Abstract
Aims: A few studies have suggested a relationship between thyroid hormones and alcohol dependence (AD) such as a blunted increase of thyroid stimulating hormone (TSH) in response to thyrotropin-releasing hormone (TRH), lower levels of circulating free triiodothyronine (fT3) and free thyroxine (fT4) levels and down regulation of the TRH receptors. The current study aimed to explore the relationship between the hormones of the thyroid axis and alcohol-seeking behaviors in a sample of alcohol-dependent patients. Methods: Forty-two treatment-seeking alcohol-dependent individuals enrolled in a 12-week treatment study were considered. The Timeline Follow Back (TLFB) was used to assess the number of drinks consumed during the 12-week period. Blood levels of thyroid hormones (TSH, fT3 and fT4) were measured prior to and at the end of treatment. Questionnaires were administered to evaluate craving for alcohol [Penn Alcohol Craving Scale (PACS) and the Obsessive Compulsive Drinking Scale (OCDS) and its two subscales ODS for obsessions and CDS for compulsions] as well as anxiety [State and Trait Inventory (STAI)], depression [the Zung Self-Rating Depression Scale (Zung)] and aggression [the Aggressive Questionnaire (AQ)]. Results: At baseline, we found significant positive correlations between fT3 and OCDS (r = 0.358, P = 0.029) and CDS (r = 0.405, P = 0.013) and negative correlations between TSH levels and STAI (r = −0.342, P = 0.031), and AQ (r = −0.35, P = 0.027). At the end of the 12-week study period, abstinent patients had a greater change in TSH than those who relapsed (−0.4 vs. −0.25, F(1,24) = 5.4, P = 0.029). Conclusion: If confirmed in larger samples, these findings could suggest that the thyroid axis might represent a biomarker of alcohol craving and drinking.
INTRODUCTION
Alcohol dependence (AD) represents one of the leading causes of mortality and morbidity in the USA (Centers for disease control and prevention, 2010). Twenty-five percent of adults in the USA report either currently having alcohol-related problems or drinking patterns that put them at risk for developing problems (National Institute on Alcohol Abuse and Alcoholism, 1995). However, Food and Drug Administration (FDA)-approved treatment options for AD remain limited and exhibit inconsistent efficacy. This has created a need to investigate new pharmacological alternatives including topiramate, baclofen, ondansetron, gabapentin, varenicline, pregabalin, nalmefene and others (for review, see Aubin and Daeppen, 2013). Therefore, a better understanding of the biological circuits that may be involved in the development of maintenance of AD is critical in order to identify and develop more effective treatments. To that end, several neuroendocrine pathways have been investigated and suggested as important biological factors or modulators that may play a role in the neurobiology of AD (Kenna et al., 2012).
Both animal and human studies have suggested a link between the hypothalamus–pituitary–thyroid (HPT) axis and AD (Hermann et al., 2002). For example, in Sprague-Dawley rats, chronic (i.e. 4 weeks) exposure to ethanol blunted thyrotropic response to cold exposure and resulted in higher levels of TRH mRNA in the paraventricular nucleus (PVN) of the hypothalamus (Zoeller et al., 1996). Additionally, an alcohol-containing diet was found to be associated with lower levels of fT3, fT4 and basal TSH levels in adult male rats compared with those fed with a non-alcohol-containing isocaloric diet (Mason et al., 1988). This observation was thought to be associated with the selective inhibition of the 5′II deiodinase by ethanol in the amygdala, where this enzyme is responsible for the conversion of T4 to T3 and its inactivation to T2 (Baumgartner et al., 1997, 1998).
Individuals with AD have a blunted increase of TSH levels in response to TRH, especially in early withdrawal, and with more severe symptoms (Välimäki et al., 1984; Baumgartner et al., 1994; Pienaar et al., 1995). This correlates with lower levels of circulating fT3 and fT4 levels (Hegedüs et al., 1988). Notably, these endocrine alterations return to normal after abstinence but recur with alcohol relapses (Liappas et al., 2006), and are thought to be mediated by the downregulation of TRH receptors (Rachdaoui and Sarkar, 2013). Consistent with this evidence, dopamine—a key neurotransmitter involved in the neurobiology of alcohol reward processing and alcohol-seeking behaviors—modulates TSH levels (Delitala et al., 1981; Cooper et al., 1983). While these studies support the involvement of the HPT axis in AD during withdrawal, the role of thyroid hormones on alcohol craving and drinking has not been fully elucidated. Previously, our group reported preliminary findings that suggested a relationship between thyroid hormones and alcohol craving (Leggio et al., 2008). Specifically, we found that TSH levels inversely correlated with measures of alcohol craving as well as psychometric variables such as anxiety, depression, and aggressiveness, and that fT3 positively correlated with some alcohol craving measurements. However, there were significant limitations in that study, namely the very small sample (42 subjects) and the fact that the post-treatment data (after 12 weeks of treatment) were only collected in those patients who maintained total alcohol abstinence. As such, that study was not able to address the question of whether results might be different in abstinent vs. non-abstinent patients. Additionally, all patients were treated off-label with baclofen for AD; the presence of a pharmacological treatment might have represented a potential confounder, since it has been demonstrated that by reducing anxiety (Drake et al., 2003) baclofen may be effective in suppressing alcohol withdrawal symptoms and lessening craving in AD patients (for reviews, see: Addolorato et al., 2009; Leggio et al., 2010). The study did not include a placebo condition to permit control for the medication condition. The limitations of the previous study (Leggio et al., 2008) were taken into consideration in this replication study where we further explored the putative relationship between the HPT axis and alcohol craving and drinking.
SUBJECTS AND METHODS
This study was conducted at the Alcoholism Treatment Unit of the Institute of Internal Medicine, ‘Agostino Gemelli’ Hospital, Catholic University of Rome (Italy), with the approval of the local Ethics Committee of the Catholic University of Rome, and performed according to the ethical standards Declaration of Helsinki of 1975, as revised in 1983. Study subjects signed an informed consent prior to participation.
Subjects
This study was part of the pilot Psychoneuroendocrinology Project (PNP), that aimed to explore secondary endocrine-related outcomes from subjects who participated in the Baclofen Intervention Study (BIS) (EudraCT Number: 2006-000713-37), a 12-week 3-arm double-blind placebo-controlled study with two doses of baclofen (10 mg t.i.d. or 20 mg t.i.d.) or placebo in treatment-seeking outpatient AD individuals diagnosed using the Diagnostic and Statistical Manual (DSM-) IV TR. Details of the parent study have been reported elsewhere (Addolorato et al., 2011; Leggio et al., 2012). Briefly, out of 94 subjects screened (inclusion/exclusion criteria are in Table 1), 42 AD patients (median age: 44 (IQR: 13), males: 76%) were enrolled. A 72-h period of complete alcohol abstinence was required for all subjects prior to initial assessment, during which subjects were monitored daily, and given benzodiazepines if needed for significant withdrawal symptoms; no benzodiazepines were administered at any other point in the study. During the study, the number of standard alcohol drinks consumed over the 28 days preceding the screening visit and between visits was recorded using the Timeline Follow Back (TLFB) method (Sobell et al., 1988). Additionally, alcohol abstinence was assessed using blood alcohol levels at each visit, as well as markers of alcohol use, including aspartate aminotransferase (AST), alanine amino transferase (ALT), gamma glutamyltransferase (GGT) and mean cellular volume (MCV).
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
|
|
DSM IV, Diagnostic and Statistical Manual of Mental Disorders; HDD, heavy drinking days; AST, aspartate aminotransferase; ALT, alanine aminotransferase; UNL, upper normal limit; AD, alcohol dependence.
aHDD is an overall intake of five or more drinks per day for men and of four or more drinks per day for women.
bWhile cannabis use was not an exclusion criterion, none of the included subjects tested positive for marijuana.
Procedures
Blood levels of thyroid hormones (TSH, fT3 and fT4) were assessed at baseline on the enrolment day (Week 01) and on the last day of the study (Week 12) by standardized techniques. Blood samples were collected at about 8 a.m., after an overnight food fast. Along with each blood collection, subjects were administered the Penn Alcohol Craving Scale (PACS) and the Obsessive Compulsive Drinking Scale (OCDS) to measure alcohol craving. The ‘appetitive urge’ and ‘emotional–motivational state’ aspects of craving are best measured with the PACS (Flannery et al., 2003), whereas the OCDS better assesses the obsessive and compulsive components of craving through its two subscales ODS for obsession and CDS for compulsion (Anton et al., 1995; Janiri et al., 2004). Both the OCDS total score, and the ODS and CDS subscales were evaluated separately. Psychometric variables pertaining to anxiety and depression were also evaluated. Specifically, the State and Trait Inventory (STAI) test was used to assess the state (STAI-Y1) and trait (STAI-Y2) components of anxiety (Spielberg et al., 1983). The Zung Self-Rating Depression Scale (Zung-SDS) was used to assess depressive mood (Zung et al., 1965) and the Aggressive Questionnaire (AQ) for aggression (Buss and Perry, 1992).
Statistical analysis
Data from all 42 subjects enrolled were analyzed. All data were expressed as mean (M) ± Standard Deviation (SD) and categorical data as percentages. Normal distribution of data was tested with the Kolmogoroff–Smirnov test. Statistical significance was accepted if a P-value <0.05 (two-sided) was obtained. Mean thyroid hormone levels at week 00 and week 12 were compared using t-tests for paired samples. Correlations between baseline hormone levels and corresponding craving or psychometric scales were assessed using Pearson's correlation coefficients. After completion of the 12-week study, and controlling for medication condition, the correlations between the changes in hormone levels and corresponding craving or psychometric scales were also evaluated.
We analyzed two subsets within our sample based on whether the subjects had remained abstinent throughout the study or had used alcohol. Using analysis of co-variance (ANCOVA) controlling for medication condition, the abstinent and the non-abstinent groups were compared for changes in the hormone levels between the time of enrolment and the end of the study.
Finally, using analysis of variance (ANOVA), the data collected throughout the study were analyzed to assess for baclofen-related dose-dependent variation in hormone blood levels.
Due to the exploratory nature and the small number of subjects enrolled in the study, we did not adjust for multiple comparisons to reduce the type I error in accordance with recommendations from both Rothman (1990) and Perneger (1998), as deflating the type I error for null associations increases the type II error for those associations that are not null (Rothman, 1990). Statistical analysis was performed using STATA version 11.2 (Stata, 2009).
RESULTS
Association between thyroid hormone levels and craving or psychometric scales at baseline and at the end of the study
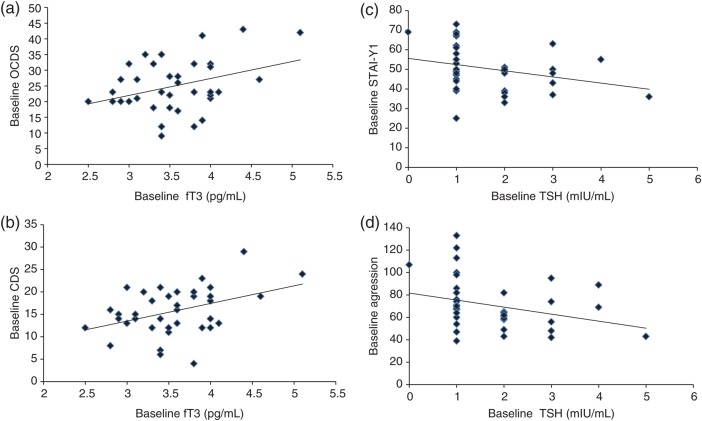
Mean levels for fT3, fT4 and TSH were not significantly different between week 0 and week 12 (Table 2). At baseline, a significant positive correlation was found between fT3 and the OCDS total craving scale (r = 0.358, P = 0.029; Fig. 1a), as well as between fT3 and the CDS compulsive subscale (r = 0.405, P = 0.013; Fig. 1b). At baseline, TSH levels were significantly and negatively correlated with both the STAI-Y1 state anxiety score (r = −0.342, P = 0.031; Fig. 1c) and the AQ aggression score (r = −0.35, P = 0.027; Fig. 1d). No other significant correlations were found at baseline between the TSH, fT3 or fT4 levels and any of the craving scales or psychometric scales assessed. At the end of the study (Week 12), after controlling for the medication condition, there were no significant correlations between TSH, fT3 or fT4 levels and the craving or psychometric scales. Additionally, we performed analyses that looked at the significance of other covariates, and their effects on thyroid hormone levels, and no such relationship was found to be significant. Particularly, we examined correlations between fT3, fT4 and TSH at Week 00 or at Week 12 and age or duration of alcohol dependence, and independent variable T-tests between gender and baseline fT3, fT4 and TSH, or analysis of covariance (ANCOVA) between gender and fT3, fT4 and TSH at Week 12. None of these analyses met statistical significance.
Table 2.
Thyroid hormone levels at baseline and at Week 12
| Hormone | Week 0 M (SD) | Week 12 M (SD) | t | Df | P |
|---|---|---|---|---|---|
| TSH (mIU/ml) | 1.61 (0.91) | 1.49 (0.90) | 0.78 | 26 | 0.44 |
| fT3 (pg/ml) | 3.44 (0.55) | 3.55 (0.56) | 1.08 | 22 | 0.29 |
| fT4 (pg/ml) | 12.11 (1.54) | 11.56 (1.51) | 2.01 | 26 | 0.06 |
TSH, thyroid stimulating hormone; fT3, triiodothyronine and fT4, thyroxine.
Fig. 1.
(a) Correlation scatter plot for baseline fT3 level and OCDS. At baseline, a significant positive correlation was found between fT3 and the OCDS total craving scale (r = 0.358, P = 0.029). (b) Correlation scatter plot for baseline fT3 level and CDS. At baseline, a significant positive correlation was found between fT3 and the CDS compulsive subscale (r = 0.405, P = 0.013). (c) Correlation scatter plot for baseline TSH level and STAI-Y1 score. At baseline, TSH levels were significantly and negatively correlated with the STAI-Y1 state anxiety score (r = −0.342, P = 0.031). (d) Correlation scatter plot for baseline TSH level and the aggression score. At baseline, TSH levels were significantly and negatively correlated with the AQ aggression score (r = −0.35, P = 0.027).
Effect of abstinent status vs. non-abstinent status on thyroid hormone levels
At the end of the study, while controlling for the medication condition, subjects who were abstinent had a greater change in TSH levels than those who were non-abstinent (−0.4 vs. −0.25, respectively; F(1,24) = 5.4, P = 0.029); no such differences in the fT3 and fT4 levels were found between the two groups.
Effect of baclofen on thyroid hormone levels
Pre-medication baseline fT3, fT4 or TSH levels were not different across the three groups (baclofen 10 mg t.i.d., 20 mg t.i.d., and placebo). Similarly, at the end of the study, we did not find any significant differences in the hormone levels across the three groups.
DISCUSSION
This study indicated that blood fT3 levels correlated positively and significantly with alcohol craving in actively drinking alcohol-dependent patients. Additionally, TSH negatively correlated with anxiety and aggression. These correlations disappeared after a 12-week period of treatment. Finally, subjects who remained abstinent during the 12-week treatment period had a steeper drop in TSH levels than those who did not.
A few reports previously highlighted alterations in thyroid hormone levels in subjects with AD (Välimäki et al., 1984; Baumgartner et al., 1994; Ozsoy et al., 2006); however, such alterations were thought to be modulated—or at least related to—psychiatric comorbidity, major surgery, severe liver or kidney diseases, caloric deprivation, psychological stress, or certain drugs (Hermann et al., 2002). In contrast, the sample studied here was a group of AD patients who enrolled in an alcoholism treatment study and no other psychiatric or medical comorbidities were present.
In our previous study (Leggio et al., 2008), negative correlations between TSH and craving scales and measures of depression, anxiety and aggression were noted; positive correlations between fT3 and craving scales, and negative correlations between fT4 and some anxiety scales at the time of enrollment were also reported; none of these correlations persisted after 12 weeks of treatment. This follow-up study replicated the positive correlation between fT3 and craving scales and the negative correlation between TSH and measures of anxiety and aggression. Furthermore, this study also replicated the observation that these correlations were specific during the active drinking phase, as they disappeared after period of treatment.
TSH negatively correlated with anxiety and aggression; the negative correlations are consistent with previously described associations between AD and mood or anxiety disorders, as well as their symptomatic manifestations (Willne et al., 1998; Ozsoy et al., 2006). It is important to note that these results represent only correlations, and therefore do not establish a causal relationship. Nonetheless, considering the consistency between these results and some previous literature, including our previous study (Leggio et al., 2008) and the fact that changes in thyroid hormones are known to cause behavioral changes (among the other symptoms present in patients with thyroid diseases), one might speculate that biochemical changes in thyroid hormones may contribute to some behavioral symptoms, including craving for alcohol, anxiety and aggression in AD patients. More specifically, one may hypothesize that lower TSH and higher fT3 levels could lead, respectively, to increased anxiety/aggression and craving, which, in turn, may be responsible for an increased risk of alcohol relapse. On a purely speculative level, one might hypothesize a role of dopaminergic signaling, as, in fact, dopamine is known to modulate TSH (Delitala et al., 1981; Cooper et al., 1983) and TRH (Przegaliński et al., 1991). Specifically, higher dopamine levels binding to striatal D2 receptors cause a reduced D2 receptor availability and lower TRH levels in the striatum (Przegaliński et al., 1991). Lower TRH levels are associated with lower TSH levels, leading to lower levels of circulating thyroid hormones. Given the existing negative feedback loop between circulating thyroid hormones and TSH, this would be consistent with the direct correlation between fT3 and measures of craving. However, while this hypothesized link is potentially intriguing, it remains speculative and the clinical significance of the present results remains preliminary. Mechanistic and molecular studies are required to demonstrate causal relationships and draw any final conclusions.
The greater drop in TSH levels in abstinent patients vs. those who were non-abstinent suggests that alcohol may affect the feedback inhibition of the thyroid hormones. Consistent with previous studies, reduction in peripheral thyroid hormones may result from the alcohol toxic effect on the thyroid gland, which induces a central compensatory activation of the HPT axis with increased TRH release (Hermann et al., 2002). It is important to note, however, that this was a sample of patients with no thyroid (or liver) diseases. Nonetheless, these non-clinical biochemical differences in TSH changes between the two groups are consistent with previous studies suggesting that there is a blunted TSH response in AD patients (Liappas et al., 2006), which might explain why changes in TSH were more pronounced in this sample vs. in those patients who abstained from alcohol.
It should be noted that, compared with the previous study (Leggio et al., 2008), the present study did not represent a mere replication in an independent sample, but addressed some of the limitations of the earlier study, i.e.: (a) a relatively larger (albeit still small) sample; (b) the presence of a placebo arm in the parent study, thus allowing us to control for the medication condition; and (c) the fact that, after the 12-week treatment period, both abstinent and non-abstinent patients were assessed, thus allowing us to explore the possible effects on drinking status. On the other hand, there are still important limitations in the present study that need to be noted. First, the sample was still small; therefore, future larger studies should be attempted. Second, these data were part of a secondary study driven from a main clinical trial; therefore, it is important to keep in mind its post hoc exploratory nature. Third, the strict inclusion/exclusion criteria applied to this study limit the generalizability of these findings. Fourth, thyroid hormones were only assessed at baseline and after a 12-week treatment period; therefore, this study does not address possible changes in thyroid hormones during the 12-week period. Finally, as mentioned previously, this study only looked at correlations between thyroid hormones and self-reported assessments of craving, anxiety, and aggression. Preclinical experiments coupled with human laboratory studies are needed to examine possible mechanisms to determine how and whether the HPT axis plays a role in alcohol-seeking behaviors. For example, human laboratory studies might assess craving and drinking in real time and under well-controlled conditions after pharmacological manipulations (e.g. acute administration of thyroid hormones, and/or TRH stimulation test) in order to investigate whether the HPT axis plays a role as a biomarker of alcohol craving and drinking, thus representing a target for AD treatment.
Funding
Support for this study was partially provided by an Exchange Award from the European Foundation for Alcohol Research (ERAB) to L.L. when he was working at Brown University, Providence, RI. At present, L.L. and M.R.L. are federal employees in the Section on Clinical Psychoneuroendocrinology and Neuropsychopharmacology, which is supported by the Division of Intramural Clinical and Biological Research of the National Institute on Alcohol Abuse and Alcoholism (NIAAA) and the Intramural Research Program of the National Institute on Drug Abuse (NIDA). E.G.A.'s work is supported by a Brown University research education grant funded by the National Institute of Mental Health (NIMH; R25MH101076). C.L.H.-K.'s work is supported by a Brown University training grant funded by NIAAA (5T32AA007459-28). G.A.K.'s work is supported by R21AA021128 from NIAAA.
Conflict of interest statement
R.M.S. has received travel and honorarium from D&A Pharma and G.A.K. received travel support from Baldacci Pharmaceuticals. G.A.K. and R.M.S. have received consultant fees from CT Laboratories. G.A. served as a consultant for Ortho-McNeil Janssen Scientific Affairs, LLC, and D&A Pharma, and was paid for his consulting services. He has received lecture fees from D&A Pharma, CT Laboratories and Lundbeck. The other authors report no financial interests or potential conflicts of interest.
References
- Addolorato G, Leggio L, Cardone S, et al. Role of the GABA(B) receptor system in alcoholism and stress: focus on clinical studies and treatment perspectives. Alcohol. 2009;43:559–63. doi: 10.1016/j.alcohol.2009.09.031. [DOI] [PubMed] [Google Scholar]
- Addolorato G, Leggio L, Ferrulli A, et al. Dose-response effect of baclofen in reducing daily alcohol intake in alcohol dependence: secondary analysis of a randomized, double-blind, placebo-controlled trial. Alcohol Alcohol. 2011;46:312–7. doi: 10.1093/alcalc/agr017. [DOI] [PubMed] [Google Scholar]
- Anton RF, Moak DH, Latham P. The Obsessive Compulsive Drinking Scale: a self-rated instrument for the quantification of thoughts about alcohol and drinking behavior. Alcohol Clin Exp Res. 1995;19:92–9. doi: 10.1111/j.1530-0277.1995.tb01475.x. [DOI] [PubMed] [Google Scholar]
- Aubin HJ, Daeppen JB. Emerging pharmacotherapies for alcohol dependence: a systematic review focusing on reduction in consumption. Drug Alcohol Depend. 2013;133:15–29. doi: 10.1016/j.drugalcdep.2013.04.025. [DOI] [PubMed] [Google Scholar]
- Baumgartner A, Heyne A, Campos-Barros A, et al. Hypothalamic-pituitary-thyroid axis in chronic alcoholism. II. Deiodinase activities and thyroid hormone concentrations in brain and peripheral tissues of rats chronically exposed to ethanol. Alcohol Clin Exp Res. 1994;18:95–304. doi: 10.1111/j.1530-0277.1994.tb00017.x. [DOI] [PubMed] [Google Scholar]
- Baumgartner A, Eravci M, Pinna G, et al. Thyroid hormone metabolism in the rat brain in an animal model of ‘behavioral dependence’ on ethanol. Neurosci Lett. 1997;227:25–8. doi: 10.1016/s0304-3940(97)00290-5. [DOI] [PubMed] [Google Scholar]
- Baumgartner A, Pinna G, Hiedra L, et al. Effects of acute administration of ethanol and the mu-opiate agonist etonitazene on thyroid hormone metabolism in rat brain. Psychopharmacol (Berl) 1998;135:63–9. doi: 10.1007/s002130050486. [DOI] [PubMed] [Google Scholar]
- Buss AH, Perry M. The aggression questionnaire. J Pers Soc Psychol. 1992;63:452–9. doi: 10.1037//0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- Centers for disease control and prevention. Summary health statistics for U.S. adults: National health interview survey. 2010. http://www.cdc.gov/nchs/fastats/alcohol.htm .
- Cooper DS, Klibanski A, Ridgway EC. Dopaminergic modulation of TSH and its subunits: in vivo and in vitro studies. Clin Endocrinol (Oxf) 1983;18:265–75. doi: 10.1111/j.1365-2265.1983.tb03211.x. [DOI] [PubMed] [Google Scholar]
- Delitala G, Devilla L, Canessa A, et al. On the role of dopamine receptors in the central regulation of human TSH. Acta Endocrinol (Copenh) 1981;98:521–7. doi: 10.1530/acta.0.0980521. [DOI] [PubMed] [Google Scholar]
- Drake RG, Davis LL, Cates ME, et al. Baclofen treatment for chronic posttraumatic stress disorder. Ann Pharmacother. 2003;37:1177–81. doi: 10.1345/aph.1C465. [DOI] [PubMed] [Google Scholar]
- Flannery BA, Poole SA, Gallop RJ, et al. Alcohol craving predicts drinking during treatment: an analysis of three assessment instruments. J Stud Alcohol. 2003;64:120–6. doi: 10.15288/jsa.2003.64.120. [DOI] [PubMed] [Google Scholar]
- Hegedüs L, Rasmussen N, Ravn V, et al. Independent effects of liver disease and chronic alcoholism on thyroid function and size: the possibility of a toxic effect of alcohol on the thyroid gland. Metabolism. 1988;37:29–33. doi: 10.1016/0026-0495(88)90100-x. [DOI] [PubMed] [Google Scholar]
- Hermann D, Heinz A, Mann K. Dysregulation of the hypothalamic-pituitary-thyroid axis in alcoholism. Addiction. 2002;97:1369–81. doi: 10.1046/j.1360-0443.2002.00200.x. [DOI] [PubMed] [Google Scholar]
- Janiri L, Calvosa F, Dario T, et al. The Italian version of the Obsessive-Compulsive Drinking Scale: validation, comparison with the other versions, and difference between type 1- and type 2-like alcoholics. Drug Alcohol Depend. 2004;74:187–95. doi: 10.1016/j.drugalcdep.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Kenna GA, Swift RM, Hillemacher T, et al. The relationship of appetitive, reproductive and posterior pituitary hormones to alcoholism and craving in humans. Neuropsychol Rev. 2012;22:211–28. doi: 10.1007/s11065-012-9209-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L, Ferrulli A, Cardone S, et al. Relationship between the hypothalamic-pituitary-thyroid axis and alcohol craving in alcohol-dependent patients: a longitudinal study. Alcohol Clin Exp Res. 2008;32:2047–53. doi: 10.1111/j.1530-0277.2008.00792.x. [DOI] [PubMed] [Google Scholar]
- Leggio L, Garbutt JC, Addolorato G. Effectiveness and safety of baclofen in the treatment of alcohol dependent patients. CNS Neurol Disord Drug Targets. 2010;9:33–44. doi: 10.2174/187152710790966614. [DOI] [PubMed] [Google Scholar]
- Leggio L, Ferrulli A, Cardone S, et al. Ghrelin system in alcohol-dependent subjects: role of plasma ghrelin levels in alcohol drinking and craving. Addict Biol. 2012;17:452–64. doi: 10.1111/j.1369-1600.2010.00308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liappas I, Piperi C, Malitas PN, et al. Interrelationship of hepatic function, thyroid activity and mood status in alcohol-dependent individuals. In Vivo. 2006;20:293–300. [PubMed] [Google Scholar]
- Mason GA, Stanley DA, Walker CH, et al. Chronic alcohol ingestion decreases pituitary-thyroid axis measures in Fischer-344 rats. Alcohol Clin Exp Res. 1988;12:731–4. doi: 10.1111/j.1530-0277.1988.tb01336.x. [DOI] [PubMed] [Google Scholar]
- National Institute on Alcohol Abuse and Alcoholism. The Physicians' Guide to Helping Patients with Alcohol Problems. Rockville, MD: U.S. Department of Health and Human Services; 1995. (NIH Publication No. 95–3769) [Google Scholar]
- Ozsoy S, Esel E, Izgi HB, et al. Thyroid function in early and late alcohol withdrawal: relationship with aggression, family history, and onset age of alcoholism. Alcohol Alcohol. 2006;41:515–21. doi: 10.1093/alcalc/agl056. [DOI] [PubMed] [Google Scholar]
- Perneger TV. What's wrong with Bonferroni adjustments. BMJ. 1998;316:1236–8. doi: 10.1136/bmj.316.7139.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pienaar WP, Roberts MC, Emsley RA, et al. The thyrotropin releasing hormone stimulation test in alcoholism. Alcohol Alcohol. 1995;30:661–7. [PubMed] [Google Scholar]
- Przegaliński E, Jaworska L, Konarska R, et al. The role of dopamine in regulation of thyrotropin-releasing hormone in the striatum and nucleus accumbens of the rat. Neuropeptides. 1991;19:189–95. doi: 10.1016/0143-4179(91)90118-3. [DOI] [PubMed] [Google Scholar]
- Rachdaoui N, Sarkar DK. Effects of alcohol on the endocrine system. Endocrinol Metab Clin North Am. 2013;42:593–615. doi: 10.1016/j.ecl.2013.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–6. [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Leo GI, et al. Reliability of a timeline method: assessing normal drinkers' reports of recent drinking and a comparative evaluation across several populations. Br J Addict. 1988;83:393–402. doi: 10.1111/j.1360-0443.1988.tb00485.x. [DOI] [PubMed] [Google Scholar]
- Spielberg CD, Gorsuch RL, Lushene RE. Manual for the State and Trait Anxiety Inventory. Paolo Alto, CA: Consulting Psychologist Press; 1983. [Google Scholar]
- Stata Statistical Software: Release 11 [computer program] College Station, TX: StataCorp LP; 2009. [Google Scholar]
- Välimäki M, Pelkonen R, Härkönen M, et al. Hormonal changes in noncirrhotic male alcoholics during ethanol withdrawal. Alcohol Alcohol. 1984;19:235–42. [PubMed] [Google Scholar]
- Willne P, Field M, Pitts K, et al. Mood, cue and gender influences on motivation, craving and liking for alcohol in recreational drinkers. Behav Pharmacol. 1998;9:631–42. doi: 10.1097/00008877-199811000-00018. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Fletcher DL, Simonyl A, et al. Chronic ethanol treatment reduces the responsiveness of the hypothalamic-pituitary-thyroid axis to central stimulation. Alcohol Clin Exp Res. 1996;20:954–60. doi: 10.1111/j.1530-0277.1996.tb05277.x. [DOI] [PubMed] [Google Scholar]
- Zung WW, Richards CB, Short MJ. Self-rating depression scale in an outpatient clinic. Further validation of the SDS. Arch Gen Psychiatry. 1965;13:508–15. doi: 10.1001/archpsyc.1965.01730060026004. [DOI] [PubMed] [Google Scholar]