (26.1) | .36 | 33.4 (12.7) | .11 |
| PERG phase, % of normal | 106.5 (7.9) | 107.2 (9.1) | .84 | 107.6 (10.1) | .58 | 101.9 (9.1) | .02 |
Abbreviations: ETDRS, Early Treatment Diabetic Retinopathy Study; OCT, optical coherence tomography; PERG, pattern electroretinogram; RNFL, retinal nerve fiber layer
Data are based on the number of eyes evaluated. P values were determined for comparison of each follow-up visit with baseline by paired t test.
Results
Between September 1, 2008, and March 31, 2012, a total of 44 patients with G11778A LHON were enrolled, regardless of the time since the onset of visual loss, and had returned for at least 1 follow-up visit as of August 31, 2012. Participant retention by visit as a percentage of possible visits was 92%(43 patients),87% (40), 79% (33), 89% (31), 81% (25), and 69% (18) for the 6-, 12-, 18-, 24-, 30-, and 36-month visits, respectively. The mean age at enrollment was 32.1 (13.0) years (range, 10–61 years), and 35 of the patients (80%) were male. The mean age at onset of acuity loss was 23.9 (11.8) years (range, 4–61 years). Males experienced onset of acuity loss at a significantly younger age compared with females (21.1 [9.5] years; range, 4–61 years; vs 34.9 [13.9]years; range, 7–51 years; P = .001). The mean time from onset of vision loss to enrollment was 97.7 (125.5) months (range, 0.8 months to 45.0 years). Males experienced a significantly longer period from onset to enrollment than did females (117.8 [133.6] months; range, 2.3 months to 45.0 years; vs 19.9 [17.7] months; range, 0.8 months to 59.5 years; P < .001). Distribution of the patient visits is reported in the Supplement (eTable 1); 18 patients (41%) had 36 months of completed visits. The intervals from onset of acuity loss in the first eye vs the fellow eye were available for 15 patients: 8 patients (53%) reported that onset of acuity loss in the second eye occurred in 2 months or less; 4 patients (27%) experienced acuity loss in less than 6 months; 2 patients (13%) had onset in 12 months or less; and 1 patient (7%) maintained good contralateral acuity 30 months after onset. Excluding the patient with unilateral involvement, the median time to fellow eye involvement was approximately 2 months and the maximum time was 8 months.
Table 1 presents a longitudinal summary of clinical factors from baseline through 36 months of follow-up. No significant changes in mean visual acuity, visual field, or PERG for all patients were identified, and no significant change was found when the data were stratified into patients with onset of visual acuity loss at 12 months or less and onset of visual acuity loss at more than 12 months. Statistically significant decreases in OCT-measured RNFL occurred over time in patients with onset of visual loss at 12 months or less; however, in those with onset of visual loss at more than 12 months, the OCT-measured RNFL was already thinned and showed no significant change over time. At the 36-month visit, the OCT-measured RNFL remained thinned regardless of the time of visual loss onset at baseline.
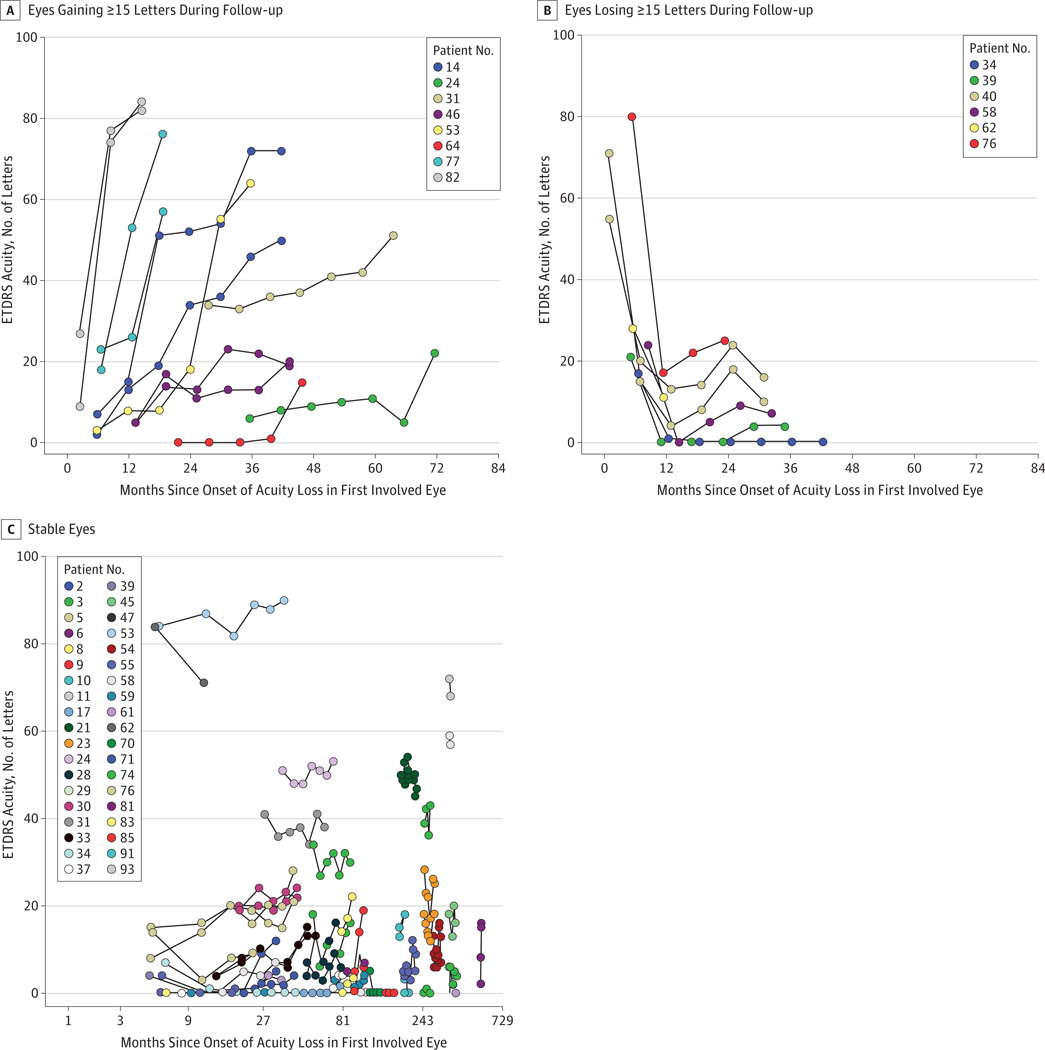
Because the end point for a phase 1/2 gene therapy clinical trial is likely a 15-letter ETDRS improvement in visual acuity (approximately 3 lines), we classified patients as improved from baseline, worsened, or stable based on this criterion. Because participants were recruited regardless of the time since visual loss, the analysis was performed using the onset of visual loss as the reference, and the figures were plotted using “months since onset of acuity loss in first involved eye” as the x-axis. The results showed that 13 eyes of 8 patients (18%) improved during follow-up, 7 eyes of 6 patients (14%) worsened, and 68 eyes of 38 patients (86%) were stable. Figure 1 displays the relationship between change in acuity and months since the onset of visual loss and length of follow-up. The plot of the 13 eyes of 8 patients that improved is shown in Figure 1A. Among the 8 patients whose vision improved, the delay after onset of acuity loss to when an eye first demonstrated a 15-letter ETDRS improvement ranged from 8.3 to 71.5 months (median, 27.5 months). Of patients with onset of acuity loss less than or equal to 12 months before enrollment, the cumulative proportion with a 15-letter ETDRS improvement in either eye was 31.6%(SE, 13.1%) at 12 months of follow-up, with no more patients subsequently experiencing improved vision. Of patients with onset of acuity loss at longer than 12 months but less than or equal to 36 months, the cumulative proportion with 15-letter ETDRS improvement increased from zero at 12 months to 54.3% (SE, 18.9%) at 36 months, with no more patients subsequently experiencing improved vision. No patients with onset of acuity loss at more than 36 months before enrollment displayed a 15-letter ETDRS improvement. Those whose vision improved late tended to have lower visual acuity. Four eyes of 3 patients (patient 24, left eye; patient 64, both eyes; and patient 31, left eye) improved by 15 ETDRS letters more than 20 months after the onset of symptoms; however, at their last visit, the acuity levels of 3 of these eyes were still worse than 30 ETDRS letters (<20/200). One eye improved to 51 letters (20/100). Taken together, patients who showed the most rapid improvements had shorter times since onset of visual loss.
Figure 1. Early Treatment Diabetic Retinopathy Study (ETDRS) Visual Acuity.
A, Eyes gaining 15 or more letters of acuity during follow-up. B, Eyes losing 15 or more letters during follow-up. C, Eyes with changes of less than 15 letters of acuity during follow-up. The x-axis uses a logarithmic scale.
Figure 1B plots the 7 eyes of 6 patients that worsened. The pattern of worsening with follow-up was more homogeneous than that of improvement. Of the eyes that worsened, all worsening occurred within 12 months after the onset of visual acuity loss and reached the lowest levels approximately 12 months after the onset of symptoms. Of the 68 eyes of the 38 patients that were stable, the eyes of 30 patients were stable bilaterally (Figure 1C). The median time since onset of visual acuity loss in these patients was 66 months (range, 9 months to 45 years). Fourteen patients (47%) were monitored for 36 months, and 21 individuals (70%) were monitored for at least 2 years.
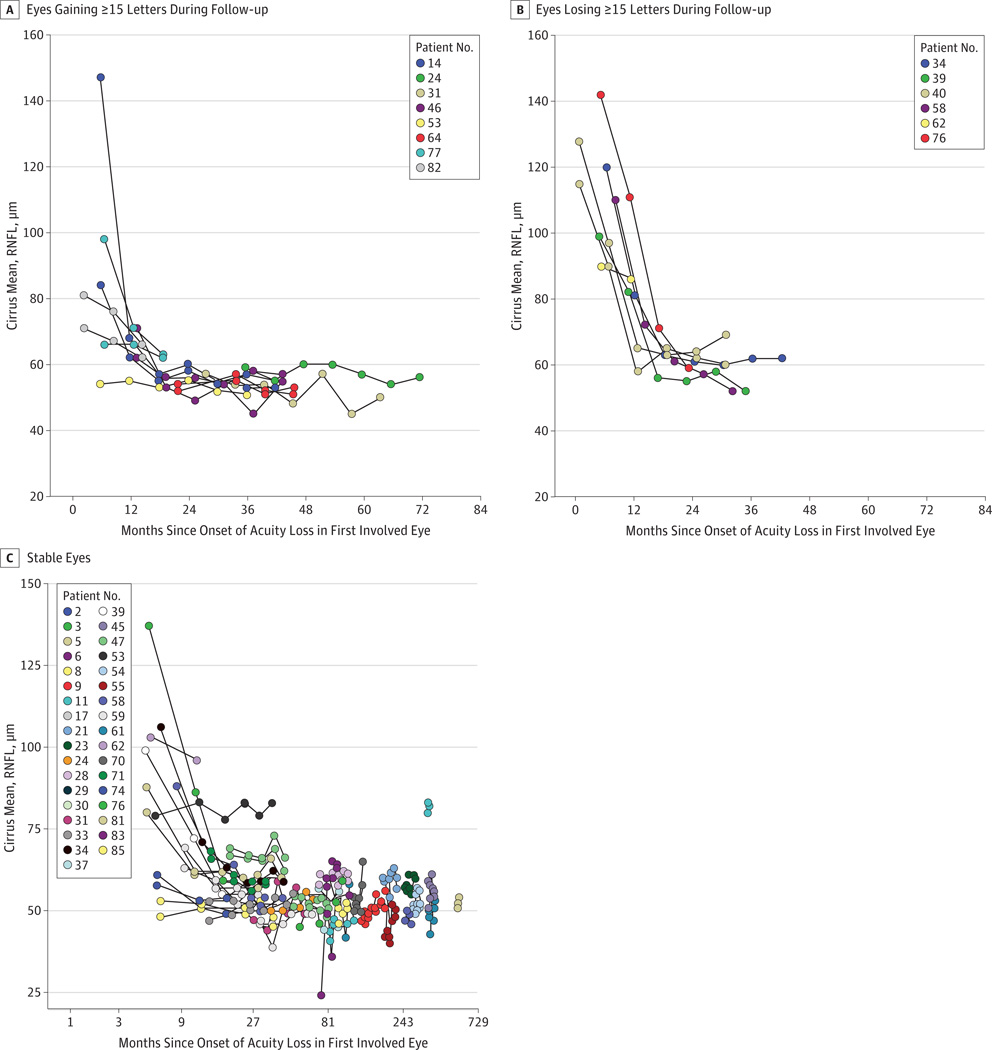
In all 13 eyes of 8 patients (18%) who had improved visual acuity, the improvements occurred despite the continued thinning of the average RNFL on OCT. Figure 2 displays changes with follow-up for mean RNFL thickness and shows RNFL thickness deteriorated in eyes that improved in a manner similar to that in eyes that worsened. Of the 8 eyes of 5 patients old enough to be represented in the age-specific normal database, the RNFL of all was decreased to less than 1% of the normal thickness range. The eyes of the patients too young to be represented in the age-specific normal database had similar thicknesses. Examples of plots of some cases demonstrating that improvements occurred despite the continued thinning of the RNFL thickness are shown in the Supplement (eFigure 1). The baseline temporal sector RNFL thickness, an indicator of the papillomacular bundle, is not related to eyes that improved (P = .48, logistic regression, less significant than mean RNFL, with P = .14). We also reviewed the Cirrus ganglion cell layer analyses for the 13 eyes with improved visual acuity. Longitudinal plotting of the average, minimum, and temporal ganglion cell–layer inner-plexiform layer data do not reveal changes over time.
Figure 2. Optical Coherence Tomography Retinal Nerve Fiber Layer (RNFL) Thickness.
A, Mean RNFL of eyes gaining 15 or more Early Treatment Diabetic Retinopathy Study (ETDRS) letters of acuity during follow-up. B, Mean RNFL of eyes losing 15 or more ETDRS letters during follow-up. C, Mean RNFL of eyes with changes of less than 15 ETDRS letters of acuity during follow-up. The x-axis uses a logarithmic scale.
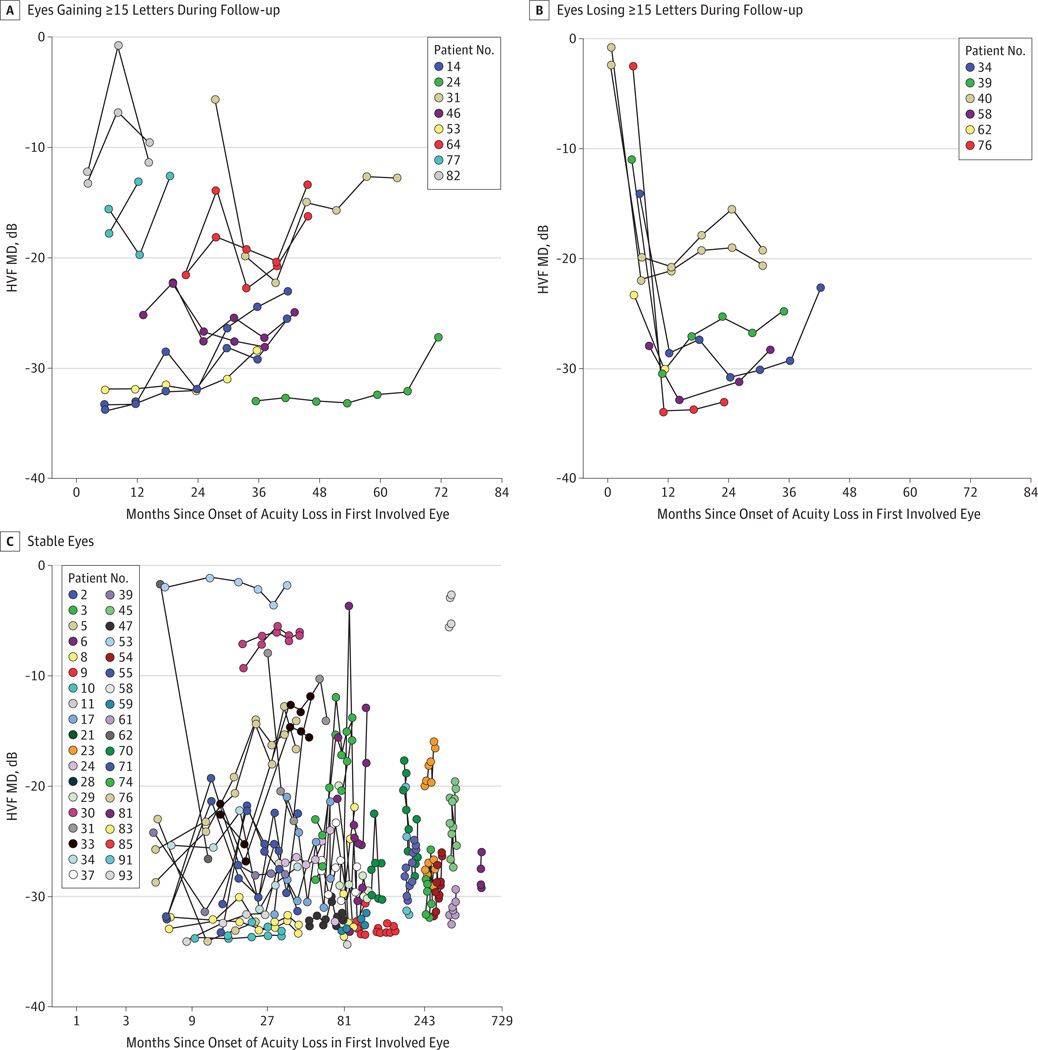
Figure 3 shows changes with follow-up for visual field mean deviation (MD). In eyes with improved visual acuity, improvements in visual field were modest and not consistently observed. In eyes with worsened visual acuity, the MD worsened correspondingly. Separation of PERG measurements into visual field change status for analysis revealed nothing note-worthy for either PERG amplitude or phase, suggesting that PERG changes occur before the onset of acuity symptoms. None of the visual function measures collected at baseline, sex, or enrollment age predicted patients who demonstrated improved central acuity (all P > .12, logistic regression), except months since onset (P = .05).
Figure 3. Humphrey Visual Field (HVF) Mean Deviation (MD) Changes.
A, The HVF MD of eyes gaining 15 or more Early Treatment Diabetic Retinopathy Study (ETDRS) letters of acuity during follow-up. B, The HVF MD of eyes losing 15 or more ETDRS letters during follow-up. C, The HVF MD of eyes with changes of less than 15 ETDRS letters of acuity during follow-up. The x-axis uses a logarithmic scale.
Among patients receiving idebenone therapy during our study period, dosages ranged from 120 to 900 mg/d. One patient received stem cells in China.22 Another patient entered into our study was still taking EPI-743, starting treatment when his vision was 20/25 OD and 20/20 OS. There was no significant difference identified in the percentages of patients improving by 15 ETDRS letters between those receiving idebenone and those who were not receiving it (Supplement [eTable 2]) (P = .41; Fisher exact test). Neither was there a significant difference among patients with onset of visual loss within 18 months of enrollment (P > .99; Fisher exact test). Because of the small sample size, however, the possibility of an effect of idebenone cannot be excluded. Changes in visual acuity and visual field data associated with respect to idebenone use for patients with recent onset of symptoms are displayed in the Supplement (eFigure 2).
Discussion
The natural history results of patients with G11778A LHON indicate that spontaneous partial recovery of vision is limited and occurs in a small percentage of affected persons. Thus, gene therapy improvements with injection of adeno-associated virus containing a normal ND4 gene will be possible to detect with a reasonable sample size. Of the visual functional and structural factors examined in the present study, visual acuity was as good, and at times better, than the other visual factors for monitoring patients, indicating that visual acuity is the most suitable primary outcome measure for detecting treatment effect in the planned gene therapy clinical trial. The end point of a 15-letter ETDRS visual acuity improvement (approximately 3 lines) is planned for the trial. Although we found that visual acuity improvement may occur up to 72 months after the onset of visual loss, patients whose vision improved late tended to have lower visual acuity. In contrast, all eyes with worsened visual acuity reached the lowest acuity levels approximately 12 months after the onset of visual loss.
Visual field, OCT-measured RNFL thickness, and PERG are reasonable secondary outcome measures for the planned clinical trial. The RNFL thickness was increased in the first months after the onset of symptoms in participants with optic disc edema (Figure 2). Subsequently, homogeneous progressive RNFL thinning was observed during the 18 months after the onset of symptoms regardless of visual acuity changes and whether optic disc edema was initially present. Consistent with our findings, previous OCT studies found thickened RNFL in the early stages of G11778A LHON, with subsequent RNFL thinning beyond 6 months of onset of visual loss.23–25 Thickened RNFL during the acute phase likely represents axoplasmic flow stasis, which masks the concomitant loss of RNFL. With time, loss of RNFL occurs and axoplasmic flow stasis subsides, resulting in decreased OCT RNFL. Despite its limitations, OCT may be a useful secondary end point for the clinical trial because if the OCT-measured RNFL is relatively more preserved after treatment compared with our natural history study, it could provide anatomical support of a therapeutic effect. Furthermore, measurement of the RNFL and macular OCT may be useful to monitor safety. We also attempted scanning laser polarimetry (GDx; Carl Zeiss Meditec) in our patients to assess axonal injury, but obtaining images of consistent quality was not possible because of poor fixation from bilateral visual loss.
Visual field improvements were modest in eyes with improved acuity but were not consistently observed (Figure 3). In contrast, the visual fields worsened correspondingly in eyes with worsened acuity, indicating the usefulness of visual fields as an adjunctive test during the 12 months after the onset of symptoms, because progressive acuity worsening in G11778A LHON in this study was found only during this period (Figure 3). Pattern electroretinogram amplitude and phase as indicators of RGC function were low in our affected cohort and showed no significant changes over time and no correlation in patients whose vision improved or worsened. However, PERG may serve as a secondary therapeutic end point if the gene therapy improves RGC function and reduces RGC degeneration.
Given our natural history result, the end point of a 15-letter ETDRS visual acuity improvement, and the need to initially establish the safety of the gene therapy, we propose to divide the LHON cohort into 2 groups for the planned phase 1/2 clinical trial in which the worse eye will be treated to establish safety: (1) group 1 (chronic poor acuity group), consisting of patients with onset of visual loss of 12 or more months with acuity loss to 30 or less ETDRS letters (20/200 OU) and (2) group 2 (acute group), consisting of patients with onset of acuity loss to less than 70 ETDRS letters (20/40 OU) within 12 months. After safety is established, treatment of the better eye in group 3 (acute better group), consisting of patients with onset of acuity loss to less than 70 ETDRS letters (20/40) in 1 eye within 12 months, but with contralateral acuity of 70 or more ETDRS letters (20/40) and monitored for at least 12 months may be considered to determine whether gene therapy is effective in maintaining vision in the better eye.Table 2 presents the calculated sample sizes for a noncontrolled phase 1/2 safety and efficacy trial comparing acuity outcomes in the injected treated eye with the contralateral eye that is not injected.26 In our natural history phase of the study, the numbers of patients who met the criteria for these groups were 25, 9, and 3, respectively. Analysis assumes comparison of dichotomous outcomes with the McNemar test. The efficacy end point in group 1 (chronic poor acuity group) is improvement of 15 ETDRS letters to acuity greater than 70 ETDRS letters (20/40) in the injected eye within 12 months. One eye of 1 patient (4%) achieved the efficacy end point in the natural history study. In group 2 (acute group), the efficacy end point is improvement of 15 ETDRS letters to acuity greater than 70 letters (20/40) in the injected eye within 12 months. Three eyes of 2 patients (22%) achieved the efficacy end point in the natural history study. In group 3 (acute better group), the efficacy end point is preservation of vision greater than 70 ETDRS letters in the injected eye compared with the unilaterally affected fellow eye. One of 3 patients (33%) in the natural history study maintained good vision in the unaffected eye beyond 12 months of follow-up.
Table 2.
Sample Sizes for a Noncontrolled Phase 1/2 Efficacy Clinical Trial
| Group Representation in Natural History Study |
No. (%) [95% CI] Patients’ Successful Outcomes at 12 mo in Natural History |
Sample Size Estimates26 for Phase 1/2 Efficacy Trial Assuming Paired Analysis With α Error of .05 |
||||||
|---|---|---|---|---|---|---|---|---|
| 50% Efficacy Power | 75% Efficacy Power | 95% Efficacy Power | ||||||
| Group | No. | 80% | 90% | 80% | 90% | 80% | 90% | |
| 1. Chronic poor VA | 25 | 1 (4) [0.1–20] | 14 | 16 | 8 | 8 | 7 | 7 |
| 2. Acute | 9 | 2 (22) [3–60] | 46 | 60 | 14 | 17 | 8 | 8 |
| 3. Acute better | 3 | 1 (33) [1–91] | 130 | 172 | 22 | 27 | 9 | 11 |
Abbreviation: VA, visual acuity.
We recognize that our estimates of natural history outcomes are not precise, particularly for groups 2 and 3. If we have substantially underestimated the incidence of successful natural history outcomes, we might fail to detect a treatment difference, even with 95% efficacy. However, our natural history data for group 1 demonstrate that long-term good acuity is rare. For example, the upper 95% confidence limit for group 1 (20%) is similar to the point estimates for groups 2 (22%) and 3 (33%). Thus, a high incidence of good acuity outcomes in the untreated eyes of cohorts 2 and 3 may suggest an additional year of follow-up to assess treatment outcomes. These data would be useful for designing an eventual phase 3 study.
Prospective longitudinal follow-up data on a large group of patients with G11778A LHON are not available in the literature for comparison with our study. Newman et al27 monitored the fellow eyes of 5 patients with G11778A LHON for more than 12 months after unilateral visual loss and documented subclinical visual loss that became symptomatic with concomitant progressive visual field loss. Compared with other forms of LHON, G11778A LHON has the worst prognosis and lowest spontaneous visual improvement.1,28–30 Previously reported1,28–31 rates of partial visual improvement in G11778A LHON ranged widely, from 4% to 33%, because of differences in sample size and methods.
Although the present study was not designed to assess the efficacy of idebenone, comparing visual acuity and visual field data (Supplement [eTable 2 and eFigure 2]) in patients who had onset of visual symptoms within 2 years of enrolment who had ever received idebenone vs those who had never received the drug we discerned no significant differences. Therefore, all patient experience was combined to estimate the incidence of visual function changes over time. Similar to what has been reported with EPI-743 treatment,7 visual acuity in one of our patients improved to 20/25 spontaneously, and we found general stabilization of disease in LHON.
Conclusions
Our natural history study indicates visual acuity to be the most suitable primary end point for the planned clinical trial. In addition, detection of gene therapy improvements may be feasible with a reasonably achievable sample size.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by National Eye Institute grants RO1EY014957 (Dr Porciatti), P30-EY014801 (Dr Porciatti), and R24EY018600 (Dr Guy), as well as by an unrestricted grant to Bascom Palmer Eye Institute from Research to Prevent Blindness.
Role of the Sponsor: The National Eye Institute approved the design and conduct of the study as part of its funding process. Research to Prevent Blindness had no direct role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Supplemental content at jamaophthalmology.com
Author Contributions: Drs Lam and Guy had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Lam.
Acquisition of data: Lam, Vandenbroucke, Rosa.
Analysis and interpretation of data: Lam, Feuer, Schiffman, Porciatti, Gregori.
Drafting of the manuscript: Lam, Feuer, Vandenbroucke.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Feuer, Schiffman.
Obtained funding: Lam.
Administrative, technical, or material support: Lam, Porciatti, Vandenbroucke, Rosa, Gregori.
Study supervision: Lam, Porciatti.
Conflict of Interest Disclosures: None reported.
REFERENCES
- 1.Riordan-Eva P, Sanders MD, Govan GG, Sweeney MG, Da Costa J, Harding AE. The clinical features of Leber's hereditary optic neuropathy defined by the presence of a pathogenic mitochondrial DNA mutation. Brain. 1995;118(pt 2):319–337. doi: 10.1093/brain/118.2.319. [DOI] [PubMed] [Google Scholar]
- 2.Sadun AA, La Morgia C, Carelli V. Leber’s hereditary optic neuropathy. Curr Treat Options Neurol. 2011;13(1):109–117. doi: 10.1007/s11940-010-0100-y. [DOI] [PubMed] [Google Scholar]
- 3.Harding AE, Sweeney MG, Govan GG, Riordan-Eva P. Pedigree analysis in Leber hereditary optic neuropathy families with a pathogenic mtDNA mutation. Am J Hum Genet. 1995;57(1):77–86. [PMC free article] [PubMed] [Google Scholar]
- 4.Bi R, Zhang AM, Yu D, Chen D, Yao YG. Screening the three LHON primary mutations in the general Chinese population by using an optimized multiplex allele-specific PCR. Clin Chim Acta. 2010;411(21–22):1671–1674. doi: 10.1016/j.cca.2010.06.026. [DOI] [PubMed] [Google Scholar]
- 5.Klopstock T, Yu-Wai-Man P, Dimitriadis K, et al. A randomized placebo-controlled trial of idebenone in Leber's hereditary optic neuropathy. Brain. 2011;134(pt 9):2677–2686. doi: 10.1093/brain/awr170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carelli V, La Morgia C, Valentino ML, et al. Idebenone treatment in Leber's hereditary optic neuropathy. Brain. 2011;134(pt 9):e188. doi: 10.1093/brain/awr180. [DOI] [PubMed] [Google Scholar]
- 7.Sadun AA, Chicani CF, Ross-Cisneros FN, et al. Effect of EPI-743 on the clinical course of the mitochondrial disease Leber hereditary optic neuropathy. Arch Neurol. 2012;69(3):331–338. doi: 10.1001/archneurol.2011.2972. [DOI] [PubMed] [Google Scholar]
- 8.Lam BL, Feuer WJ, Abukhalil F, Porciatti V, Hauswirth WW, Guy J. Leber hereditary optic neuropathy gene therapy clinical trial recruitment: year 1. Arch Ophthalmol. 2010;128(9):1129–1135. doi: 10.1001/archophthalmol.2010.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glick B, Wachter C, Schatz G. Protein import into mitochondria: two systems acting in tandem? Trends Cell Biol. 1991;1(4):99–103. doi: 10.1016/0962-8924(91)90037-a. [DOI] [PubMed] [Google Scholar]
- 10.Neupert W. Protein import into mitochondria. Annu Rev Biochem. 1997;66:863–917. doi: 10.1146/annurev.biochem.66.1.863. [DOI] [PubMed] [Google Scholar]
- 11.Manfredi G, Fu J, Ojaimi J, et al. Rescue of a deficiency in ATP synthesis by transfer of MTATP6, a mitochondrial DNA-encoded gene, to the nucleus. Nat Genet. 2002;30(4):394–399. doi: 10.1038/ng851. [DOI] [PubMed] [Google Scholar]
- 12.Guy J, Qi X, Pallotti F, et al. Rescue of a mitochondrial deficiency causing Leber hereditary optic neuropathy. Ann Neurol. 2002;52(5):534–542. doi: 10.1002/ana.10354. [DOI] [PubMed] [Google Scholar]
- 13.Guy J, Qi X, Koilkonda RD, et al. Efficiency and safety of AAV-mediated gene delivery of the human ND4 complex I subunit in the mouse visual system. Invest Ophthalmol Vis Sci. 2009;50(9):4205–4214. doi: 10.1167/iovs.08-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koilkonda RD, Chou TH, Porciatti V, Hauswirth WW, Guy J. Induction of rapid and highly efficient expression of the human ND4 complex I subunit in the mouse visual system by self-complementary adeno-associated virus. Arch Ophthalmol. 2010;128(7):876–883. doi: 10.1001/archophthalmol.2010.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ellouze S, Augustin S, Bouaita A, et al. Optimized allotopic expression of the human mitochondrial ND4 prevents blindness in a rat model of mitochondrial dysfunction. Am J Hum Genet. 2008;83(3):373–387. doi: 10.1016/j.ajhg.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stone EM. Genetic testing for inherited eye disease. Arch Ophthalmol. 2007;125(2):205–212. doi: 10.1001/archopht.125.2.205. [DOI] [PubMed] [Google Scholar]
- 17.Vizzeri G, Weinreb RN, Gonzalez-Garcia AO, et al. Agreement between spectral-domain and time-domain OCT for measuring RNFL thickness. Br J Ophthalmol. 2009;93(6):775–781. doi: 10.1136/bjo.2008.150698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porciatti V, Ventura LM. Normative data for a user-friendly paradigm for pattern electroretinogram recording. Ophthalmology. 2004;111(1):161–168. doi: 10.1016/j.ophtha.2003.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fredette MJ, Anderson DR, Porciatti V, Feuer W. Reproducibility of pattern electroretinogram in glaucoma patients with a range of severity of disease with the new glaucoma paradigm. Ophthalmology. 2008;115(6):957–963. doi: 10.1016/j.ophtha.2007.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ventura LM, Porciatti V, Ishida K, Feuer WJ, Parrish RK., II Pattern electroretinogram abnormality and glaucoma. Ophthalmology. 2005;112(1):10–19. doi: 10.1016/j.ophtha.2004.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ventura LM, Sorokac N, De Los Santos R, Feuer WJ, Porciatti V. The relationship between retinal ganglion cell function and retinal nerve fiber thickness in early glaucoma. Invest Ophthalmol Vis Sci. 2006;47(9):3904–3911. doi: 10.1167/iovs.06-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abukhalil F, Lam BL, Guy J. Visual observations of an American patient with Leber hereditary optic neuropathy after purported injections of stem cells in China. Arch Ophthalmol. 2012;130(4):532–534. doi: 10.1001/archophthalmol.2011.1665. [DOI] [PubMed] [Google Scholar]
- 23.Seo JH, Hwang JM, Park SS. Comparison of retinal nerve fibre layers between 11778 and 14484 mutations in Leber’s hereditary optic neuropathy. Eye (Lond) 2010;24(1):107–111. doi: 10.1038/eye.2009.36. [DOI] [PubMed] [Google Scholar]
- 24.Savini G, Barboni P, Valentino ML, et al. Retinal nerve fiber layer evaluation by optical coherence tomography in unaffected carriers with Leber’s hereditary optic neuropathy mutations. Ophthalmology. 2005;112(1):127–131. doi: 10.1016/j.ophtha.2004.09.033. [DOI] [PubMed] [Google Scholar]
- 25.Barboni P, Savini G, Valentino ML, et al. Retinal nerve fiber layer evaluation by optical coherence tomography in Leber’s hereditary optic neuropathy. Ophthalmology. 2005;112(1):120–126. doi: 10.1016/j.ophtha.2004.06.034. [DOI] [PubMed] [Google Scholar]
- 26.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 27.Newman NJ, Biousse V, Newman SA, et al. Progression of visual field defects in Leber hereditary optic neuropathy: experience of the LHON treatment trial. Am J Ophthalmol. 2006;141(6):1061–1067. doi: 10.1016/j.ajo.2005.12.045. [DOI] [PubMed] [Google Scholar]
- 28.Oostra RJ, Bolhuis PA, Wijburg FA, Zorn-Ende G, Bleeker-Wagemakers EM. Leber’s hereditary optic neuropathy: correlations between mitochondrial genotype and visual outcome. J Med Genet. 1994;31(4):280–286. doi: 10.1136/jmg.31.4.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stone EM, Newman NJ, Miller NR, Johns DR, Lott MT, Wallace DC. Visual recovery in patients with Leber’s hereditary optic neuropathy and the 11778 mutation. J Clin Neuroophthalmol. 1992;12(1):10–14. [PubMed] [Google Scholar]
- 30.Mashima Y, Sato EA, Ohde H, Oguchi Y. Macular nerve fibers temporal to fovea may have a greater potential to recover function in patients with Leber’s hereditary optic neuropathy. Jpn J Ophthalmol. 2002;46(6):660–667. doi: 10.1016/s0021-5155(02)00562-2. [DOI] [PubMed] [Google Scholar]
- 31.Nakamura M, Yamamoto M. Variable pattern of visual recovery of Leber’s hereditary optic neuropathy. Br J Ophthalmol. 2000;84(5):534–535. doi: 10.1136/bjo.84.5.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.