Abstract
Background
Previous studies have shown that the expression level of γ-synuclein (SNCG) is associated with progression of many different malignant tumors. In this study, we discuss and assess the prognostic ability of SNCG in bladder cancer.
Material/Methods
Medical records (2005–2013) were retrospectively reviewed for the population of interest. SNCG expression was identified immunohistochemically from bladder cancer tissues of 113 bladder cancer patients. The survival rate was calculated by the Kaplan-Meier method. Cox proportional hazard regression model was used for analysis of predictors of bladder cancer.
Results
SNCG was overexpressed in bladder cancer tissues compared with the normal bladder tissues (p<0.0001). SNCG expression in bladder cancer tissue was strongly related to tumor stage. However, SNCG level was not a prognostic factor of survival.
Conclusions
Our results demonstrate that SNCG is highly expressed in bladder cancer tissue and its expression is stage-specific, but it is not helpful for predicting outcome in bladder cancer patients.
MeSH Keywords: gamma-Synuclein, Prognosis, Urinary Bladder Neoplasms
Background
Bladder cancer is the seventh most common cancer in men and the seventeenth most common cancer in women worldwide [1]. The American Cancer Society estimated that in 2013 there were 54 610 men and 17 960 women first diagnosed with bladder cancer, with a total of 72 570 new cases and 15 210 deaths [2]. About 80% of patients had bladder tumors that had not invaded the detrusor termed non-muscle-invasive tumors, and in general the prognosis is good. The vast majority of bladder cancer patients have a 30–70% recurrence rate, and the tumor may become invasive in 10–30% of patients [3]. The high recurrence rate makes bladder cancer the most socioeconomically expensive malignant tumor [4]. Early detection and timely intervention can increase patient survival. Consequently, it is important to find associated markers for estimating bladder cancer prognosis to predict and assess responses to various preventive and therapeutic interventions [5].
Synucleins are several small soluble proteins expressed primarily in the neural tissues and certain in tumors. The family of synuclein proteins contains α-synuclein (SNCA), β-synuclein (SNCB), and SNCG [6]. The previous 2 proteins are ubiquitously detected in both neuronal cells and presynaptic nerve terminals, where their main physiological functions are the regulation of synaptic levels of monoamine neurotransmitters through modulation of vesicular release [7]. These 2 proteins are considered to be important in certain neurodegenerative diseases, including Parkinson’s disease, Alzheimer’s disease, and dementia with Lewy bodies [8–10]. SNCG was first found to be stage-specific upregulation in breast carcinoma, and it was previously termed breast cancer-specific gene 1 (BCSG1) [11]. The human SNCG gene is localized at 10q23.20–23.3, and cDNA of SNCG is 5 kb in length, composed of 5 exons that translate into a protein of 127 amino acids [12]. SNCG has been reported to have a strong connection with malignancies such as breast, gastric, pancreatic, esophagus, colon, and prostate cancers [13–18]. Many genes, including SNCG, are involved in DNA methylation, which plays an important role in the occurrence and development of tumors [19–21]. Furthermore, SNCG is also involved in resistance to certain chemotherapeutic or antimicrotubule agents in breast cancer treatment [22]. Recent studies further indicated that SNCG overexpression stimulated tumor progression through a series of mechanisms, including the promotion of cell proliferation, chromosomal instability, cell invasion, and metastasis [12,23].
SNCG has been shown to be an unfavorable prognostic marker in breast cancer in clinical follow-up studies [24]. In breast cancer, the patients with a positive expression of SNCG were observed to have a shorter DFS and OS compared with those with a negative expression of SNCG. However, there are no studies on the prognostic significance of SNCG in bladder cancer. In this study, the expression of SNCG proteins was detected by immunohistochemistry (IHC) and we assessed whether SNCG was a predictor of survival for patients with bladder cancer.
Material and Methods
Patients and tissue samples
From the human bladder cancer patients in this study, 113 specimens were obtained at the time of surgery at the Department of Urology in Chaoyang Hospital, Capital Medical University, between June 2005 and April 2013. The samples of normal bladder tissue were obtained from 15 in-patients with bladder stones. Among 113 bladder cancer patients, the age range was 34–89 years (mean ±SD, 65.7±33.4) and the range of follow-up was 10–116 months (mean ±SD, 56.4±25.4). During follow-up, 12.4% (14/113) of patients died of bladder cancer and 27.4% (31/113) of patients had recurrence after surgery. Patient who accepted any radiotherapy or chemotherapy before surgery were excluded. Disease-free survival was defined as the period from time of surgery to evidence of treatment failure (recurrence or progression). Overall survival was defined as the period of time from bladder cancer confirmation to death from any cause, or to the last follow-up. Clinicopathological information was collected from medical records of the patients. Tumor histology was confirmed independently by 2 pathologists.
Immunohistochemistry for SNCG
All 113 bladder cancer tissues and 15 normal bladder tissues were analyzed by immunohistochemistry. Each tissue was embedded with paraffin and cut serially in 4-μm-thick sections. The antibody for SNCG [25] staining was obtained from the Laboratory of Biochemistry and Molecular Biology, Peking University. The sample was incubated with 3% hydrogen peroxidase for 10 min at room temperature and then samples were incubated with the antibody for SNCG for 2 h at room temperature. Light microscopy (Applied Imaging Company) at 200× was used to detect SNCG expression. We used a 4-value classification method [26] for grading the bladder samples. If the score of a section was more than 3, we defined it as SNCG-positive and if the score was less than 3, we defined it as SNCG-negative.
Statistical analysis
The differences between tumor and normal tissues in SNCG protein expression were evaluated with Student’s t test. Pearson chi-square test was performed for evaluating the correlation between SNCG levels and patients’ clinical pathological features. Survival rates were estimated by the Kaplan-Meier method and the log-rank test was performed for evaluating the differences. The Cox proportional hazard model was used for multivariate analysis for the independent risk factors. Statistical significance in this study was set at p<0.05, and all reported p values were 2-sided. All analyses were performed with SPSS v.18 for Windows.
Results
SNCG is highly expressed in bladder cancer tissues and is related to the tumor stage
None of the 15 benign bladder tissues was detected SNCG-positive staining, but 83 SNCG-positive cases were detected in 113 bladder cancer tissues (73.5%) (Table 1). SNCG overexpressed in the bladder cancer tissues, but no SNCG expression was detected in the normal bladder tissues (Figure 1). As shown in Table 2, we evaluated the correlation between SNCG level and clinicopathologic features in 113 bladder cancer patients. Overexpression of SNCG was only strongly correlated with the tumor stage (p=0.011). We also estimated the correlation between these features and tumor recurrence (Table 2). We found the tumor size, number of tumors, and tumor grade were significantly associated with recurrence of bladder cancer (p=0.001, 0.001, 0.015, respectively), whereas, gender, age, and tumor stage were not associated with recurrence (p>0.05). There were 31 patients with evidence of tumor recurrence in the study, and importantly, we found for the first time that there was not correlation between the SNCG overexpression and bladder cancer recurrence (p=0.123).
Table 1.
SNCG expressing profile in bladder cancer.
| Cases | SNCG expression | Positive rate (%) | p value | ||
|---|---|---|---|---|---|
| (−) | (+) | ||||
| Normal bladder tissues | 15 | 15 | 0 | 0 | <0.0001 |
| Bladder cancer tissues | 113 | 30 | 83 | 73.5 | |
Figure 1.
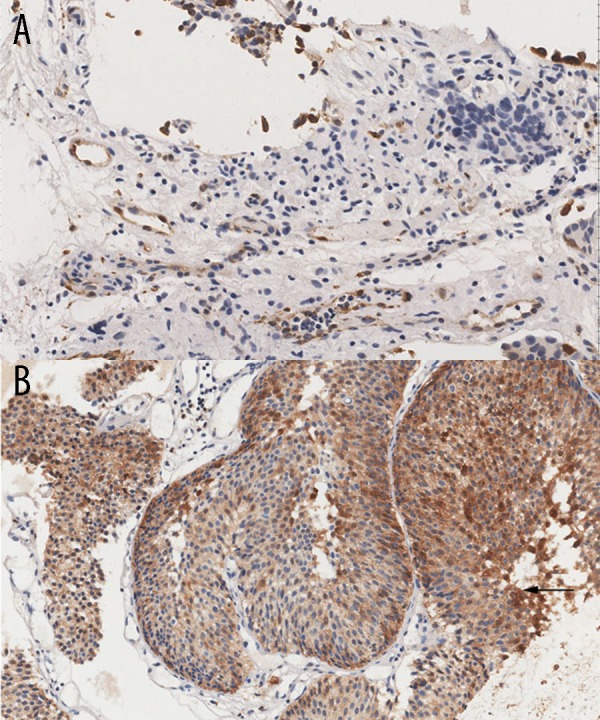
Immunohistochemical staining for SNCG in human bladder cancer tissues (A, B). (A) Normal bladder samples showed negative staining intensity; (B) Bladder cancer samples showed strong positive staining intensity (original magnification 200×).
Table 2.
Correlations of SNCG expression with clinicopathologic factors and their influences on postoperative recurrence.
| Characteristics | No. of cases | SNCG expression | No. of recurrence (%) | HR (95%CI) | p value | |
|---|---|---|---|---|---|---|
| SNCG+ (%) | p value | |||||
| Gender | ||||||
| Male | 87 | 64 (73.6) | 0.961 | 22 (25.3) | 1 | 0.350 |
| Female | 26 | 19 (73.1) | 9 (34.6) | 1.564 (0.610–4.010) | ||
| Age (yrs) | ||||||
| ≤60 | 48 | 31 (64.6) | 0.067 | 12 (25.0) | 1 | 0.618 |
| >60 | 65 | 52 (80.0) | 19 (29.2) | 1.239 (0.533–2.882) | ||
| Size (cm) | ||||||
| ≤3 | 75 | 55 (73.3) | 0.968 | 13 (17.3) | 1 | 0.001 |
| ≥3 | 38 | 28 (73.7) | 18 (47.4) | 4.292 (1.792–10.281) | ||
| Tumor number | ||||||
| Solitary | 57 | 41 (71.9) | 0.712 | 8 (14.0) | 1 | 0.001 |
| Multiple | 56 | 42 (75.0) | 23 (41.1) | 4.269 (1.705–10.686) | ||
| Tumor stage | ||||||
| MIBC | 8 | 6 (75.0) | 0.011 | 2 (25.0) | 1 | 0.823 |
| NMIBC | 73 | 22 (30.1) | 21 (28.8) | 1.212 (0.226–6.492) | ||
| Tx | 32 | |||||
| Tumor grade | ||||||
| Low | 81 | 62 (76.5) | 0.236 | 17 (21.0) | 1 | 0.015 |
| High | 32 | 21 (65.7) | 14 (43.8) | 2.928 (1.215–7.057) | ||
| SNCG | ||||||
| Negative | 30 | ---- | ---- | 5 (16.7) | 1 | 0.123 |
| Positive | 83 | ---- | 26 (31.3) | 2.281 (0.785–6.625) | ||
NMIBC – non-muscle invasive bladder cancer; MIBC – muscle invasive bladder cancer; Tx – primary tumor cannot be assessed; HR – hazard ratio; CI – confidence interval.
Highly expressed SNCG is not a predictor of progression in bladder cancer
The median follow-up of all bladder cancer patients was 56±25 months (range 10–116 months) after surgery and we correlated the period with SNCG expression in order to determine if SNCG can be a predictor of progression of bladder cancer.
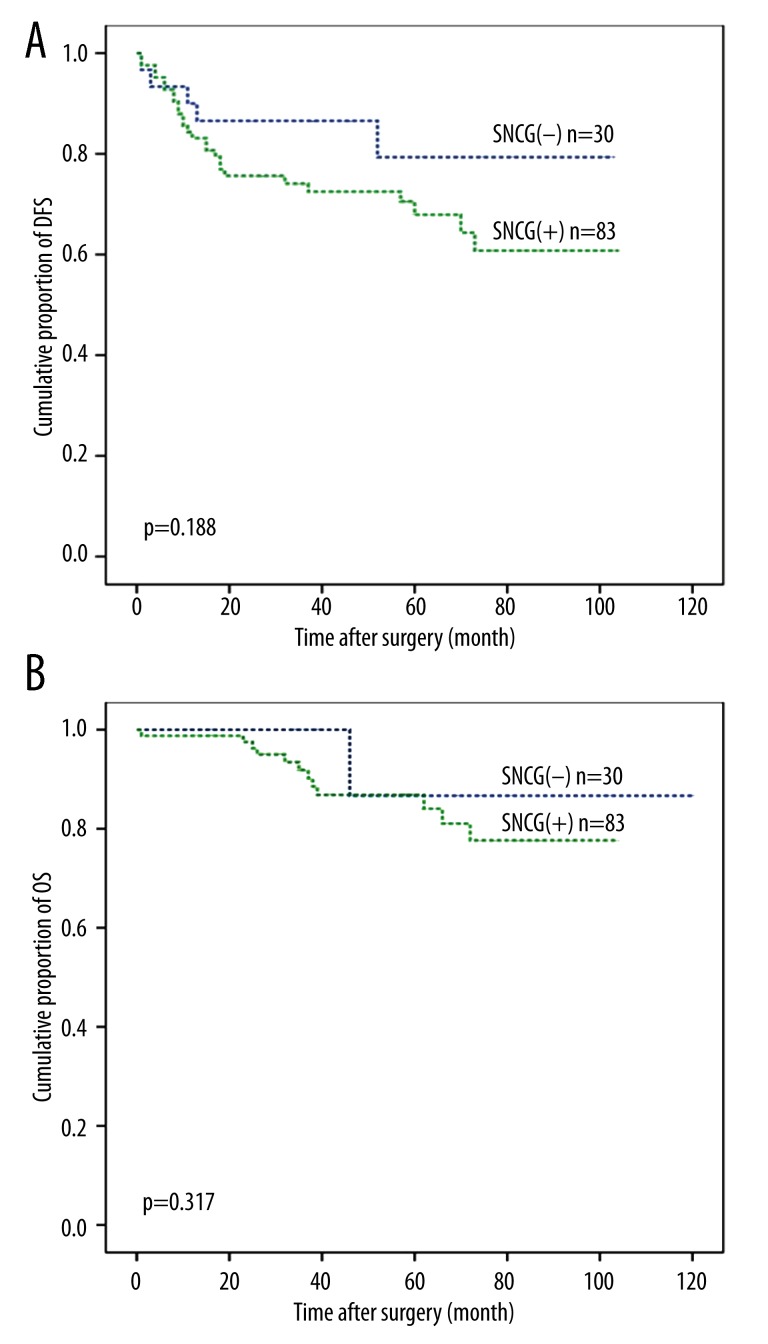
In the present study, there was no correlation between SNCG and survival. There seemed to be better prognosis in SNCG-negative patients than those who were SNCG-positive in disease-free survival and overall survival calculated by Kaplan-Meier analysis, but the differences were not statistically significant (Figure 2). The DFS was 86.4±6.7 months (95% confidence interval (CI), 73.2–99.6 months) in SNCG-negative patients compared with 74.0±4.7 months (95% CI, 65.2–83.8 months) in SNCG-positive patients. OS was 110.1±6.5 months (95% CI, 97.4–122.9 months) in SNCG-negative patients compared with 90.8±3.4 months (95% CI, 84.1–97.6 months) in SNCG-positive patients (P=0.188 and 0.317, respectively).
Figure 2.
Kaplan-Meier estimation of disease-free survival (A) and overall survival (B). The SNCG-negative group (blue line) was compared with SNCG-positive group (green line). The SNCG-positive group demonstrated no significantly differences in survival rates compared to the SNCG-negative group (p>0.05 by log-rank test).
We next assessed whether SNCG could serve as an independent predictor of survival, by performing multivariate Cox proportional hazards analysis (Table 3). The potential prognostic factors were chosen after univariate analysis, including size, number, stage, grade of tumor, and SNCG expression. The analysis demonstrated that both DFS and OS were no different in the SNCG-positive group and SNCG-negative group (p=0.139 and 0.447, respectively). The tumor grade is an independent predictor for both DFS and OS (p=0.015 and 0.021, respectively). The number of tumors is an independent predictor only for DFS (p=0.004). The analysis identified that SNCG expression in bladder cancer tissue was not a predictor for DFS and OS.
Table 3.
Univariate and multivariate analyses of factors associated with survival and recurrence of 113 patients with bladder cancer.
| Variables | DFS | p value | OS | p value |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | |||
| Univariate analysis | ||||
| Tumor size (cm) (≤3/>3) | 1.324 (0.507–3.455) | 0.566 | 0.715 (0.155–3.305) | 0.349 |
| Tumor number (solitary/multiple) | 0.387 (0.158–0.952) | 0.039 | 0.702 (0.189–2.608) | 0.597 |
| Tumor stage (MIBC/NMIBC) | 2.874 (0.602–13.723) | 0.186 | 3.599 (0.410–31.558) | 0.248 |
| Tumor grade (low/high) | 0.236 (0.090–0.615) | 0.003 | 0.145 (0.030–0.701) | 0.016 |
| SNCG (negative/positive) | 0.467 (0.170–1.282) | 0.139 | 0.544 (0.113–2.616) | 0.447 |
| Multivariate analysis | ||||
| Tumor number (multiple/solitary) | 0.306 (0.136–0.688) | 0.004 | --- | --- |
| Tumor grade (low/high) | 0.411 (0.201–0.841) | 0.015 | 0.290 (0.101–0.831) | 0.021 |
NMIBC – non-muscle invasive bladder cancer; MIBC – muscle invasive bladder cancer; HR – hazard ratio; CI – confidence interval; DFS – disease-free surviva; OS – overall survival.
Discussion
In this study, we found that SNCG expression was not a predictor of prognosis for the bladder cancer patients. A similar result was reported in breast cancer [27]. However, conflicting results were also found in many other malignant tumors [13–18]. Due to its important role in neural tissue, SNCG highly expressed in tumor tissue might be associated with cancer-related neurogenesis, a factor recently related to aggressive tumors and indicating unfavorable prognosis [28]. In the present study, we also found that SNCG expression is strongly related to bladder tumor stage. Mhawech-Fauceglia et al. [29] first found the protein synuclein highly expressed in non-muscle invasive bladder tumors and reported a statistically significant association between synuclein expression and tumor stage (p=0.029). They also found that synuclein failed to predict recurrence/progression in pTa/pT1 bladder tumors. However, our study indicated that the SNCG-positive bladder cancer patients have higher clinical stage tumors. Similar results also were reported that SNCG expression was related to the tumor stage in many other different cancers [14]. We grouped the bladder patients according to whether the tumor had invaded into the bladder muscles but not according to the TNM classification, because the former classification in wide clinical use. Moreover, the muscle-invasive bladder cancer (MIBC) has obviously poorer prognosis and more limited options for durable disease control than the non-muscle-invasive bladder cancer (NMIBC) [30]. This suggested that SNCG may be a malignant risk factor in patients with bladder cancer.
Bladder cancer has a high recurrence rate and needs regular follow-up. Surveillance strategies for bladder cancer recurrence have historically relied on the combination of cystoscopy and urinary cytology. However, only 40% of patients actually comply with a standard surveillance protocol in clinical practice [31] because cystoscopy can be expensive, invasive, and lead to problems in compliance, and cytology has low sensitivity in low-grade bladder tumors. In our study, we did not find that SNCG-positive patients had higher recurrence rates than compared to SNCG-negative patients. Therefore, SNCG does not predict the probabilities of recurrence in bladder cancer.
Tissue-based markers are effective prognostic tools are used to identify groups of patients at risk of relapse or metastasis or to monitor cancer survivors following treatment. The widely studied tissue-based markers in bladder cancer are p53, p21, p16, Rb, and Tip60, but none of these markers has been used in clinical diagnosis and prediction because they lacked significant power in validation studies to reach clinical testing [32,33]. Therefore, finding new effective and sensitive prognostic markers in bladder cancer is of high importance. In the present study, we found that SNCG was overexpressed in bladder cancer tissues. A previous study [29] demonstrated the protein synuclein was highly expressed in non-muscle invasive bladder tumors. However, the study did not compare the expression of SNCG in the tumor tissue with normal bladder tissue. In our study, we detected highly expressed SNCG in bladder cancer tissue compared with normal bladder tissue (p<0.001), suggesting that SNCG could be valuable as a molecular marker for bladder cancer.
Conclusions
Our study demonstrated that the SNCG protein was overexpressed in bladder cancer tissue and that the expression was related to tumor stage. However, SNCG cannot predict survival in patients with bladder cancer.
Footnotes
Conflict of interest
None of the authors have personal financial interests related to this research.
Source of support: Departmental sources
References
- 1.Murta-Nascimento C, Schmitz-Drager BJ, Zeegers MP, et al. Epidemiology of urinary bladder cancer: from tumor development to patient’s death. World J Urol. 2007;25:285–95. doi: 10.1007/s00345-007-0168-5. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 3.Dalbagni G, Herr HW. Current use and questions concerning intravesical bladder cancer group for superficial bladder cancer. Urol Clin North Am. 2000;27:137–46. doi: 10.1016/s0094-0143(05)70241-x. [DOI] [PubMed] [Google Scholar]
- 4.Cheluvappa R, Smith DP, Cerimagic S, Patel MI. A comprehensive evaluation of bladder cancer epidemiology and outcomes in Australia. Int Urol Nephrol. 2014;46:1351–60. doi: 10.1007/s11255-014-0643-z. [DOI] [PubMed] [Google Scholar]
- 5.Zargar H, Espiritu PN, Fairey AS, et al. Multicenter Assessment of Neoadjuvant Chemotherapy for Muscle-invasive Bladder Cancer. Eur Urol. 2014 doi: 10.1016/j.eururo.2014.09.007. pii: S0302-2838(14)00893-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clayton DF, George JM. The synucleins: a family of proteins involved in synaptic function, plasticity, neurodegeneration and disease. Trends Neurosci. 1998;21:249–54. doi: 10.1016/s0166-2236(97)01213-7. [DOI] [PubMed] [Google Scholar]
- 7.Surgucheva I, Newell KL, Burns J, Surguchov A. New alpha- and gamma-synuclein immunopathological lesions in human brain. Acta Neuropathol Commun. 2014;2:132. doi: 10.1186/s40478-014-0132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Bohlen und Halbach O. Synucleins and their relationship to Parkinson’s disease. Cell Tissue Res. 2004;318:163–74. doi: 10.1007/s00441-004-0921-7. [DOI] [PubMed] [Google Scholar]
- 9.Iwai A. Properties of NACP/alpha-synuclein and its role in Alzheimer’s disease. Biochim Biophys Acta. 2000;1502:95–109. doi: 10.1016/s0925-4439(00)00036-3. [DOI] [PubMed] [Google Scholar]
- 10.Lavedan C. The synuclein family. Genome Res. 1998;8:871–80. doi: 10.1101/gr.8.9.871. [DOI] [PubMed] [Google Scholar]
- 11.Ji H, Liu YE, Jia T, et al. Identification of a breast cancer-specific gene, BCSG1, by direct differential cDNA sequencing. Cancer Res. 1997;57:759–64. [PubMed] [Google Scholar]
- 12.Jiang Y, Liu YE, Goldberg ID, Shi YE. Gamma synuclein, a novel heat-shock protein-associated chaperone, stimulates ligand-dependent estrogen receptor alpha signaling and mammary tumorigenesis. Cancer Res. 2004;64:4539–46. doi: 10.1158/0008-5472.CAN-03-3650. [DOI] [PubMed] [Google Scholar]
- 13.Wu K, Huang S, Zhu M, et al. Expression of synuclein gamma indicates poor prognosis of triple-negative breast cancer. Med Oncol. 2013;30:612. doi: 10.1007/s12032-013-0612-x. [DOI] [PubMed] [Google Scholar]
- 14.Liu H, Liu W, Wu Y, et al. Loss of epigenetic control of synuclein-gamma gene as a molecular indicator of metastasis in a wide range of human cancers. Cancer Res. 2005;65:7635–43. doi: 10.1158/0008-5472.CAN-05-1089. [DOI] [PubMed] [Google Scholar]
- 15.Hibi T, Mori T, Fukuma M, et al. Synuclein-gamma is closely involved in perineural invasion and distant metastasis in mouse models and is a novel prognostic factor in pancreatic cancer. Clin Cancer Res. 2009;15:2864–71. doi: 10.1158/1078-0432.CCR-08-2946. [DOI] [PubMed] [Google Scholar]
- 16.Amsterdam A, Shezen E, Raanan C, et al. Differential staining of gamma synuclein in poorly differentiated compared to highly differentiated colon cancer cells. Oncol Rep. 2012;27:1451–54. doi: 10.3892/or.2012.1658. [DOI] [PubMed] [Google Scholar]
- 17.Chen J, Jiao L, Xu C, et al. Neural protein gamma-synuclein interacting with androgen receptor promotes human prostate cancer progression. BMC Cancer. 2012;12:593. doi: 10.1186/1471-2407-12-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yanagawa N, Tamura G, Honda T, et al. Demethylation of the synuclein γ gene CpG island in primary gastric cancers and gastric cancer cell lines. Clin Cancer Res. 2004;10:2447–51. doi: 10.1158/1078-0432.ccr-03-0107. [DOI] [PubMed] [Google Scholar]
- 19.Lin YL, Xie PG, Ma JG. Aberrant methylation of CDH13 is a potential biomarker for predicting the recurrence and progression of non muscle invasive bladder cancer. Med Sci Monit. 2014;20:1572–77. doi: 10.12659/MSM.892130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang L, Xie PG, Lin YL, et al. Aberrant methylation of PCDH10 predicts worse biochemical recurrence-free survival in patients with prostate cancer after radical prostatectomy. Med Sci Monit. 2014;20:1363–68. doi: 10.12659/MSM.891241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dokun OY, Florl AR, Seifert HH, et al. Relationship of SNCG, S100A4, S100A9 and LCN2 gene expression and DNA methylation in bladder cancer. Int J Cancer. 2008;123:2798–807. doi: 10.1002/ijc.23893. [DOI] [PubMed] [Google Scholar]
- 22.Singh VK, Jia Z. Targeting synuclein-gamma to counteract drug resistance in cancer. Expert Opin Ther Targets. 2008;12:59–68. doi: 10.1517/14728222.12.1.59. [DOI] [PubMed] [Google Scholar]
- 23.Jiang Y, Liu YE, Lu A, et al. Stimulation of estrogen receptor signaling by gamma synuclein. Cancer Res. 2003;63:3899–903. [PubMed] [Google Scholar]
- 24.Wu K, Quan Z, Weng Z, et al. Expression of neuronal protein synuclein gamma gene as a novel marker for breast cancer prognosis. Breast Cancer Res Treat. 2007;101:259–67. doi: 10.1007/s10549-006-9296-7. [DOI] [PubMed] [Google Scholar]
- 25.Liu C, Guo J, Qu L, et al. Applications of novel monoclonal antibodies specific for synuclein-γ in evaluating its levels in sera and cancer tissues from colorectal cancer patients. Cancer Lett. 2008;269:148–58. doi: 10.1016/j.canlet.2008.04.037. [DOI] [PubMed] [Google Scholar]
- 26.Liu C, Dong B, Lu A, et al. Synuclein gamma predicts poor clinical outcome in colon cancer with normal levels of carcinoembryonic antigen. BMC Cancer. 2010;10:359. doi: 10.1186/1471-2407-10-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin TA, Gomez K, Watkins G, et al. Expression of breast cancer specific gene-1 (BCSG-1/gamma-synuclein) is associated with tumour grade but not with clinical outcome of patients with breast cancer. Oncology reports. 2006;16:207–12. [PubMed] [Google Scholar]
- 28.Zhu J, Jiang Z, Gao F, et al. A systematic analysis on DNA methylation and the expression of both mRNA and microRNA in bladder cancer. PloS One. 2011;6:e28223. doi: 10.1371/journal.pone.0028223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mhawech-Fauceglia P, Ali L, Cheney RT, et al. Prognostic significance of neuron-associated protein expression in non-muscle-invasive urothelial bladder cancer. J Clin Pathol. 2009;62:710–14. doi: 10.1136/jcp.2009.066159. [DOI] [PubMed] [Google Scholar]
- 30.Shah JB, McConkey DJ, Dinney CP. New strategies in muscle-invasive bladder cancer: on the road to personalized medicine. Clin Cancer Res. 2011;17:2608–12. doi: 10.1158/1078-0432.CCR-10-2770. [DOI] [PubMed] [Google Scholar]
- 31.Schrag D, Hsieh LJ, Rabbani F, et al. Adherence to surveillance among patients with superficial bladder cancer. J Natl Cancer Inst. 2003;95:588–97. doi: 10.1093/jnci/95.8.588. [DOI] [PubMed] [Google Scholar]
- 32.Matsushita K, Cha EK, Matsumoto K, et al. Immunohistochemical biomarkers for bladder cancer prognosis. Int J Urol. 2011;18:616–29. doi: 10.1111/j.1442-2042.2011.02809.x. [DOI] [PubMed] [Google Scholar]
- 33.Shariat SF, Tokunaga H, Zhou J, et al. p53, p21, pRB, and p16 expression predict clinical outcome in cystectomy with bladder cancer. J Clin Oncol. 2004;22:1014–24. doi: 10.1200/JCO.2004.03.118. [DOI] [PubMed] [Google Scholar]