Abstract
Context
The evolving concept of more rigorously coordinated and integrated perioperative management, often referred to as the perioperative surgical home (PSH), parallels the well-known concept of a patient-centered medical home (PCMH), as they share a vision of improved clinical outcomes and reductions in cost of care through patient engagement and care coordination. Elements of the PSH and similar surgical care coordination models have been studied in the United States and other countries.
Methods
This comprehensive review of peer-reviewed literature investigates the history and evolution of PSH and PSH-like models and summarizes the results of studies of PSH elements in the United States and in other countries. We reviewed more than 250 potentially relevant studies. At the conclusion of the selection process, our search had yielded a total of 152 peer-reviewed articles published between 1980 and 2013.
Findings
The literature reports consistent and significant positive findings related to PSH initiatives. Both US and non-US studies stress the role of anesthesiologists in perioperative patient management. The PSH may have the greatest impact on preparing patients for surgery and ensuring their safe and effective transition to home or other postoperative rehabilitation. There appear to be some subtle differences between US and non-US research on the PSH. The literature in non-US settings seems to focus strictly on the comparison of outcomes from changing policies or practices, whereas US research seems to be more focused on the discovery of innovative practice models and other less direct changes, for example, information technology, that may be contributing to the evolution toward the PSH model.
Conclusions
The PSH model may have significant implications for policymakers, payers, administrators, clinicians, and patients. The potential for policy-relevant cost savings and quality improvement is apparent across the perioperative continuum of care, especially for integrated care organizations, bundled payment, and value-based purchasing.
Keywords: perioperative management, enhanced recovery after surgery, early patient engagement, care coordination
The United States spends about $180 billion per year on inpatient surgical procedures in nonfederal hospitals alone.1 The average cost of surgery continues to climb—from $13,000 per hospitalization in 2000 to $18,000 (inflation adjusted) in 2010—and patient safety, outcomes, and readmissions are ongoing concerns.1 Is the perioperative surgical home (PSH) a part of the solution? The concept of a more rigorously coordinated and integrated perioperative patient management system has been implemented, studied, and reported primarily in Canada, Europe, Australia, and the United States within the last 40 years, but the evolution of the PSH concept in the United States seems to be more recent. Earlier, surgical care in the United States followed a general trend of surgical specialties and capabilities moving toward same-day surgery admissions2; market expectations for high-quality surgical outcomes while controlling cost of surgeries by pursuing service-line strategies3; and value-based payment programs launched as a result of the Patient Protection and Affordable Care Act, which could yield significant savings for payers.4
The PSH continues to be defined in both the literature and clinical practice. One of its most recent definitions is based on the PSH model adopted by the University of Alabama at Birmingham, which describes the PSH model as “an innovative, patient-centered, surgical continuity of care model that incorporates shared decision making.”5 PSH programs in the United States have a variety of names, such as “center for perioperative services,” “reengineered perioperative services,” and “perioperative care pathways.” An initial examination of the literature suggests that most definitions feature 2 points of emphasis: stronger continuity, coordination, and integration of surgical care; and greater patient-focused and shared decision making. Because the terminology used to describe the PSH varies widely, we looked at the most recent comprehensive reviews and definitions of this new concept of perioperative medicine and surgical care. In one, Lee and colleagues broadly use the umbrella term “perioperative system” to encompass all the PSH's activities and developments.6
Consistently emerging evidence in the health care literature supports care coordination models like the well-established patient-centered medical home (PCMH), with its underlying principle of a single physician who coordinates the patient's care and engages a team of health care providers and their patient in an individualized treatment and management plan.7 The PCMH embodies principles laid out by the Institute of Medicine intended to improve care coordination and patient satisfaction.8 The PCMH and the PSH share a vision of higher quality and lower cost while at the same time incorporating similar elements of patient engagement and care coordination.5 Unfortunately, surgical care often is not standardized or coordinated, resulting in duplicative or unnecessary preoperative testing and procedures, which cost an estimated $18 billion annually in the United States alone.9 The PSH concept provides a model that addresses this need for perioperative care standardization and coordination, and its impact on both clinical outcomes and cost has recently been evaluated.10
Key Elements of PSH Models
Specific activities associated with the PSH concept facilitate the transition from the traditionally reactive and siloed surgical processes to a more proactive, coordinated, and team-based approach to surgery.6 Our comprehensive literature review identified key elements of the PSH, which are summarized in Table 1.
Table 1.
Perioperative Surgical Home Elements Identified in the Literature
| Preoperative | Intraoperative | Postoperative |
|---|---|---|
| Key Elements | Key Elements | Key Elements |
|
|
|
Those hospitals engaging in these new systems of coordinated perioperative care vary dramatically in their stage of implementation, operations, team composition, program leadership, scope and depth of coordination, and communication among care providers. But as Table 1 shows, some elements are associated with PSH models integrating preoperative, intraoperative, and postoperative activities. Indeed, the PSH is a much broader and all-encompassing model that goes beyond concepts such as enhanced recovery after surgery (ERAS) programs, bundled payment models, the PCMH, and other care coordination and patient engagement models cited in the literature.
For this article, we compared the evolution of the PSH model in the United States with that of other countries; identified key elements of PSH models studied in the literature; and summarized and compared international findings related to the cost of surgery-related care and clinical outcomes in various surgical homes. To do this, we conducted a comprehensive review of the peer-reviewed literature on the evolution and implementation of perioperative systems. A comparative and evidence-based understanding of various perioperative management models can accelerate the path toward effective PSH design, making operating rooms more efficient and improving surgical outcomes in the United States.
Literature Review Approach and Methodology
We performed a literature search between June 2013 and September 2013 using a broad set of terms to maximize sensitivity. The search used 3 means of article retrieval: (1) a comprehensive search of peer-reviewed literature using PubMed and Google Scholar, (2) reference crawling of all selected articles, and (3) additional articles identified and suggested by experts in the field. Our research team retrieved and evaluated a total of 261 potentially relevant studies related to the general topics of perioperative management, the PSH concept, the history and evolution of PSH-like programs, and key elements of surgical systems studied. These articles were identified using search terms like preoperative, intraoperative, and postoperative process and activities. We then reviewed the abstract and methods sections of each paper to determine whether its content fit the selection criteria for the following 7 general topics related to the PSH concept and surgical care: (1) surgical home, (2) early patient engagement, (3) preoperative testing, (4) intraoperative initiatives leading to reductions in cost of care or care efficiencies, (5) postoperative care initiatives, (6) reduced postoperative complications, and (7) care coordination and transition planning. In this study, we defined cost as overall treatment and surgical costs and efficiencies related to case flow (throughput), case duration, case delays, and case cancellations. For the topic of “reduced postoperative complications,” we excluded articles published before 2005, as more stringent restrictions were necessary for this category to ensure its relevance to the general concept of the PSH. For the other 6 categories, we excluded articles published before 1975.
At the conclusion of the selection process, our search yielded a total of 152 peer-reviewed articles published between 1980 and 2013; collectively as of September 2013, they had been cited 28,725 times.
Classification of Articles
We distinguished between research studies and review articles to accurately categorize content. Furthermore, we included only relevant reviews and original studies found in peer-reviewed literature. The 35 review articles (11 US and 24 non-US) are summarized in Appendix Table 1A (available online) and cover the development and evolution of the PSH model and the history of perioperative management structures. Appendix Tables 2A and 3A (available online) summarize the 117 original PSH studies (63 US and 54 non-US), recording the setting, sample size, PSH elements evaluated (categorized by preoperative, intraoperative, and postoperative elements studied), metrics used to measure cost and efficiencies and/or surgical and clinical outcomes, and reported study results. These tables provided the basis for our content analysis and comparison of US and non-US study results.11
Coding and Counting of Studies
Our research team analyzed the 2 summary tables of the 63 US and the 54 non-US studies to determine the stage of the perioperative process at which each intervention was held and also to define the outcomes for each study. We applied summative content analysis methodology to count and compare studies that reported significant results of preoperative, intraoperative, and postoperative initiatives.12
First we coded the studies as preoperative, intraoperative, and/or postoperative based on the “location” of the intervention in these 3 phases of perioperative care. A study could be coded as both preoperative and intraoperative or as both intraoperative and postoperative, depending on the nature of PSH initiatives and elements evaluated. We then evaluated the study results (outcome measures) so that we could code each study as reporting significant positive results, nonsignificant results, or significant negative results for 2 outcomes: (1) reported cost and efficiencies and (2) clinical outcomes. The most commonly used clinical outcome measures were readmission rates, morbidity (complication) rates, and mortality rates. Other commonly used cost and efficiency measures were overall treatment cost, case flow (throughput), case duration, case delays, and case cancellations. During the coding process, Yichen Zhang and Kayla Cline made an initial pass through the summary tables in order to clarify the coding and assignment criteria.11
We then reevaluated the code assignments and made corrections based on the definitions resulting from discussions in research meetings with the entire project team. We ensured coding reliability by having the 2 primary researchers (Zhang and Cline) code both summary tables independently. A third researcher (Bita Kash) then independently coded the summary tables following the agreed-on coding criteria to ensure triangulation. A study was considered as reporting statistically significant results if it reported a p-value of less than 0.05, and any reported study results with a p-value greater than 0.05 were coded as nonstatistically significant. Also, if a study reported mixed results in the same category of intervention (preoperative, intraoperative, and postoperative) and outcomes dimension (cost/efficiency and clinical outcomes), it was coded as nonstatistically significant.
General Findings
The Evolution and History of the PSH in the United States
In the United States, the “perioperative process” of surgery is currently often referred to and studied as a “surgical home” or a “perioperative management program.” Scholars have recently described the PSH model for the United States as “an innovative, patient-centered, surgical continuity of care model that incorporates shared decision making.”5
The PSH concept has evolved over the last 3 decades. During the 1970s and 1980s, innovations in information technology improved capabilities that in turn facilitated the continuity of care and patient engagement.6 In the 1990s, driven by the emphasis on cost containment in the health care delivery system, the Department of Anesthesiology at the University of Pittsburgh Medical School helped develop the concept of the “perioperative process,”13 and researchers started developing associated benchmarks and measures. In the early 1990s, we also learned about the structure and potential value of preoperative clinics. For example, a study of the preoperative clinic at Stanford University described its development and operating structure and showed that after implementation of the clinic, the average length of stay decreased 87.9%.14 This US study also provided evidence supporting the selective ordering of tests by anesthesiologists, a practice resulting in the cost of tests ordered to be reduced $112.09 per patient, for a total savings of $1.01 million over the course of the study. At about the same time, 2 other, non-US, studies describe similar work and results related to the selective ordering of tests.15,16 In addition, the national Veterans Affairs Surgical Quality Improvement Program (VASQIP) created reliable data on patient risk factors, surgery process of care, and postsurgical morbidity and mortality rates for major surgical procedures in the 1990s.17 By the turn of the millennium, the Institute of Medicine was defining patient-centered care as care that establishes a partnership between providers and patients and gives patients the support they need to make decisions and participate in their own care.18 This set the stage for a further refinement of the perioperative management concept.
In 2006, the American Society of Anesthesiologists (ASA) Task Force on Future Paradigms in Anesthesia Practice advised anesthesiology training programs to expand their focus beyond the operating room to include perioperative management.19 Some of the impetus for these recommendations was the trend toward new practice arrangements, such as anesthesiologists being employed by hospitals or corporations, and new payment structures, such as bundled payments for surgical procedures, which include quality, safety, satisfaction, and other performance metrics considerations.20 For example, in the United States, the Centers for Medicare & Medicaid Services launched several value-based purchasing programs and created the Bundled Payments for Care Improvement Initiative to enter into payment arrangements with hospitals that included financial and performance accountability for episodes of care.21–23 In addition, there are major initiatives by commercial payers and large employers that focus on bundled care and payments for common and/or costly surgical procedures.24 This trend away from fee-for-service and toward bundled or integrated payments requires integrated and coordinated care, the cornerstones of the PSH model for surgical patients. The alignment of delivery and payment models seeks to enhance operations and logistics, increase efficiencies, and improve quality.25–27
Perhaps having the greatest influence on PCMH and PSH development was the Patient Protection and Affordable Care Act (PPACA) of 2010, in its introduction of accountable care organizations (ACOs), which are generally designed to hold multiple providers jointly accountable for achieving quality targets and reductions in the cost of care per capita for a designated patient population in a health system. Because two-thirds of hospital costs are directly related to surgical care, operating room efficiency is one of the primary objectives of current and future ACO initiatives.28
There is clearly an external environmental push from both regulatory and market forces toward greater care coordination within the perioperative continuum as envisioned by the PSH models. At the same time, recent US studies reveal the environmental difficulties of establishing practice guidelines when implementing the key elements of a comprehensive PSH model.29
The Evolution and History of the PSH Outside the United States
Outside the United States, application of the ERAS guidelines is quite prevalent, and the ERAS guidelines employ some of the principal elements of the more comprehensive PSH models pursued in the United States. In 2009, Eskicioglu and colleagues described ERAS programs as “multimodal perioperative programs that aim to accelerate recovery, shorten hospital stay, and reduce complication rates following surgery.”30
The non-US studies with a preoperative intervention focus that we included examined preoperative counseling; avoidance of mechanical bowel preparation, fasting, and premedication; administration of pre- and probiotics; and preoperative carbohydrate loading.31 The intraoperative interventions included goal-directed fluid management, normothermia, hyperoxia, and tailored analgesia;32 and the postoperative interventions included early routine mobilization, early enteral nutrition, avoidance of nasogastric tubes and peritoneal drains, and early removal of catheters.31
Summary of Specific PSH Studies in the United States and Other Countries
Studies of Preoperative Elements
Figure 1 provides a synopsis of the results of all US and non-US studies of preoperative initiatives that we identified in our review of the literature. Overall, 82% of preoperative studies reported significant positive results; 23 studies measuring cost and 32 reporting clinical outcomes reported significantly positive results associated with preoperative initiatives. Only 1 study of cost and 1 study of clinical outcomes found significant negative results.
Figure 1.
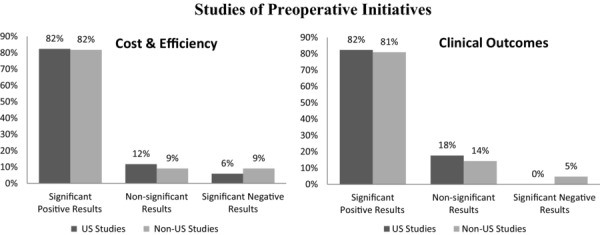
Studies of Preoperative Initiatives Reporting Significant Positive, Nonsignificant, and Negative Results
Research studies from the United States and other countries describe those efforts to engage patients early as being beneficial to both the patient and the health care enterprise, by creating open communication, educating patients to make decisions regarding the process of undergoing surgery, and reducing patients’ unrealistic expectations about surgical outcomes.33–37
An important European study describes the results of preoperative initiatives, such as educating patients with chronic obstructive pulmonary disease (COPD) about postoperative instruction compliance, smoking cessation, and weight reduction.34 Preoperative risk assessment and education are important, as well, to achieve appropriate perioperative management strategies, resulting in optimal outcomes.34 This seminal study by Ergina and colleagues was an impetus for the emphasis on preoperative risk assessment in subsequent patient engagement literature,38,39 having been cited 110 times since its publication in 1993. Besides risk assessment, anesthesiologists are becoming increasingly more engaged in shared decision making processes in the preoperative phase of surgery and beyond, through initiatives like the PSH and presurgical education.38,39
Other large studies have shown preoperative education and counseling to improve surgical outcomes. For example, a study of knee joint arthroplasty patients showed that a preoperative education program significantly reduced their length of stay (LOS) and readmission rates.40 Similar results (reduced readmission rates) were observed in a Canadian study of the impact of a series of coordinated patient education and nutrition programs on colonic surgery outcomes.37 Furthermore, results of a meta-analysis show that short-term preoperative smoking cessation programs decreased the risk of postoperative respiratory complications.41
Because the cost of preoperative testing is high,42 minimizing the number of unnecessary preoperative tests is estimated to reduce nationwide health care costs by at least $10 billion annually in the United States, while improving patients’ experience.43 Researchers outside the United States also have paid increasing attention to the costs of preoperative assessment. A 2005 Canadian study found that anesthesiologists’ selective ordering of preoperative tests decreases the number and overall cost of testing, saving an average of $73 per patient.44 Various other studies have demonstrated that routine testing, rather than selective testing, offers no patient benefits but does drive up costs.43,45,46
Finally, the latest non-US studies reported that abnormal preoperative tests have only a limited ability to predict adverse perioperative outcomes.47 Canadian and European researchers are investigating the possibility of eliminating most preoperative tests without escalating adverse events.42 A European study also has advocated this concept of eliminating preoperative tests for at-risk populations.48
Conversely, though, US studies have found that preoperative evaluations generally were beneficial to surgical outcomes. Recently, Vazirani and colleagues noted that “a structured medical preoperative evaluation may benefit medically complex patients and improve perioperative processes outcomes.”49 The researchers found a significant reduction in LOS for inpatients with an ASA physical status of 3 or higher (p < 0.0001). Among complex patients, medication reconciliation also can improve quality and outcomes, and medication reconciliation has remained one of the Joint Commission's National Patient Safety Goals since 2005.50–55 The PSH can also serve as the primary way of reducing unnecessary interventions that do not benefit patients while also lowering costs and enhancing patient safety by mitigating risks to patients.56 Furthermore, US scholars have demonstrated how specific early patient engagement, such as exercise and targeted evaluations, can improve surgical outcomes.36,57 Other US research has looked at reducing the number of operating room (OR) cancellations, OR turnover time, and surgical case delays as a result of preoperative programs.33,58,59 Finally, a recent US study found evidence of excessive use of preoperative testing for low-risk surgical patients in a national random sample of Medicare claims data.60
Whereas non-US studies primarily examined the feasibility of eliminating selective preoperative testing, US studies investigated factors contributing to excessive preoperative testing by employing provider surveys and evaluating the effects of data-sharing activities.61,62
Studies of Intraoperative Elements
Figure 2 summarizes the results of all studies of intraoperative initiatives included in our review. Some 82% of studies reported significantly positive effects of intraoperative interventions on clinical outcomes and efficiencies or cost reductions. Globally, 17 studies of the cost of surgery and efficiencies and 29 studies of clinical and surgical outcomes reported significantly positive results of intraoperative interventions. Only 3 studies reported negative results.
Figure 2.
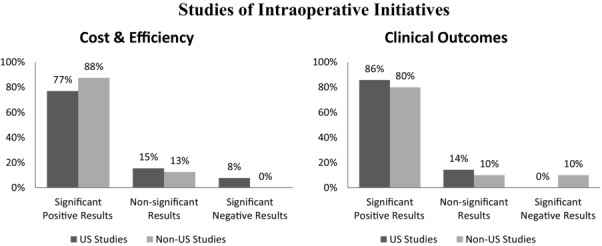
Studies of Intraoperative Initiatives Reporting Significant Positive, Nonsignificant, and Negative Results
Researchers generally agree on the benefits of a PSH model that incorporates intraoperative efforts, such as goal-directed fluid management and process flow and design initiatives, which often lead to lower surgery costs and greater efficiencies.
Real-time patient-routing systems have been found to be effective in reducing OR delays and improving perioperative cost efficiencies.13 For example, the PSH model mitigates the risk of case delays and cancellations by using a real-time electronic dashboard to ensure access to the electronic medical record and the findings from the preoperative assessment, consultation, and treatment clinic.5
A US study examined the influence of parallel perioperative system design on OR throughput.63 The 3-room suite, consisting of a preoperative room, an OR, and an early recovery area, replaced the traditional sequential activities with a parallel design. The new workflow processed more cases per day and used less time for both operative and nonoperative processes. The results demonstrated that a deliberate OR and perioperative process redesign can improve throughput without sacrificing quality.63 Furthermore, preanesthesia clinics can increase efficiencies if they function in close conjunction with the perioperative process.29
These workflow and OR system design results are consistent with studies of the PSH model in other countries. Non-US studies also have reported significant reductions in surgery delays and waiting times and a significant increase in surgeon availability for urgent surgery.64 In general, non-US studies have shown that the PSH model can improve perioperative efficiencies and result in cost savings.
Studies of Postoperative Elements
Figure 3 provides a synopsis of the results of all the studies of postoperative initiatives identified in our comprehensive review. Almost 9 in 10 studies reported significantly positive effects of postoperative interventions on outcomes, and studies with significantly positive effects on clinical outcomes outnumbered studies of efficiencies or cost reductions. Fifteen US and 8 non-US studies reported significantly positive cost and efficiency outcomes of postoperative initiatives, and 33 US and 38 non-US studies reported significantly positive clinical outcomes. Only 5 studies worldwide reported negative results.
Figure 3.
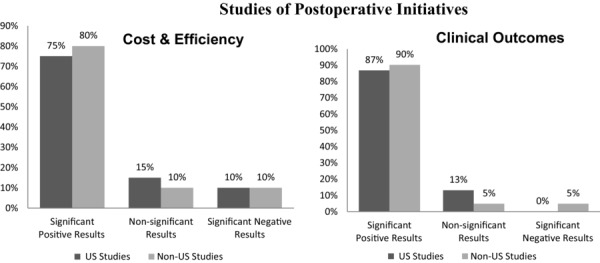
Studies of Postoperative Initiatives Reporting Significant Positive, Nonsignificant, and Negative Results
Currently, the leading postprocedural initiatives globally are the fast-track protocols and ERAS programs, which, based on a recent meta-analysis of randomized trials of colorectal surgery, reduce postoperative complications, LOS, and associated costs.30
Fast-track surgery was introduced in Denmark in the early 1990s to improve patient recovery and reduce LOS.65,66 The fast-track protocols have been integral to ERAS programs, which were developed to decrease complications and hasten patient recovery, by promoting early discharge37,40,42 and shorter postoperative hospital stays.67–70 Numerous studies have reported that ERAS programs reduce hospital LOS.62–65,67
The ERAS initiative has gained increasing support since its introduction. The Enhanced Recovery Partnership Programme in the United Kingdom highlights the necessity of consistently tracking measures of patient outcomes and standardized ERAS protocols,68 and the ERAS Society recently published 3 new and updated surgery-specific guidelines.71–73
These ERAS protocols are gradually becoming more accepted by the US surgical community and researchers, and they have achieved similar outcomes in reducing postoperative complications and overall perioperative costs. For example, the Blue Cross Blue Shield Cardiovascular Consortium of Efforts decreased hospital mortality by 25% in the first 4 years,74 and the Keystone Collaborative lowered the number of bloodstream-associated infections by 66% in 2 years, at an estimated cost savings of $160 million.74 Hospitals participating in the Michigan Surgical Quality Collaborative saw fewer complications and less morbidity after introducing the ERAS program.75 Note, though, that the successes reported in Michigan or other states and regions may be affected by local culture and payment models not present in all US states.
New and evolving PSH models are being implemented in the United States that apply the ERAS protocols and guidelines, such as the PSH programs at the University of Alabama at Birmingham and the University of California, Irvine.5,76 Scholars agree that ERAS initiatives can reduce postoperative complications and lower medical expenditures. Furthermore, both US and non-US health services researchers are interested in the unique role of the anesthesiologist in postoperative care initiatives.77 As part of a more comprehensive PSH program, successful ERAS implementation requires multidisciplinary support to overcome any hurdles along the way.78
Limitations
Our comprehensive review of PSH programs has 2 potential limitations: (1) publication bias and (2) the lack of study design inclusion criteria. Publication bias is always a concern with literature reviews, since negative study results often are not reported or are published less frequently than positive study results are. Also, to date, most implemented PSH models are characterized as new and promising change initiatives by early adopters, who most likely approach the PSH study design looking for measurable benefits and not adverse effects. We also did not use in our inclusion and exclusion criteria the type of study design, study sample, nature of outcomes and cost metrics, or setting.
Conclusion
Studies of evolving PSH model elements have consistently emphasized opportunities for reducing unnecessary preoperative tests, which could significantly reduce surgery-related costs without affecting clinical outcomes. In general, the international literature reports consistent and significant positive findings related to perioperative management. Many US hospitals have considerable opportunities to improve surgical cost efficiencies using PSH strategies in accordance with surgical specialty.4
The PSH model may have significant implications for policymakers, payers, administrators, clinicians, and patients. Certainly, policy-relevant cost-saving and quality improvement potential is apparent in all 3 PSH phases of care, as defined in Table 1. Their importance is obvious for integrated care, bundled payment, and value-based purchasing-related policies. The relevant roles of various health care providers in the PSH are evolving, with significant evidence of a greater role for anesthesiologists in coordination across the 3 phases of surgical care and all elements of the PSH. Finally, the PSH may have the greatest impact on the patient-centered arena, by both preparing patients for surgery and ensuring their safe and effective transition to home or other postoperative rehabilitation.
There are some subtle differences between US and non-US research on the PSH. The literature in non-US settings seems to focus on strict comparison of the outcomes of changing policies or practices, whereas in the United States, research seems to focus more on discovering innovative practice models and other, less direct changes, like information technology, that may be contributing to the evolution toward the PSH model.
Although the PSH is a nascent perioperative practice model,38 it is worth considering the future of PSH implementation, just as researchers and practitioners are considering the next generation of the PCMH.79 For the PSH to evolve successfully, there will likely be several milestones in its development:
Early PSH models are disseminated and implemented effectively. As noted by Vetter and colleagues, “Dissemination and implementation of a PSH model will require that its broad set of stakeholders want it (‘pull’) and that systematic, organizational efforts help adopters apply (‘push’) this innovation.”80
PSH implementers lead in the development and acceptance of meaningful perioperative quality and outcome measures and, more importantly, in improving the fidelity of these quality measures.81,82
PSH implementers lead in the adoption of evidence-based perioperative guidelines and protocols, and work to reduce surgical variation resulting from nonpatient differences.39,83
PSHs are formally recognized and/or accredited based on accepted standards and levels of development, similar to the recognition and accreditation process for PCMHs.
The successful evolution of the PSH will require the concomitant expansion of perioperative clinicians’ roles in providing leadership in the organizational change represented by the PSH and in other transformative initiatives undertaken by the hospital or health system to help realize the Triple Aim: to improve care quality, patient satisfaction, and population health while reducing the cost of health care.80 Anesthesiologists, hospitalists, surgeons, and nurses will need to be actively involved in organization-wide strategy development and initiatives to improve care quality and the health systems's financial position. As noted by the American College of Physician Executives, the top 5 hospitals ranked by US News & World Report in 2013 were led by physicians.84 Accordingly, the PSH may represent the beginning of a long, collaborative journey for many physicians and health systems.
Acknowledgments
The authors thank Tiffany Radcliff, Thomas Vetter, Rebecca Wells, and Larry Gamm for their guidance and for reviewing drafts of this manuscript. The authors also thank the anonymous reviewers for their comments and suggestions on original drafts.
Funding/Support
The preparation of this article is based on work supported by the National Science Foundation (NSF) under grant no. IIP-0832439. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation. This project was cofunded by NSF's Center for Health Organization Transformation's industry members, including the American Society of Anesthesiologists.
Conflict of Interest Disclosures
All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. No disclosures were reported.
Policy Points
The perioperative surgical home (PSH) is complementary to the patient-centered medical home (PCMH) and defines methods for improving the patient experience and clinical outcomes, and controlling costs for the care of surgical patients.
The PSH is a physician-led care delivery model that includes multispecialty care teams and cost-efficient use of resources at all levels through a patient-centered, continuity of care delivery model with shared decision making.
The PSH emphasizes “prehabilitation” of the patient before surgery, intraoperative optimization, improved return to function through follow-up, and effective transitions to home or post-acute care to reduce complications and readmissions.
Supplementary Material
Additional supporting information may be found in the online version of this article at http://onlinelibrary.wiley.com/journal/10.1111/(ISSN)1468-0009:
Review Articles
Appendix Table 2A: US Original Studies
Appendix Table 3A: International Original Studies
References
- 1.National Center for Health Statistics. Health, United States, 2012: With Special Feature on Emergency Care. Hyattsville, MD: National Center for Health Statistics; 2013. [PubMed] [Google Scholar]
- 2.Reed WA, Ford JL. Development of an independent outpatient surgical center. Int Anesthesiol Clin. 1976;14(2):113–130. doi: 10.1097/00004311-197614020-00007. [DOI] [PubMed] [Google Scholar]
- 3.Robinson JC. Case studies of orthopedic surgery in California: the virtues of care coordination versus specialization. Health Aff (Millwood) 2013;32(5):921–928. doi: 10.1377/hlthaff.2012.1119. May. [DOI] [PubMed] [Google Scholar]
- 4.Miller DC, Gust C, Dimick JB, Birkmeyer N, Skinner J, Birkmeyer JD. Large variations in Medicare payments for surgery highlight savings potential from bundled payment programs. Health Aff (Millwood) 2011;30(11):2107–2115. doi: 10.1377/hlthaff.2011.0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vetter TR, Goeddel LA, Boudreaux AM, Hunt TR, Jones KA, Pittet J-F. The perioperative surgical home: how can it make the case so everyone wins? BMC Anesthesiol. 2013;13(1):6. doi: 10.1186/1471-2253-13-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee A, Kerridge RK, Chui PT, Chiu CH, Gin T. Perioperative systems as a quality model of perioperative medicine and surgical care. Health Policy. 2011;102(2):214–222. doi: 10.1016/j.healthpol.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Pourat N, Lavarreda SA, Snyder S. Patient-centered medical homes improve care for adults with chronic conditions. Los Angeles, CA: UCLA Center for Health Policy Research; 2013. [PubMed] [Google Scholar]
- 8.The patient centered medical home: history, seven core features, evidence and transformational change. Washington, DC: Robert Graham Center; 2007. Robert Graham Center. [Google Scholar]
- 9.Pasternak LR. Preoperative testing: moving from individual testing to risk management. Anesth Analg. 2009;108(2):393–394. doi: 10.1213/ane.0b013e31819278ea. [DOI] [PubMed] [Google Scholar]
- 10.Warner M. Surgical Home. ASA Newsletter: Academic Anesthesia. 2012;76(5):30–32. [Google Scholar]
- 11.Miles MB, Huberman AM. Qualitative Data Analysis: An Expanded Sourcebook. Thousand Oaks, CA: Sage; 1994. [Google Scholar]
- 12.Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–1288. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- 13.Rotondi AJ, Brindis C, Cantees KK. Benchmarking the perioperative process. I. Patient routing systems: a method for continual improvement of patient flow and resource utilization. J Clin Anesth. 1997;9(2):159–169. doi: 10.1016/s0952-8180(96)00242-5. [DOI] [PubMed] [Google Scholar]
- 14.Fischer SP. Development and effectiveness of an anesthesia preoperative evaluation clinic in a teaching hospital. Anesthesiology. 1996;85(1):196–206. doi: 10.1097/00000542-199607000-00025. [DOI] [PubMed] [Google Scholar]
- 15.Finegan B. Preadmission and outpatient consultation clinics. Can J Anaesth. 1992;39(10):1009–1011. doi: 10.1007/BF03008367. [DOI] [PubMed] [Google Scholar]
- 16.Kerridge R, Lee A, Latchford E, Beehan S, Hillman K. The perioperative system: a new approach to managing elective surgery. Anaesth Intensive Care. 1995;23(5):591–596. doi: 10.1177/0310057X9502300511. [DOI] [PubMed] [Google Scholar]
- 17.Khuri SF, Daley J, Henderson W. The Department of Veterans Affairs’ NSQIP: the first national, validated, outcome-based, risk-adjusted, and peer-controlled program for the measurement and enhancement of the quality of surgical care (National VA Surgical Quality Improvement Program) Ann Surg. 1998;228(4):491–507. doi: 10.1097/00000658-199810000-00006. http://journals.lww.com/annalsofsurgery/Abstract/1998/10000/The_Department_of_Veterans_Affairs__NSQIP__The.6.aspx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oates J, Weston WW, Jordan J. The impact of patient-centered care on outcomes. Fam Pract. 2000;49:796–804. [PubMed] [Google Scholar]
- 19.Fleisher LA, Beckman JA, Brown KA. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology / American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) Circulation. 2007;116(17):e418–e499. doi: 10.1161/CIRCULATIONAHA.107.185699. [DOI] [PubMed] [Google Scholar]
- 20.Johnstone RE. Anesthesia Practice Trends: What Is Changing? What Does It Mean? Morgantown, WV: American Society of Anesthesiologists; 2012. [Google Scholar]
- 21.Froimson MI, Rana A, White RE., Jr Bundled payments for care improvement initiative: the next evolution of payment formulations: AAHKS bundled payment task force. J Arthroplasty. 2013;28(8):157–165. doi: 10.1016/j.arth.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 22.Tompkins CP, Higgins AR, Ritter GA. Measuring outcomes and efficiency in Medicare value-based purchasing. Health Aff (Millwood) 2009;28(2):w251–w261. doi: 10.1377/hlthaff.28.2.w251. [DOI] [PubMed] [Google Scholar]
- 23.Van Lare JM, Conway PH. Value-based purchasing—national programs to move from volume to value. N Engl J Med. 2012;367(4):292–295. doi: 10.1056/NEJMp1204939. [DOI] [PubMed] [Google Scholar]
- 24.Commerical Bundled Payment Tracker. Washington, DC: Advisory Board Company; 2014. Advisory Board Company. [Google Scholar]
- 25.Cutler DM, Ghosh K. The potential for cost savings through bundled episode payments. N Engl J Med. 2012;366(12):1075–1077. doi: 10.1056/NEJMp1113361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mechanic RE, Altman SH. Payment reform options: episode payment is a good place to start. Health Aff (Millwood) 2009;28(2):w262–w271. doi: 10.1377/hlthaff.28.2.w262. [DOI] [PubMed] [Google Scholar]
- 27.Mechanic RE. Opportunities and challenges for episode-based payment. N Engl J Med. 2011;365(9):777–779. doi: 10.1056/NEJMp1105963. [DOI] [PubMed] [Google Scholar]
- 28.Johnstone RE. 59th Annual American Urological Association Meeting. Cleveland, OH: 2012. [Google Scholar]
- 29.Newman MF, Mathew JP, Aronson S. The evolution of anesthesiology and perioperative medicine. Anesthesiology. 2013;118(5):1005–1007. doi: 10.1097/ALN.0b013e31828ea5cb. [DOI] [PubMed] [Google Scholar]
- 30.Eskicioglu C, Forbes SS, Aarts MA, Okrainec A, McLeod RS. Enhanced recovery after surgery (ERAS) programs for patients having colorectal surgery: a meta-analysis of randomized trials. J Gastrointest Surg. 2009:2321–2329. doi: 10.1007/s11605-009-0927-2. 13(12) [DOI] [PubMed] [Google Scholar]
- 31.Wind J, Polle S, Fung Kon Jin P. Systematic review of enhanced recovery programmes in colonic surgery. Br J Surg. 2006;93(7):800–809. doi: 10.1002/bjs.5384. [DOI] [PubMed] [Google Scholar]
- 32.Fearon K, Ljungqvist O, Von Meyenfeldt M. Enhanced recovery after surgery: a consensus review of clinical care for patients undergoing colonic resection. Clin Nutr. 2005;24(3):466–477. doi: 10.1016/j.clnu.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Ferschl MB, Tung A, Sweitzer B, Huo D, Glick DB. Preoperative clinic visits reduce operating room cancellations and delays. Anesthesiology. 2005;103(4):855–859. doi: 10.1097/00000542-200510000-00025. [DOI] [PubMed] [Google Scholar]
- 34.Ergina PL, Gold SL, Meakins JL. Perioperative care of the elderly patient. World J Surg. 1993;17(2):192–198. doi: 10.1007/BF01658926. [DOI] [PubMed] [Google Scholar]
- 35.Knox M, Myers E, Wilson I, Hurley M. The impact of pre-operative assessment clinics on elective surgical case cancellations. The Surgeon. 2009;7(2):76–78. doi: 10.1016/s1479-666x(09)80019-x. [DOI] [PubMed] [Google Scholar]
- 36.Leichtle SW, Mouawad NJ, Welch KB, Lampman RM, Cleary RK. Risk factors for anastomotic leakage after colectomy. Dis Colon Rectum. 2012;55(5):569. doi: 10.1097/DCR.0b013e3182423c0d. [DOI] [PubMed] [Google Scholar]
- 37.Carli F, Charlebois P, Baldini G, Cachero O, Stein B. An integrated multidisciplinary approach to implementation of a fast-track program for laparoscopic colorectal surgery. Can J Anaesth. 2009;56(11):837–842. doi: 10.1007/s12630-009-9159-x. [DOI] [PubMed] [Google Scholar]
- 38.Kain ZN, Vakharia S, Garson L. The perioperative surgical home as a future perioperative practice model. Anesth Analg. 2014;118(5):1126–1130. doi: 10.1213/ANE.0000000000000190. [DOI] [PubMed] [Google Scholar]
- 39.Garson L, Schwarzkopf R, Vakharia S. Implementation of a total joint replacement-focused perioperative surgical home: a management case report. Anesth Analg. 2014;118(5):1081–1089. doi: 10.1213/ANE.0000000000000191. R1010.1213/ANE.0000000000000191. [DOI] [PubMed] [Google Scholar]
- 40.Jones S, Alnaib M, Kokkinakis M, Wilkinson M, Gibson ASC, Kader D. Pre-operative patient education reduces length of stay after knee joint arthroplasty. Ann R Coll Surg Engl. 2011;93(1):71. doi: 10.1308/003588410X12771863936765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wong J, Lam D, Abrishami A, Chan MV, Chung F. Short-term preoperative smoking cessation and postoperative complications: a systematic review and meta-analysis. Can J Anaesth. 2012;59(3):268–279. doi: 10.1007/s12630-011-9652-x. [DOI] [PubMed] [Google Scholar]
- 42.Chung F, Yuan H, Yin L, Vairavanathan S, Wong DT. Elimination of preoperative testing in ambulatory surgery. Anesth Analg. 2009;108(2):467–475. doi: 10.1213/ane.0b013e318176bc19. [DOI] [PubMed] [Google Scholar]
- 43.Brown SR, Brown J. Why do physicians order unnecessary preoperative tests? A qualitative study. Fam Med–Kansas City. 2011;43(5):338. [PubMed] [Google Scholar]
- 44.Finegan BA, Rashiq S, McAlister FA, O'Connor P. Selective ordering of preoperative investigations by anesthesiologists reduces the number and cost of tests. Can J Anaesth. 2005;52(6):575–580. doi: 10.1007/BF03015765. [DOI] [PubMed] [Google Scholar]
- 45.Mantha S, Roizen MF, Madduri J, Rajender Y, Shanti Naidu K, Gayatri K. Usefulness of routine preoperative testing: a prospective single-observer study. J Clin Anesth. 2005;17(1):51–57. doi: 10.1016/j.jclinane.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 46.Kaplan EB, Sheiner LB, Boeckmann AJ. The usefulness of preoperative laboratory screening. JAMA. 1985;253(24):3576–3581. [PubMed] [Google Scholar]
- 47.Fritsch G, Flamm M, Hepner D, Panisch S, Seer J, Soennichsen A. Abnormal pre-operative tests, pathologic findings of medical history, and their predictive value for perioperative complications. Acta Anaesthesiol Scand. 2012;56(3):339–350. doi: 10.1111/j.1399-6576.2011.02593.x. [DOI] [PubMed] [Google Scholar]
- 48.Aarts M-A, Okrainec A, Glicksman A, Pearsall E, Victor JC, McLeod RS. Adoption of enhanced recovery after surgery (ERAS) strategies for colorectal surgery at academic teaching hospitals and impact on total length of hospital stay. Surg Endosc. 2012;26(2):442–450. doi: 10.1007/s00464-011-1897-5. [DOI] [PubMed] [Google Scholar]
- 49.Vazirani S, Lankarani-Fard A, Liang LJ, Stelzner M, Asch SM. Perioperative processes and outcomes after implementation of a hospitalist-run preoperative clinic. J Hosp Med. 2012;7(9):697–701. doi: 10.1002/jhm.1968. [DOI] [PubMed] [Google Scholar]
- 50.Burda S, Hobson D, Pronovost P. What is the patient really taking? Discrepancies between surgery and anesthesiology preoperative medication histories. Quality Safety Health Care. 2005;14(6):414–416. doi: 10.1136/qshc.2005.014738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cornish PL, Knowles SR, Marchesano R. Unintended medication discrepancies at the time of hospital admission. Arch Intern Med. 2005;165(4):424–429. doi: 10.1001/archinte.165.4.424. [DOI] [PubMed] [Google Scholar]
- 52.Leung V, Mach K, Charlesworth E, Hicks S, Kizemchuk K, Stumpo C. Perioperative Medication Management (POMM) pilot: integrating a community-based medication history (MedsCheck) into medication reconciliation for elective orthopedic surgery inpatients. Can.Pharmacists J. 2010;143(2):82–87. [Google Scholar]
- 53.Tam VC, Knowles SR, Cornish PL, Fine N, Marchesano R, Etchells EE. Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review. Can Med Assoc J. 2005;173(5):510–515. doi: 10.1503/cmaj.045311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Joint Commission. Oakbrook Terrace, IL: Joint Commission; 2014. January 1, 2014. National Patient Safety Goals Effective. [Google Scholar]
- 55.van den Bemt PM, van den Broek S, van Nunen AK, Harbers JB, Lenderink AW. Medication reconciliation performed by pharmacy technicians at the time of preoperative screening. Ann Pharmacother. 2009;43(5):868–874. doi: 10.1345/aph.1L579. [DOI] [PubMed] [Google Scholar]
- 56.Dexter F, Wachtel RE. Strategies for net cost reductions with the expanded role and expertise of anesthesiologists in the perioperative surgical home. Anesth Analg. 2014;118(5):1062–1071. doi: 10.1213/ANE.0000000000000173. [DOI] [PubMed] [Google Scholar]
- 57.Mayo NE, Feldman L, Scott S. Impact of preoperative change in physical function on postoperative recovery: argument supporting prehabilitation for colorectal surgery. Surgery. 2011;150(3):505–514. doi: 10.1016/j.surg.2011.07.045. [DOI] [PubMed] [Google Scholar]
- 58.Correll DJ, Bader AM, Hull MW, Hsu C, Tsen LC, Hepner DL. Value of preoperative clinic visits in identifying issues with potential impact on operating room efficiency. Anesthesiology. 2006;105(6):1254–1259. doi: 10.1097/00000542-200612000-00026. [DOI] [PubMed] [Google Scholar]
- 59.Glick DB, Tung A, Ferschl MB, Sweitzer B. Anesthesia preoperative medicine clinic: beyond surgery cancellations. Anesthesiology. 2006;105(1):225–226. doi: 10.1097/00000542-200607000-00039. [DOI] [PubMed] [Google Scholar]
- 60.Thilen S, Treggiari M, Weaver E. An opportunity for anesthesiologists to add value by controlling preoperative resources. Orlando, FL: Paper presented at: ASA Practice Management Meeting; 2012. [Google Scholar]
- 61.Huesch MD. Are there always synergies between productive resources and resource deployment capabilities. Strategic Manage J. 2013;34(11):1288–1313. [Google Scholar]
- 62.Frost EA. Integrating perioperative information from divergent sources. Mount Sinai J Med. 2012;79(1):3–12. doi: 10.1002/msj.21282. [DOI] [PubMed] [Google Scholar]
- 63.Sandberg WS, Daily B, Egan M. Deliberate perioperative systems design improves operating room throughput. Anesthesiology. 2005;103(2):406–418. doi: 10.1097/00000542-200508000-00025. [DOI] [PubMed] [Google Scholar]
- 64.Cosgrove J, Gaughan M, Snowden C, Lees T. Decreasing delays in urgent and expedited surgery in a university teaching hospital through audit and communication between perioperative and surgical directorates. Anaesthesia. 2008;63(6):599–603. doi: 10.1111/j.1365-2044.2008.05441.x. [DOI] [PubMed] [Google Scholar]
- 65.Kehlet H MogensenT. Hospital stay of 2 days after open sigmoidectomy with a multimodal rehabilitation programme. Br J Surg. 1999;86(2):227–230. doi: 10.1046/j.1365-2168.1999.01023.x. http://onlinelibrary.wiley.com/doi/10.1046/j.1365-2168.1999.01023.x/abstract. [DOI] [PubMed] [Google Scholar]
- 66.White PF, Kehlet H, Neal JM. The role of the anesthesiologist in fast-track surgery: from multimodal analgesia to perioperative medical care. Anesth Analg. 2007;104(6):1380–1396. doi: 10.1213/01.ane.0000263034.96885.e1. [DOI] [PubMed] [Google Scholar]
- 67.Sammour T, Zargar-Shoshtari K, Bhat A, Kahokehr A, Hill AG. A programme of Enhanced Recovery After Surgery (ERAS) is a cost-effective intervention in elective colonic surgery. N Zealand Med J. 2010;123(1319):61–70. [PubMed] [Google Scholar]
- 68.Knott A, Pathak S, McGrath JS. Consensus views on implementation and measurement of enhanced recovery after surgery in England: Delphi study. BMJ Open. 2012;2(6) doi: 10.1136/bmjopen-2012-001878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ren L, Zhu D, Wei Y. Enhanced Recovery After Surgery (ERAS) program attenuates stress and accelerates recovery in patients after radical resection for colorectal cancer: a prospective randomized controlled trial. World J Surg. 2012;36(2):407–414. doi: 10.1007/s00268-011-1348-4. [DOI] [PubMed] [Google Scholar]
- 70.Ramirez JM, Blasco JA, Roig JV. Enhanced recovery in colorectal surgery: a multicentre study. BMC Surg. 2011;11:9. doi: 10.1186/1471-2482-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lassen K, Coolsen MM, Slim K. Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr. 2012;31:817–830. doi: 10.1016/j.clnu.2012.08.011. [DOI] [PubMed] [Google Scholar]
- 72.Gustafsson U, Scott M, Schwenk W. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr. 2012;31:783–800. doi: 10.1016/j.clnu.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 73.2013. ERAS Society. ERAS guidelines. http://www.erassociety.org/index.php/eras-guidelines. Accessed August 23, 2014.
- 74.Campbell DA., Jr Quality improvement is local. J Am Coll Surg. 2009;209(1):141–143. doi: 10.1016/j.jamcollsurg.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 75.Campbell DA, Jr, Englesbe MJ, Kubus JJ. Accelerating the pace of surgical quality improvement: the power of hospital collaboration. Arch Surg. 2010;145(10):985. doi: 10.1001/archsurg.2010.220. [DOI] [PubMed] [Google Scholar]
- 76.Vakharia SB. Irvine, CA: University of California; 2013. Urology surgical home a transformative model of perioperative care. [Google Scholar]
- 77.Kahokehr A, Sammour T, Zargar-Shoshtari K, Thompson L, Hill AG. Implementation of ERAS and how to overcome the barriers. Int J Surg. 2009;7(1):16–19. doi: 10.1016/j.ijsu.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 78.Melnyk M, Casey RG, Black P, Koupparis AJ. Enhanced Recovery After Surgery (ERAS) protocols: time to change practice. Can Urological Assoc J. 2011;5(5):342. doi: 10.5489/cuaj.11002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Keckley PH. Medical home 2.0: the present, the future. Washington, DC: Deloitte Center for Health Solutions; 2010. [Google Scholar]
- 80.Vetter TR, Boudreaux AM, Jones KA, Hunter Jr JM, Pittet J-F. The perioperative surgical home: how anesthesiology can collaboratively achieve and leverage the Triple Aim in health care. Anesth Analg. 2014;118(5):1131–1136. doi: 10.1213/ANE.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 81.Hawkins RB, Levy SM, Senter CE. Beyond surgical care improvement program compliance: antibiotic prophylaxis implementation gaps. Am J Surg. 2013;206(4):451–456. doi: 10.1016/j.amjsurg.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 82.Levy SM, Senter CE, Hawkins RB. Implementing a surgical checklist: more than checking a box. Surgery. 2012;152(3):331–336. doi: 10.1016/j.surg.2012.05.034. [DOI] [PubMed] [Google Scholar]
- 83.Birkmeyer JD, Reames BN, McCulloch P, Carr AJ, Campbell WB, Wennberg JE. Understanding of regional variation in the use of surgery. Lancet. 2013;382(9898):1121–1129. doi: 10.1016/S0140-6736(13)61215-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Angood P, Birk S. The value of physician leadership. Tampa, FL: American College of Physician Executives; 2014. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Review Articles
Appendix Table 2A: US Original Studies
Appendix Table 3A: International Original Studies


