Abstract
Background and Objectives:
Routine drainage after laparoscopic cholecystectomy is still controversial. This meta-analysis was performed to assess the role of drains in reducing complications in laparoscopic cholecystectomy.
Methods:
An electronic search of Medline, Science Citation Index Expanded, Scopus, and the Cochrane Library database from January 1990 to June 2013 was performed to identify randomized clinical trials that compare prophylactic drainage with no drainage in laparoscopic cholecystectomy. The odds ratio for qualitative variables and standardized mean difference for continuous variables were calculated.
Results:
Twelve randomized controlled trials were included in the meta-analysis, involving 1939 patients randomized to a drain (960) versus no drain (979). The morbidity rate was lower in the no drain group (odds ratio, 1.97; 95% confidence interval, 1.26 to 3.10; P = .003). The wound infection rate was lower in the no drain group (odds ratio, 2.35; 95% confidence interval, 1.22 to 4.51; P = .01). Abdominal pain 24 hours after surgery was less severe in the no drain group (standardized mean difference, 2.30; 95% confidence interval, 1.27 to 3.34; P < .0001). No significant difference was present with respect to the presence and quantity of subhepatic fluid collection, shoulder tip pain, parenteral ketorolac consumption, nausea, vomiting, and hospital stay.
Conclusion:
This study was unable to prove that drains were useful in reducing complications in laparoscopic cholecystectomy.
Keywords: Cholecystectomy, Laparoscopy, Drainage
INTRODUCTION
Prophylactic drains in abdominal surgery are widely used either to detect early complications, such as postoperative hemorrhage or leakage, or to remove collections that might be toxic, such as bile, and become infected. However, evidence-based data do not support the use of prophylactic drainage in the majority of abdominal surgery procedures.1,2
Cholecystectomy is the second most common operation in gastrointestinal surgery after appendectomy. In the era of open cholecystectomy, a meta-analysis showed that drains increased morbidity without providing any additional benefit for patients.3 At present, laparoscopic cholecystectomy (LC) is the preferred method for either elective cholecystectomy or emergent cholecystectomy.4,5 With the advent of LC, the use of drains may be justified because of the increased incidence of biliary injury and, consequently, bile leakage. The use of prophylactic drainage in LC to avoid bile and blood collection requiring subsequent treatment is largely diffuse. In a recent Australian survey, surgeons were evenly divided into those who used drains routinely, those who always used drains, and those who never used drains after LC.6 Moreover, when LC for acute cholecystitis is considered, 74.9% of surgeons insert an abdominal drain, as reported in a recent prospective multicenter survey in Belgium.7 However, in the literature there is no evidence to support the use of drains in LC, according to the results of a Cochrane Database Systematic Review.8 The 6 studies comparing groups with and without placement of an abdominal drain in LC included 741 patients, but they had important methodologic limitations regarding, in particular, quality and heterogeneity in the measurement of outcomes.9–14 The authors of the review claimed that high-quality randomized studies according to the CONSORT (Consolidated Standards of Reporting Trials) statement (www.consortstatement.org) were needed to improve evidence, in particular for cholecystectomy performed for acute cholecystitis.
After the publication of the aforementioned review, 7 other randomized trials have been published.15–21 The aim of our study was to perform a systematic review and meta-analysis including these new studies to assess the utility of drainage in reducing complications after both emergent and elective LC.
METHODS
The methods for the analysis and generation of inclusion criteria were based on the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement recommendations.22 According to population, interventions, comparators, outcome measures, and setting (PICOS) criteria, patients were included if they had benign gallbladder pathologies for which LC was indicated. The study methods were documented in a protocol registered and accessible at http://www.crd.york.ac.uk/prospero/ (registration CRD42013004056).
Types of Studies, Participants, and Interventions
Randomized clinical trials (irrespective of language, blinding, or publication status) that compare prophylactic subhepatic drainage with no drainage in LC were included in this analysis.
Types of Outcome Measures
The primary outcomes were mortality rate at maximal follow-up, morbidity rate, and presence and quantity (in milliliters) of subhepatic fluid collection 24 hours after surgery, measured by ultrasonographic examination.
The secondary outcomes were wound infection (as reported by the authors), abdominal and shoulder tip pain 24 hours after surgery (measured with a visual analog scale), analgesic (ketorolac) consumption (in milligrams), nausea and vomiting (at times reported by the authors), and hospital stay (in days).
Subgroup Analyses
We intended to perform the following subgroup analyses: trials with high methodologic quality compared with trials with low methodologic quality, drainage in emergency compared with elective LC, and suction drainage compared with no drainage.
Search Strategy and Data Collection
An electronic search of Medline, Science Citation Index Expanded, Scopus, and the Cochrane Library database from January 1990 to March 2013 was performed.
The studies in Medline were identified by use of the following search terms: ((((laparoscop* or celioscop* or coelioscop* or abdominoscop* or peritoneoscop*) AND (cholecystecto* or colecystecto*)) OR “Cholecystectomy, Laparoscopic” [MeSH]) AND (“Drainage” [MeSH] OR drain*)) AND randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized controlled trials [mh] OR random allocation [mh] OR double-blind method [mh] OR single-blind method [mh] OR clinical trial [pt] OR clinical trials [mh] OR (“clinical trial” [tw]) OR ((singl* [tw] OR doubl* [tw] OR trebl* [tw] OR tripl* [tw]) AND (mask* [tw] OR blind* [tw])) OR (placebos [mh] OR placebo* [tw] OR random* [tw] OR research design [mh:noexp]) NOT (animals [mh] NOT human [mh] AND (“1990” [PDAT]: “3000” [PDAT]). In the other databases, a Boolean search was performed as follows: (laparoscop* or celioscop* or coelioscop* or abdominoscop* or peritoneoscop*) AND (cholecystecto* or colecystecto*) AND (drain*) AND (random*). References of the identified trials were analyzed to collect further relevant trials.
Data Extraction
All data were extracted independently by 3 reviewers (M.P., P.L., and F.D.A.) using a paper data extraction pro forma. The accuracy of the extracted data was further confirmed by a third author (E.S.).
The information collected from each study was as follows: year of publication, study design, inclusion criteria, exclusion criteria, and outcomes.
Assessment of Risk of Bias
Two raters (F.S. and A.D.F.) independently assessed the methodologic quality of the included studies according to The Cochrane Collaboration guidelines.23
Statistical Analysis
We performed the meta-analyses using the software package Review Manager, version 5.2 (The Cochrane Collaboration). For dichotomous variables, we calculated the odds ratios (OR) with the 95% confidence interval (CI). For continuous variables, we calculated the standardized mean difference (SMD) with the 95% CI. Medians were converted to means by use of the technique described by Hozo et al.24 We used the Mantel-Haenszel method for calculating the weighted summary OR. Heterogeneity was assessed by the I2 measure of inconsistency; I2 > 50% was defined as statistically significant. Whenever I2 was < 50%, the fixed-effects model results were used; otherwise, the random-effects model results were preferred. Trials with no events in any treatment arm were excluded from the analysis because they are uninformative. For all the outcomes, P < .05 was considered statistically significant. Forest plots were used for graphical display of the results.
RESULTS
Study Selection
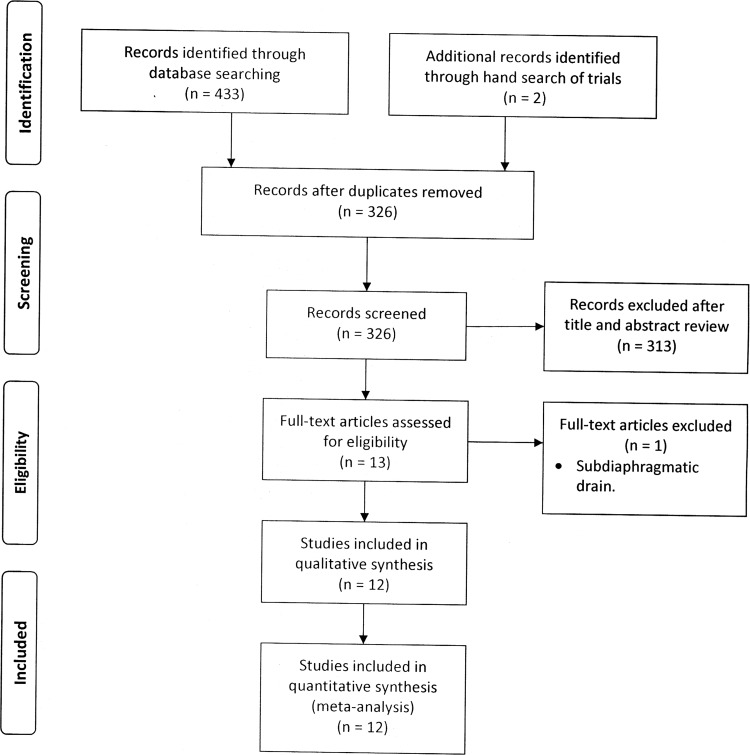
The database search retrieved 433 records. Two further records were identified in the reference lists. After deletion of duplicate results, a total of 322 records remained for title and abstract review. Of these, 13 randomized controlled trials were selected for full-text examination. One study was excluded because the drain was positioned between the diaphragm and liver.12 Twelve studies in all fulfilled the inclusion criteria and were suitable for the meta-analysis.9–11,13–21 All trials compared the use of prophylactic drainage with no drainage in elective LC with the exception of the study of Lucarelli et al,21 which included only emergency procedures for acute calculous cholecystitis. Figure 1 shows the PRISMA flowchart for study inclusion and exclusion.
Figure 1.
Article identification and selection algorithm.
Characteristics of Included Studies
Table 1 summarizes the characteristics of the 12 selected studies. A total of 1939 patients were included in the meta-analysis: 960 in the drain group and 979 in the no drain group.
Table 1.
Summary of characteristics of the included studies.
| Study | No. of patients | Intervention | Inclusion criteria | Exclusion criteria | Main outcomes |
|---|---|---|---|---|---|
| Capitanich13 | 93 (55 F) | Passive open drain (n=40) no drain (n=53) | Elective laparoscopic cholecystectomy. | Emergency cholecystectomy, jaundice, injury to cystic artery, choledocholithiasis, sclerotic gallbladder. | Pain at different times (8 hours, 16 hours, 24 hours) |
| El-labban18 | 160 (116 F) | Passive open drain (n= 80) no drain (n=80) | Elective laparoscopic cholecystectomy | > 70 years old, acute cholecystitis, cholangitis, or pancreatitis, absence of any contraindication for the laparoscopic approach; no need for common bile duct exploration or any other additional procedure. | Pain at different times (24 hours, 48 hours, 1 week), nausea, vomiting, wound infection, abdominal collection, hospital stay |
| Georgiou17 | 116 (79 F) | Passive open drain (n=63) no drain (n=53) | Elective laparoscopic cholecystectomy | Acute cholecystitis, empyema of gallbladder, exploration of the common bile duct. | The length of hospital stay, postoperative pain, the existence of subhepatic fluid on the 1st postoperative day postoperative complications, shoulder pain, nausea, vomiting, wound infections. |
| Hawasli9 | 100 (79 F) | Suction drain (n=50) no drain (n=50) | Elective laparoscopic cholecystectomy | Acute cholecystitis, required cholangiogram, complicated procedure. | Wound infection, same day discharge, abdominal pain, shoulder pain, nausea. |
| Lucarelli21 | 30 (20 F) | suction drain (n=15) no drain (n=15) | Laparoscopic cholecystectomy for acute calculous cholecystitis | Symptoms present for >1 week, gangrenous or emphysematous cholecystitis, previous upper abdominal surgery, presence of significant medical diseases, coexisting common bile duct stones with ductal dilatation, acute cholangitis, acutepancreatitis. | Presence of subhepatic fluid collection at ultrasonographic examination of the abdomen 24 hours after surgery, postoperative abdominal and shoulder tip pain, useof analgesics, and morbidity. |
| Mrozowicz14 | 150 (117 F) | Closed passive drain (n=80). no drain (n=70). | Symptomatic cholelithiasis. | Conversion to open surgery, obstructive jaundice, peritonitis, suspicion of cancer, intra-operative injury, intra-operative hemorrhage. | Mortality, abdominal collections, wound infection, chest infection, hospital stay, abdominal pain, shoulder pain, nausea and vomiting. |
| Nomdedeu11 | 50 (35 F) | Suction drain (n=25) no drain (n=25) | Symptomatic gallstones. | Cholecystitis, coagulopathy, abnormal cholangiogram, conversion to open cholecystectomy, biliary colic, gallbladder rupture during surgery, Liver disease. | Abdominal collections and hospital stay. |
| Picchio.20 | 106 (82 F) | suction drain (n=53) no drain (n=53) | Elective laparoscopic cholecystectomy | Patients with acute cholecystitis, cholangitis, or pancreatitis, intraoperative common bile duct exploration or any other additional procedure performed. | Presence of subhepatic fluid collection at ultrasonographic examination of the abdomen 24 hours after surgery postoperative abdominal and shoulder tip pain, use of analgesics, nausea, vomiting, and morbidity. |
| Shamim19 | 170 (136 F) | Passive open drain (n=79) no drain (n=76) | Patients with chronic calculous cholecystitis were included in the study. | Acute cholecystitis, choledocholithiasis, acute pancreatitis, previous upper abdominal surgery, patients who require conversion and elective subhepatic drainage, cases with incomplete patients' data, and patients who were lost to follow-up. | Subdiaphragmatic air volume, drainage volume, subhepatic fluid collections, postoperative complications. |
| Thiebe.10 | 279 | Closed passive drain (n=131). no drain (n=148). | Elective laparoscopic cholecystectomy | Conversion to open surgery, liver bed bleeding, acute cholecystitis. | Abdominal collections, chest infection, other chest complications, pain, analgesic requirements, and hospital stay. |
| Tzovaras.16 | 565 (393 F) | Passive open drain (n=284) no drain (n=281) | Elective laparoscopic cholecystectomy | Acute cholecystitis, cholangitis, or pancreatitis, contraindication for the laparoscopic approach, common bile duct exploration or any other additional procedure | Morbidity, postoperative pain, hospital stay. |
| Uchiyama.15 | 120 (66 F) | Passive open drain (n=60) no drain (n=60) | Elective laparoscopic cholecystectomy for cholecystolithiasis or gallbladder polyp | > 80 years, acute cholecystitis and choledocholithiasis, history of upper laparotomy, severe comorbidities including cardiopulmonary disease or a hemorrhagic tendency due to cirrhosis were also excluded. | Postoperative pain 6, 48, 72, 96 hours after surgery, number of patients requiring analgesics, postoperative complications, changes in maximum body temperature for 4 consecutive days postoperatively. |
Risk of Bias
All the trials had adequate follow-up. Eleven trials had adequate generation of randomization.9,11,14–21 Allocation concealment was adequate in 9 studies.11,13,15–21 The trials of Picchio et al20 and Lucarelli et al21 had adequate blinding of patients, surgeons, and assessors. Sample size calculation was reported in 7 trials.15–17,19–21 Intention-to-treat analysis was used in 10 trials.9,11,13,15–21 In one trial 7 patients were excluded because of conversion to open cholecystectomy.14 In the study of Georgiou et al,17 14 patients were excluded because an inflamed gallbladder was present at the operation. Another trial had 6 crossovers from the no drain group to the drain group and 3 crossovers from the drain group to the no drain group and used a per-protocol analysis.10 The trials of Picchio et al and Lucarelli et al had no risk of bias and were considered of high quality.
Mortality and Morbidity Rates
The only trial that reported deaths in both groups was the trial by Mrozowicz et al.14 One patient in the drain group died of a respiratory infection. Two patients in the no drain group died of a stroke and cardiac failure, which were considered unrelated to surgery (OR, 0.43; 95% CI, 0.04 to 4.85; P = .5).
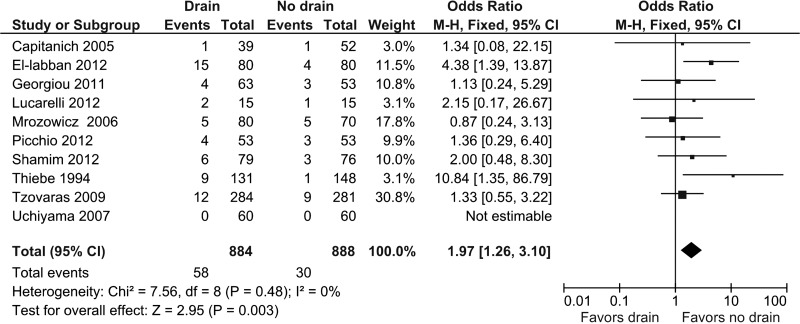
Ten studies provided information about overall complications.10,13–21 Complications were present in 58 of 884 patients (6.6%) in the drain group and 30 of 888 patients (3.4%) in the no drain group. Pooled analysis showed a statistically significant OR in favor of the no drain group (OR, 1.97; 95% CI, 1.26 to 3.10; P = .003) (Figure 2). Heterogeneity was not statistically significant (I2 = 0%, P = .48).
Figure 2.
Forest plot showing overall complications in drain group compared with no drain group. M-H, Mantel-Haenszel.
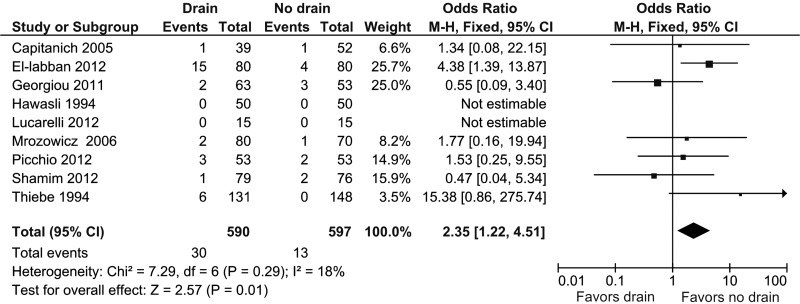
Nine studies provided information about wound infection.13,14,17–21 Wound infection was present in 30 of 590 patients (5.1%) in the drain group and 13 of 597 patients (2.2%) in the no drain group. Pooled analysis showed a statistically significant OR in favor of the no drain group (OR, 2.35; 95% CI, 1.22 to 4.51; P = .01) (Figure 3). Heterogeneity was not statistically significant (I2 = 18%, P = .29).
Figure 3.
Forest plot showing wound infection in drain group compared with no drain group. M-H, Mantel-Haenszel.
Presence of Subhepatic Fluid Collection
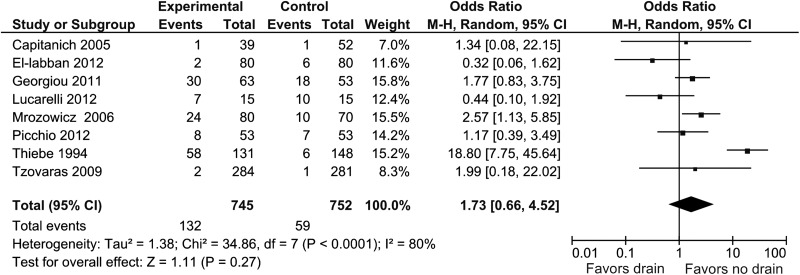
Eight studies provided information about the presence of subhepatic fluid collection on the first postoperative day.10,13,14,16–18,20,21 Subhepatic fluid collection was present in 132 of 745 patients (17.7%) in the drain group and 59 of 752 patients (7.8%) in the no drain group. Pooled analysis showed no statistically significant difference (OR, 1.73; 95% CI, 0.66 to 4.52; P = .27) (Figure 4). Heterogeneity was statistically significant (I2 = 80%, P < .0001).
Figure 4.
Forest plot showing presence of subhepatic fluid collection in drain group compared with no drain group. M-H, Mantel-Haenszel.
Three studies provided information about the quantity of subhepatic fluid collection on the first postoperative day.19–21 No difference was detected in both groups (SMD, –0.14; 95% CI, –0.56 to 0.28; P = .50) (Figure 5). Heterogeneity was statistically significant (I2 = 63%, P < .07).
Figure 5.
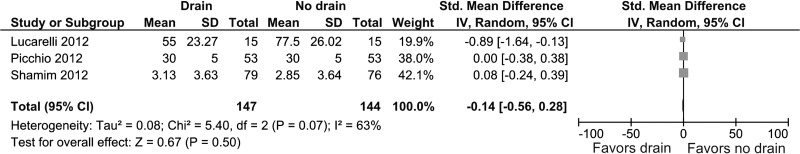
Forest plot showing quantity of subhepatic fluid collection (in milliliters) in drain group compared with no drain group.
Pain After Surgery
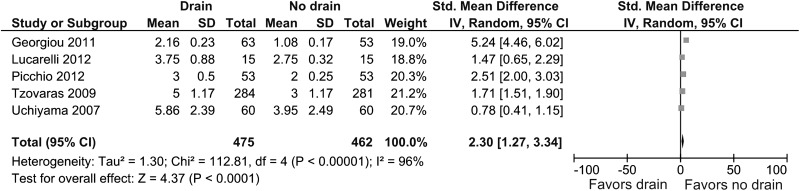
Five studies provided information about the severity of abdominal pain 24 hours after surgery.15–17,20,21 Abdominal pain 24 hours after surgery was less severe in the no drain group (SMD, 2.30; 95% CI, 1.27 to 3.34; P < .0001) (Figure 6). Heterogeneity was statistically significant (I2 = 96%, P < .00001).
Figure 6.
Forest plot showing severity of abdominal pain 24 hours after surgery in drain group compared with no drain group.
Two studies provided information about the severity of shoulder tip pain 24 hours after surgery.20,21 No significant difference was found between the two groups (SMD, 0.93, 95% CI, –0.98 to 2.85; P = .34) (Figure 7). Heterogeneity was statistically significant (I2 = 96%, P < .00001).
Figure 7.
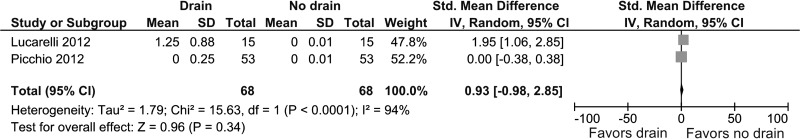
Forest plot showing severity of shoulder tip pain 24 hours after surgery in drain group compared with no drain group.
Two studies provided information about the requirement of parenteral ketorolac between the two groups.20,21 No difference was detected in both groups (SMD, 2.98; 95% CI, –2.09 to 8.04; P = .25) (Figure 8). Heterogeneity was statistically significant (I2 = 99%, P < .00001).
Figure 8.
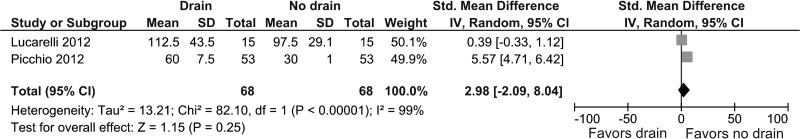
Forest plot showing parenteral ketorolac consumption (in milliliters) in drain group compared with no drain group.
Other Outcomes
The other outcomes assessed are reported in Table 2. The occurrences of nausea and vomiting were similar in both groups. Similarly, hospital stay did not differ between the two groups.
Table 2.
Meta-analysis of other outcomes.
| Outcome | No. of studies | Incidence/duration |
OR or SMD |
95 % CI | P | I2 (%) | |
|---|---|---|---|---|---|---|---|
| Drain (%) | No drain (%) | ||||||
| Nausea | 59,14,17,20,21 | 16.9 | 15.2 | 1.12 | 0.71–1.77 | 0.62 | 0 |
| Vomiting | 317,20,21 | 14,9 | 12.1 | 1.20 | 0.62–2.33 | 0.58 | 0 |
| Hospital stay | 710,11,14–16,20,21 | – | – | 0.37 | –0.16–0.89 | 0.17 | 95 |
OR, odds ratio; SMD, standardized mean difference; CI, confidence interval.
Subgroup Analysis
Two studies were considered of high quality.20,21 The outcomes analyzed are reported in Table 3. Pooled data showed no significant difference between the two groups with the exception of the severity of abdominal pain, which was significantly lower in the no drain group.
Table 3.
| Outcome | Incidence/mL/severity/duration |
OR or SMD |
95 % CI | P | I2 (%) | |
|---|---|---|---|---|---|---|
| Drain (%) | No drain (%) | |||||
| Morbidity | 8.8 | 5.9 | 1.55 | 0.42–5.76 | 0.51 | 0 |
| Presence of abdominal collection | 22.0 | 25.0 | 0.03 | −0.16–0.10 | 0.65 | 30 |
| Quantity of abdominal collection | – | – | −0.38 | −1.24–0.48 | 0.39 | 76 |
| Severity of abdominal pain | – | – | 2.04 | 1.03–3.06 | <0.0001 | 78 |
| Severity of shoulder tip pain | – | – | 0.93 | −0.98–2.85 | 0.34 | 94 |
| Hospital stay | – | – | –0.05 | −0.39–0.29 | 0.77 | 0 |
OR, odds ratio; SMD, standardized mean difference; CI, confidence interval.
There was only 1 trial that compared drains versus no drains in the setting of emergency LC for acute calculous cholecystitis.21 This trial found no significant difference between the two groups with respect to morbidity rate, presence and quantity of abdominal fluid collection, severity of abdominal and shoulder tip pain, parenteral ketorolac consumption, and hospital stay.
Four studies compared suction drainage and no drainage.9,11,20,21 However, only 2 studies reported data suitable for our meta-analysis,20,21 so the results are equal to those reported in Table 3.
DISCUSSION
The main reason to use prophylactic drainage in LC is to reduce complications such as intra-abdominal collections that require treatment and to detect bile leak, thereby decreasing the overall mortality and morbidity rates. At present, the rate of biliary complications after LC is 0.4% (range, 0.1% to 0.9%)25 and postoperative hemorrhagic complications are similarly very rare. Nevertheless, this meta-analysis showed a consistent trend in favor of the no drain approach in terms of overall morbidity, a result that may be attributed to the combined effect of other more common complications.
In particular, the wound infection rate was lower in the no drain group. Port-site infection is a minor complication that affects 1.1% to 7.9% of patients after LC.26,27 The use of drains seems to improve the incidence of this complication, possibly related to the presence of a foreign body.8 Reducing the permanence of the drain after surgery is a valid method to decrease wound infection rates.20
The use of drains may reduce complications because drains can evacuate subhepatic collections. However, an experimental study showed that, when a drain is inserted in the peritoneal cavity that contains no fluids, it is quickly surrounded by omentum and completely occluded within 48 hours.28 The absence of any significant difference with respect to either the presence or the quantity of subhepatic collections in the two groups, as shown in our meta-analysis, confirms that drains have no effect on fluid evacuation in the abdominal cavity. The tendency toward larger subhepatic collections in the no drain group in the study of Lucarelli et al21 seems to support the use of drainage in LC for acute calculous cholecystitis. However, the limited number of patients analyzed in this trial should be confirmed by further larger studies.
There is no general consensus on the use of one scale for measuring pain. We analyzed only studies using a validated scale.29 Moreover, we reported postoperative pain at a fixed time: 24 hours after surgery. Despite the few studies analyzed, in particular for shoulder tip pain, our results are relevant because of their reduced amount of assessment bias. In particular, the studies of Picchio et al20 and Lucarelli et al21 are significant because they represent a rare case in surgery in which adequate blinding of investigators, participants, and assessors was possible because sham drainage was used in the no drain group.
LC has progressed dramatically in the past decade because the period of hospitalization has been shortened and quick recovery has become possible as a result of small operative wounds and mild postoperative pain. In the literature the effect of subhepatic drains on postoperative pain is controversial. This datum is confirmed by the heterogeneity of the results of the studies included in our meta-analysis. However, the overall outcomes clearly showed a significant reduction in the severity of abdominal pain when drainage was not used. The increase in pain in patients with drains is probably because of irritation of the peritoneum and skin at the entry point of the drains. When postoperative pain was studied in depth by assessing visual analog scale scores at different times after surgery, the results favored the no drain group, thus confirming the finding of our meta-analysis.15 However, the difference is not sufficiently significant to lead to different postoperative analgesic requirement recommendations.
Postoperative nausea and vomiting have been reported with an incidence of 53% to 72% after LC.30 This meta-analysis confirms the relevant presence of both vomiting and nausea after LC without any significant difference between the drain and no drain groups.
Assessment of recovery by analyzing hospital stay is not fully reliable because many factors are not directly related to the operation (eg, patient's motivation, external uncontrolled advice, and insurance coverage for disability) and may influence the results in different studies. In meta-analyses with different studies from different health care systems, this is particularly true. This might be a reason why the lower rates of morbidity and abdominal pain do not lead to early postoperative discharge, as shown in our meta-analysis. However, it should be emphasized that the routine placement of subhepatic drains for at least 24 hours may hinder the early discharge proposed for day-case LC to save costs.8
Subgroup analysis confirmed the results of the global analysis. In particular, our data were unable to prove that use of suction drains has any significant effect on either abdominal or shoulder tip pain after LC, as was shown in the trial of Jorgensen et al.31
With respect to the previous meta-analysis by Gurusamy et al,8 the main strength of our meta-analysis is the considerable number of new trials included with satisfactory methodologic quality. However, a claim should be made that high-quality randomized trials should be performed, according to what was suggested by Gurusamy et al and performed in the studies of Picchio et al21 and Lucarelli et al.21 With respect to the role of drainage in LC for acute calculous cholecystitis, no definitive conclusion can be drawn based on our meta-analysis because only 1 small study without a power analysis calculation was included.21 We are waiting for the results of a randomized trial being conducted in patients with acute cholecystitis that is currently recruiting participants.32
CONCLUSION
This study was unable to prove that drains were useful in reducing complications in LC. There is still no evidence present in the setting of emergent LC. However, it is reasonable to avoid drain insertion when a dry operatory field is obtained at the end of the procedure.
Contributor Information
Marcello Picchio, Department of Surgery, Hospital “P. Colombo,” Velletri, Italy..
Pierino Lucarelli, Basildon Hospital, Basildon, England..
Annalisa Di Filippo, Department of Surgery, University of Rome “La Sapienza,” Terracina, Italy..
Francesco De Angelis, Department of Surgery, University of Rome “La Sapienza,” Terracina, Italy..
Francesco Stipa, Department of Surgery, Hospital “S. Giovanni-Addolorata,” Rome, Italy..
Erasmo Spaziani, Department of Surgery, University of Rome “La Sapienza,” Terracina, Italy..
References:
- 1. Petrowsky H, Demartines N, Rousson V, Clavien PA. Evidence-based value of prophylactic drainage in gastrointestinal surgery: a systematic review and meta-analyses. Ann Surg. 2004;240:1074–1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Launay-Savary MV, Slim K. Evidence-based analysis of prophylactic abdominal drainage. Ann Chir. 2006;131:302–305. [DOI] [PubMed] [Google Scholar]
- 3. Gurusamy KS, Samraj K. Routine abdominal drainage for uncomplicated open cholecystectomy. Cochrane Database Syst Rev. 2007;(2):CD006003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gallstones and Laparoscopic Cholecystectomy. National Institutes of Health Consensus Development Conference Statement. September 14–16, 1992. Available at: http://consensus.nih.gov/1992/1992GallstonesLaparoscopy090html.htm Accessed July 24, 2013.
- 5. Strasberg SM. Clinical practice. Acute calculous cholecystitis. N Engl J Med. 2008;358:2804–2811. [DOI] [PubMed] [Google Scholar]
- 6. Askew J. A survey of the current surgical treatment of gallstones in Queensland. Aust N Z J Surg. 2005;75:1086–1089. [DOI] [PubMed] [Google Scholar]
- 7. Navez B, Ungureanu F, Michiels M, et al. Surgical management of acute cholecystitis: results of a 2-year prospective multicenter survey in Belgium. Surg Endosc. 2012;26:2436–2445. [DOI] [PubMed] [Google Scholar]
- 8. Gurusamy KS, Samraj K, Mullerat P, Davidson BR. Routine abdominal drainage for uncomplicated laparoscopic cholecystectomy. Cochrane Database Syst Rev. 2007;3:CD006004. [DOI] [PubMed] [Google Scholar]
- 9. Hawasli A, Brown E. The effect of drains in laparoscopic cholecystectomy. J Laparoendosc Surg. 1994;4:393–398. [DOI] [PubMed] [Google Scholar]
- 10. Thiebe U, Eggert A. Drainage after laparoscopic cholecystectomy. Minim Invasive Chir. 1994;3:90–92. [Google Scholar]
- 11. Nomdedeu J, Escrig J, Salvador JL. Systematic placement of drains in laparoscopic cholecystectomy. A prospective study. Rev Soc Valencia Patol Dig. 1996;15:299–300. [Google Scholar]
- 12. Nursal TZ, Yildirim S, Tarim A, et al. Effect of drainage on postoperative nausea, vomiting, and pain after laparoscopic cholecystectomy. Langenbecks Arch Surg. 2003;388:95–100. [DOI] [PubMed] [Google Scholar]
- 13. Capitanich P, Segundo UL, Malizia P, Herrera J, Iovaldi ML. Usefulness of prophylactic drainage in laparoscopic cholecystectomy. Randomized prospective report. Prensa Med Argent. 2005;92:623–627. [Google Scholar]
- 14. Mrozowicz A, Rucinski P, Polkowski WP. Routine drainage of the subhepatic area after laparoscopic cholecystectomy. Prospective, controlled study with random patient selection. Pol Przegl Chir. 2006;78:597–609. [Google Scholar]
- 15. Uchiyama K, Tani M, Kawai M, Terasawa H, Hama T, Yamaue H. Clinical significance of drainage tube insertion in laparoscopic cholecystectomy: a prospective randomized controlled trial. J Hepatobiliary Pancreat Surg. 2007;14:551–556. [DOI] [PubMed] [Google Scholar]
- 16. Tzovaras G, Liakou P, Fafoulakis F, Baloyiannis I, Zacharoulis D, Hatzitheofilou C. Is there a role for drain use in elective laparoscopic cholecystectomy? A controlled randomized trial. Am J Surg. 2009;197:759–763. [DOI] [PubMed] [Google Scholar]
- 17. Georgiou C, Demetriou N, Pallaris T, Theodosopoulos T, Katsouyanni K, Polymeneas G. Is the routine use of drainage after elective laparoscopic cholecystectomy justified? A randomized trial. J Laparoendosc Adv Surg Tech A. 2011;21:119–123. [DOI] [PubMed] [Google Scholar]
- 18. El-Labban G, Hokkam E, El-Labban M, Saber A, Heissam K, El-Kammash S. Laparoscopic elective cholecystectomy with and without drain: a controlled randomised trial. J Minim Access Surg. 2012;8:90–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shamim M. Routine sub-hepatic drainage versus no drainage after laparoscopic cholecystectomy: open, randomized, clinical trial. Indian J Surg. 2013;75:22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Picchio M, De Angelis F, Zazza S, et al. Drain after elective laparoscopic cholecystectomy. A randomized multicentre controlled trial. Surg Endosc. 2012;26:2817–2822. [DOI] [PubMed] [Google Scholar]
- 21. Lucarelli P, Picchio M, Martellucci J, et al. Drain after laparoscopic cholecystectomy for acute calculous cholecystitis. A pilot randomized study. Indian J Surg. 2012;74:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151:65–94. [DOI] [PubMed] [Google Scholar]
- 23. Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions, version 5.1.0. Available at: http://www.cochrane-handbook.org. Published 2011 Accessed July 24, 2013.
- 24. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chiche L, Letoublon C. Traitment des Complications de la Cholecystctomie. In: EMC, Techniques Chirurgicales-Appareil Digestif. Paris: Elsevier Masson SAS; 2010. 40–960. [Google Scholar]
- 26. The Southern Surgeons Club. A prospective analysis of 1518 laparoscopic cholecystectomies. N Engl J Med. 1991;324:1073–1078. [DOI] [PubMed] [Google Scholar]
- 27. Harling R, Moorjani N, Perry C, MacGowan AP, Thompson MH. A prospective, randomised trial of prophylactic antibiotics versus bag extraction in the prophylaxis of wound infection in laparoscopic cholecystectomy. Ann R Coll Surg Engl. 2000;82:408–410. [PMC free article] [PubMed] [Google Scholar]
- 28. Agrama HM, Blackwood JM, Brown CS, Machiedo GW, Rush BF. Functional longevity of intraperitoneal drains: an experimental evaluation. Am J Surg. 1976;132:418–421. [DOI] [PubMed] [Google Scholar]
- 29. Huskisson EC. Measurement of pain. Lancet. 1974;2:1127–1131. [DOI] [PubMed] [Google Scholar]
- 30. Feo CV, Sortini D, Ragazzi R, De Palma M, Liboni A. Randomized clinical trial of the effect of preoperative dexamethasone on nausea and vomiting after laparoscopic cholecystectomy. Br J Surg. 2006;93:295–299. [DOI] [PubMed] [Google Scholar]
- 31. Jorgensen JO, Gillies RB, Hunt DR. A simple and effective way to reduce postoperative pain after laparoscopic cholecystectomy. Aust N Z J Surg. 1955;65:466–469. [DOI] [PubMed] [Google Scholar]
- 32. Drainage is not necessary procedure after laparoscopic cholecystectomy due to severe acute cholecystitis. ClinicalTrials.gov identifier NCT01625247. http://clinicaltrials.gov/show/NCT01625247 Accessed July 24, 2013.