Abstract
Septins are filament-forming GTP-binding proteins that act as scaffolds in diverse cell functions including division, polarity and membrane remodeling. In a variety of fungal pathogens, it has been observed that septins are required for virulence because cells are unable to survive or are misshapen when septins are mutated. Cell morphology is interconnected with pathogenesis and thus septin mutants displaying aberrant cell morphologies are commonly deficient in host tissue invasion. The degree to which septins orchestrate versus maintain changes in fungal cell morphology during pathogenesis remains to be determined. Aside from the importance of septins in the process of pathogenesis, animal and plant fungal pathogens display complexity in septin form, dynamics, and function not seen in S. cerevisiae making these organisms important models for uncovering diversity in septin behavior. Additionally, host septins have recently been implicated in the process of C. albicans invasion, motivating the need to examine host septins in fungal pathogenesis. Understanding the role of septins in the host-pathogen interaction not only illuminates pathogenesis mechanisms but importantly also expands our understanding of septin biology in general.
Keywords: Septin, morphology, cytoskeleton, fungal pathogens
How is septin function linked to assembly of filaments?
Septins are a family of filament-forming GTP-binding proteins that function in eukaryotic cell compartmentalization [1-4]. In Saccharomyces cerevisiae, the organism in which septins were discovered and have been most intensively studied, five mitotic septin proteins (Cdc3, Cdc10, Cdc11, Cdc12, Shs1) form an hourglass structure associated with the plasma membrane at the mother-bud neck (Figure 1a) [5,6]. At this junction they act as a scaffold for proteins involved in cell division and septation [3,7]. Additionally, both in yeast and at the base of primary cilia, higher order septin structures can compartmentalize membranes by acting as diffusion barriers [8-10]. Recently, septins have been also been implicated in cell functions as diverse as calcium signaling, membrane remodeling, and cell morphology [11-13]. Additionally, both host and microbial septins have newly emerging roles in pathogenesis that are related to cell shape and invasion of host tissues [14]. How a single class of proteins touches on such diverse cell functions remains a critical open question.
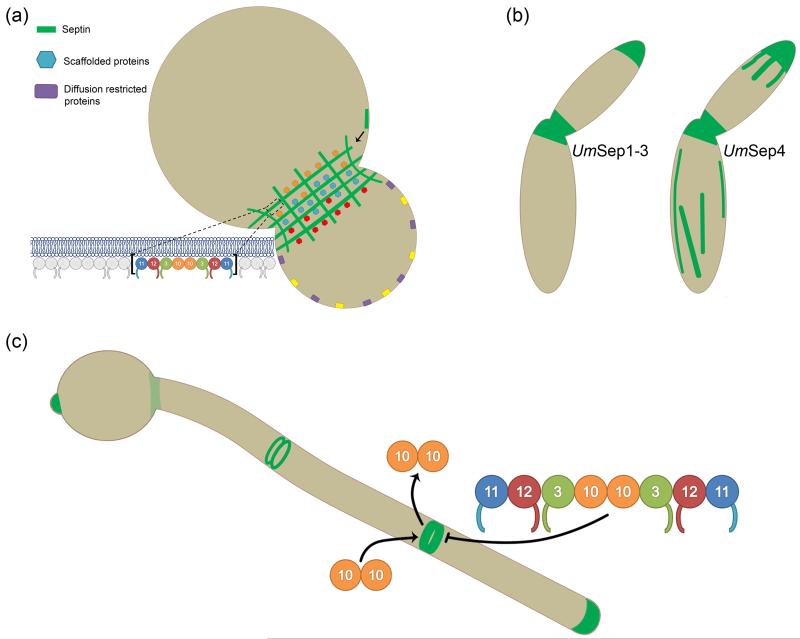
Figure 1.
Septin localization and dynamics. (a) In S. cerevisiae, septins localize to the bud-neck plasma membrane in a gauze-like arrangement of filaments that act as molecular scaffolds for many proteins, some of which preferentially localize to one side of the bud-neck [4]. Septin higher-order structures assemble from palindromic rod subunits via a process involving filament annealing and once assembled are capable of restricting membrane associated proteins to one side of the cell [3,22]. (b) In the plant pathogen U. maydis, septins Sep1, Sep2, and Sep3 localize to the cell middle and at growing tips [30]. Notably, Sep4 also localizes at these sites but additionally forms fibers structures running throughout the cell, which partially colocalize with, but do not depend on, microtubules [30]. (c) Though all septins colocalize in C. albicans hyphae, their dynamics are separable. While Cdc3, Cdc11, Cdc12 and Shs1 assembled into higher order structures do not readily exchange with cytoplasmic septins as determined by FRAP, Cdc10 does. Interestingly, Cdc10, the only septin lacking a c-terminal coiled-coil domain, was found to recover after photobleaching only when Shs1 was not mutated [29].
The number of septin genes varies widely between eukaryotic organisms, from two in nematodes to 13 in humans [15-17]. Despite this variability in number, septins have been found to assemble into soluble hetero-oligomeric rod shaped complexes, typically containing two copies of each septin protein when immunoprecipitated from different cell types (Figure 1a) [18-21]. Septins interact with one another via two interfaces, one of which is a surface created by the N- and C- termini that are brought into proximity when the polypeptide is folded, and the other surface is the GTP binding domain [18]. In the formation of higher order structures such as rings and hourglasses involved in cytokinesis, septin rods associate with the plasma membrane then diffuse and collide to form short filaments by end-on association, termed annealing. These short filaments subsequently merge together in the plane of the membrane to form the functional higher order structure [22]. Transmission electron microscopy and electron tomography of septin higher-order structures in S. cerevisiae has revealed that septin filaments may be paired and run in two orthogonal arrays, forming assemblies resembling “gauzes” (Figure 1a) [5,6,23]. At cytokinesis, the septin hourglass rapidly rearranges, as demonstrated by fluorescence polarization microscopy and fluorescence recovery after photobleaching (FRAP), likely by a process that involves the loss of a large proportion of septins via filament fragmentation [24-27]. How the fundamental characteristics of septin complexes and filaments relate to their broad cellular functions remains to be described.
The links between septin properties such as filament formation and functions such as scaffolding are ready to be investigated at both the molecular and biophysical level. For example, what role, if any does the GTPase cycle play in dynamic rearrangements of septin structures? Is there exchange of either rods or individual monomers within filaments? Why is a filament-forming protein that makes flexible filaments used to build scaffolds and barriers [22]? What direct interactions occur at septin assemblies and what is the molecular basis for scaffolding? Do septins restrict membrane-associated protein to specific regions of the cell by influencing membranes or by acting as a physical barrier? Functions have been ascribed mostly based on phenotypes observed upon deletion of septin genes, many of which could be indirect consequences of losing septins. Thus, many questions regarding the molecular mechanism of septin function are ripe for examination.
In order to understand the fundamental properties and mechanistic function of septin assemblies, work must be performed in a variety of eukaryotic organisms. Septins in fungal pathogens are not only important for their involvement in virulence, but also because they serve as a great comparison to S. cerevisiae and other systems. Septin form and dynamics appear different in even somewhat closely related fungal models making fungi powerful systems for analyzing septin regulation. Furthermore, unlike mammals which express over 10 different septins, most fungi have a minimal set of septins simplifying functional analysis. We review here how septins are involved in fungal pathogenesis and how our understanding of septins in this context expands our understanding of septins in eukaryotes as a whole.
How do septin localizations and dynamics vary in different pathogenic fungi?
The localization and dynamics of septin structures have been examined in a number of pathogenic fungal organisms. When growing as yeast, all Candida albicans mitotic septins co-localize at the bud neck in much the same way as septins in S. cerevisiae [13,28]. Likewise, after bud emergence in both organisms, FRAP studies have shown little or no exchange with cytoplasmic septin subunits or rearrangement within the higher order structure [29]. Interestingly, as a point of contrast, septins in several other fungal pathogens localize somewhat independently of one another and/or form additional assemblies. In the plant pathogen Ustilago maydis, septins collectively localize to a variety of structures, from collars at the bud-neck to filaments concentrated at growing cell tips that can span the length of the cell [30,31]. Interestingly, UmSep4 (a Cdc10 ortholog), the only septin protein lacking a C-terminal coiled-coil domain, forms fibers running along the length of the cell that partially co-localize with microtubules yet do not depend on microtubules for localization (Figure 1b) [30]. Notably, mammalian septins have been found to associate with microtubules and microtubule associated proteins [32,33]. Do examples of independent septin localizations imply separable functions for individual septins? Are heteromeric complexes of different composition assembled in the same cytoplasm and are monomeric septin proteins able to function on their own? Do distinct septin structures turn over at different rates? Because of the apparent uniformity of septins in S. cerevisiae, fungal pathogens serve as a good model for the diverse septin localizations and function, an understanding that could translate to mammalian cells that contain many more septin proteins.
In most cases, septin localization changes upon the induction of hyphal growth. When stimulated to grow as invasive hyphae, C. albicans septins localize diffusely at the base of the hypha, but are enriched at the growing hyphal tip and sites of septation [34]. As in the yeast state, FRAP analysis of C. albicans septins has revealed that Cdc3, Cdc12 and Sep7/Shs1 are frozen in higher-order structures at sites of septation during hyphal growth [29]. Notably however, Cdc10 appears to turn over at a much faster rate in the same structures and these dynamics are dependent on Sep7/Shs1, again raising the possibility of some unique properties of each septin protein (Figure 1c) [29]. Similarly, individual septins have been localized to distinct structures in hyphae and conidiophores of the infectious basidiomycete Aspergillus fumigatus including rings, puncta, and patches [35]. How do multiple higher order septin structures coexist in the same cytoplasm in pathogenic morphologies? Are different septin structures in the same cytoplasm post-translationally modified to control their localization or do septin interacting proteins dictate the distinct localizations and properties? Interestingly, when the filamentous fungal specific AspE is deleted from Aspergillus nidulans, a nonpathogenic relative of A. fumigatus, AspB-GFP septin structures disappear after septum formation [36]. Does AspE play a role in establishing and maintaining septin structures during hyphal growth? Septins in fungal pathogens provide diversity in localization and dynamics not found in S. cerevisiae and these can serve as a model for how distinct assemblies coexist and function in a single cell.
How do septins influence cell morphology and participate in morphological transitions?
S. cerevisiae cells containing temperature sensitive septin alleles shifted to the restrictive temperature display abnormal elongated bud morphology [37]. The elongated buds form because of a persistent G2 state in which the switch from apical to isotropic growth is blocked (Figure 2a) [38]. The G2 arrest is primarily, if not entirely, due to the stabilization of the Wee1-kinase, Swe1, which requires localization to the septin hourglass for degradation [38-40]. Even subtle defects in septin organization are sufficient to stabilize Swe1 and thus delay the cell cycle in G2 through inhibition of CDK/cylin complexes [41]. Thus, S. cerevisiae cells in which septin rings are improperly formed display an elongated bud phenotype due to persistent presence of the inhibitory Swe1 kinase. Additionally, septin defects can lead to changes in the shape or curvature of the neck suggesting a possible role in local exocytosis [42,43]. Like budding yeast, many fungal pathogens display abnormal morphologies when septins are mutated and serve as a point of comparison for conserved septin function in influencing cell shape.
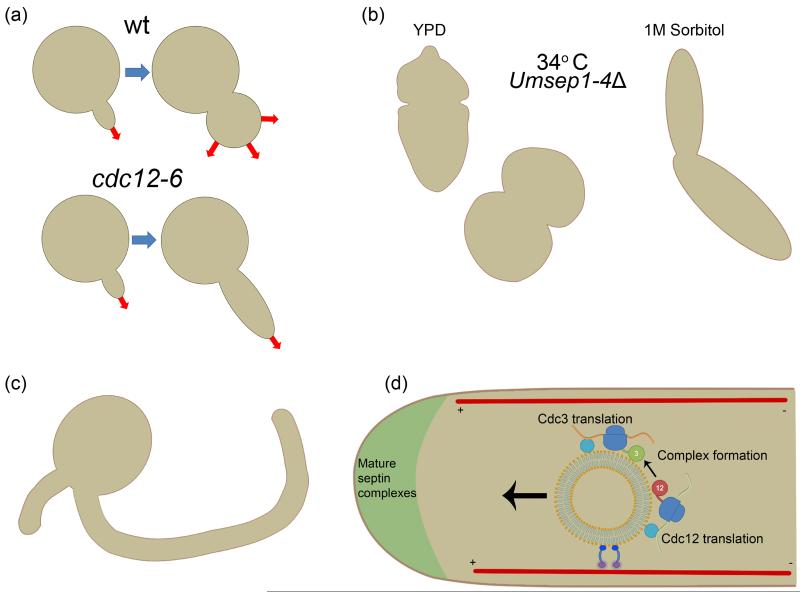
Figure 2.
Septins are required for proper cell morphology. (a) While wild type S. cerevisiae cells undergo an apical to isotropic growth transition early in budding, temperature sensitive septin mutants skip this Swe1 mediated checkpoint [41]. (b) U. maydis cells in which individual septin genes have been deleted remain viable yet display strong morphology defects that are not restricted to one region of the cell. These defects are alleviated upon growth in media containing sorbitol, suggesting aberrant shapes could be due to cell wall imperfections [30]. (c) In C. albians cdc3Δ, cdc12Δ, and cdc12-6 mutants grow with curved hyphae that are unable to appreciably invade host tissues. Additionally, these mutants form new germ tubes axial to the original, unlike wild type cells in which the site of polarity is random, suggesting a role for septins in establishing or maintaining cell polarity [13,44*]. (d) In U. maydis, Cdc3 is translated on endosomes traveling to growing cell tips [48**]. Are the other septins translated on the same endosomes? Could this be the basis for septin complex formation?
Septin deletion mutants in many fungal pathogens resemble the temperature-sensitive S. cerevisiae strains in terms of morphology but remarkably the pathogenic fungal cells are frequently still viable without septins. For example, while Cdc3 and Cdc12 appear to be essential for C. albicans viability, cdc10Δ and cdc11Δ and cdc12-6 cells are viable but mothers are rounded and form elongated buds that display aberrant cell wall deposition [13,44*]. Though no individual septin gene deletions are lethal for vegetative growth of the pathogen Cryptococcus neoformans at 24° C, at 37° C each septin except Cdc10 is required for viability and mutant cells are swollen and frequently form multiple buds [45]. When individual septin genes are deleted in U. maydis, vegetatively growing cells remain viable at low temperatures but display bud-neck and cell wall deposition defects and become abnormally rounded [30,31]. Notably, aberrant cell morphologies in U. maydis and C. neoformans mutant growth are partially rescued by 1M sorbitol, suggesting that cell wall deposition or integrity is compromised in septin mutants (Figure 2b)[30,45]. Are the molecular origins of aberrant cell shape the same in fungal pathogens as they are in S. cerevisiae? Do these organisms use septins as a platform for Swe1 kinase degradation? Does the non-essential function of septins in some systems support that there may be roles for individual septins outside of the complex? Changes in cell morphology are linked to fungal pathogenesis and it is clear that septins play a central role in fungal cell shape.
Many fungal pathogens undergo morphology transitions that are essential for invasion and pathogenesis and septins have been implicated in these transitions. The degree to which septins coordinate these events versus respond to them remains unresolved. Interestingly, in C. albicans, Cdc11 is phosphorylated directly by the cyclin-dependent kinase Cdc28 complexed with hyphal-specific cyclins and the septin-associated kinase Gin4 during hyphal growth [46]. Mutagenesis of these phosphorylation sites results in aberrant hyphal morphologies suggesting a direct role for septins in controlling hyphal morphogenesis in a cyclin/Cdk dependent manner. What is different about septin structures in invasive states that promotes a different form of growth? Future work focused on how post-translational modifications influence septin form and function will be illuminating.
What is the role of septins in determining the stability and number of polarity sites in hyphal cells?
Many fungal pathogens in which septins have been mutated display aberrant hyphal morphologies during invasive growth and this defect is thought to account largely for decreased virulence. Interestingly, there are multiple examples of septin mutants that fail to initiate or maintain polarity, initiate too many sites of polarity, or locate new sites of polarity improperly. When initiated to grow in the hyphal state, C. albicans cdc10Δ, cdc11Δ and cdc12-6 temperature sensitive mutants develop curved hyphae and cell wall deposition is abnormal (Figure 2c)[34,44*]. Likewise, when septins are depleted from Blastomyces dermatitidis, hyphae are curved and thicker than control cells [47]. In A. nidulans, aspbΔ mutant cells display the emergence of bent germ tubes in contrast to wild-type conidia which generally grow via a single straight germ tube [36]. Why are septins required for straight hyphae? Could this be a function to restrict the polarity machinery at the apex of hyphae? In wild-type C. albicans, second germ tubes are placed randomly while septin mutants place second germ tubes proximal to the original implying a role for septins in polarity establishment [13,44*]. In A. nidulans, conidia lacking AspB display the emergence of multiple instead of a single straight germ tube and hyphae contain shorter and more frequent branches suggesting a defect both in selecting sites and in polarity maintenance [36]. Why, in this case, are septins required to suppress sites of polarity? Similarly, U. maydis septins are required for unipolar hyphal growth, and U. maydis frequently grow from two sites of polarity when individual septin genes are deleted [30,48**]. Interestingly, during Magnaporthe oryzae penetration of the rice leaf, septins assemble into a unique ring in the M. oryzae appressorium, a pressurized cell structure responsible for penetrating the rice leaf for invasion [49]. When any septin gene is deleted, the invasive peg is not generated further implicating septins in a function in polarity establishment [49*]. Collectively, these morphology and polarity defects demonstrate a role for septins in producing and maintaining cell shapes required for virulence. Do observed morphology and cell wall defects result solely from misguided cell polarity in the absence of septins or do septins play a more direct role?
How is septin function important on the host side of fungal pathogenesis?
In recent years, septins in host cells have been implicated in bacterial entry and motility but the role of host septins in fungal tissue invasion has remained largely unexplored [50-52]. Exciting and pioneering work has revealed a role for host septins in endocytosis of C. albicans by endothelial cells which occurs during aggressive hematogenously disseminated infections [53**]. During C. albicans invasion, human SEPT7 was found to co-localize with actin and N-cadherin, the cell surface receptor to which C. albicans invasins bind for internalization. Host septins were found to interact with N-cadherin in an actin-dependent manner and N-cadherin knockdown was found to reduce septin recruitment to sites of C. albicans endocytosis. Likewise, SEPT7 knockdown was found to reduce N-cadherin accumulation at sites of C. albicans endocytosis and to significantly reduce the ability of endothelial cells to internalize C. albicans [53**]. This work indicates that host septins could be important for host penetration by diverse intracellular fungal pathogens, a possibility that is worthy of future exploration. Additionally, it is worth investigating which host septin proteins are important for fungal cell entry and how each might behave individually during internalization. Although actin has been explored in great detail in the context of intracellular pathogen invasion, work on host septins in infection lags behind. Additionally, the work done to this point on pathogen invasion motivates further investigation into the mechanistic details of interactions between septins, N-cadherin, and actin.
Why is study of septins in fungal pathogens important?
In short, septins in fungal pathogens are not only relevant for their role in pathogenesis but also because they serve as diverse systems for understanding general septin biology. Unlike S. cerevisiae, septins display a certain degree of independence from one another in fungal pathogens and a better understanding in these organisms could translate well to mammalian systems where there is a more varied milieu of individual septins coexisting in one cell. Additionally, the more complex cell types seen in fungal pathogens present challenges more reminiscent to those experienced by mammalian cells. For example, it was shown in U. maydis that Cdc3 is translated on endosomes traveling along microtubules to cell tips (Figure 2d)[48**]. It was demonstrated that this is the basis for tip localization of septin filaments, but could this also be the basis for septin complex formation? To what degree are the processes conserved in human neurons? In summary, continued mechanistic experiments in fungal pathogens will be important for our general understanding of septin function. These diverse fungal systems often face different problems than S. cerevisiae cells and as such present unique uses of the septin cytoskeleton. The combination of tractability and complex septin biology make fungal pathogens critical models for future study of septins.
Acknowledgements
We thank the laboratory for thoughtful discussion. This work was supported by funding from the National Science Foundation (MCB-507511, to A.S.G.) and by National Institute of General Medical Sciences (T32GM008704, to A.A.B.).
References
- 1.Beise N, Trimble W. Septins at a glance. J Cell Sci. 2011;124:4141–4146. doi: 10.1242/jcs.087007. [DOI] [PubMed] [Google Scholar]
- 2.Spiliotis ET, Gladfelter AS. Spatial guidance of cell asymmetry: septin GTPases show the way. Traffic. 2012;13:195–203. doi: 10.1111/j.1600-0854.2011.01268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mostowy S, Cossart P. Septins: the fourth component of the cytoskeleton. Nat Rev Mol Cell Biol. 2012;13:183–194. doi: 10.1038/nrm3284. [DOI] [PubMed] [Google Scholar]
- 4.Gladfelter AS, Pringle JR, Lew DJ. The septin cortex at the yeast mother-bud neck. Curr Opin Microbiol. 2001;4:681–689. doi: 10.1016/s1369-5274(01)00269-7. [DOI] [PubMed] [Google Scholar]
- 5.Byers B, Goetsch L. A highly ordered ring of membrane-associated filaments in budding yeast. J Cell Biol. 1976;69:717–721. doi: 10.1083/jcb.69.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bertin A, McMurray MA, Pierson J, Thai L, McDonald KL, Zehr EA, Garcia G, 3rd, Peters P, Thorner J, Nogales E. Three-dimensional ultrastructure of the septin filament network in Saccharomyces cerevisiae. Mol Biol Cell. 2012;23:423–432. doi: 10.1091/mbc.E11-10-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh Y, Bi E. Septin structure and function in yeast and beyond. Trends Cell Biol. 2011;21:141–148. doi: 10.1016/j.tcb.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barral Y, Mermall V, Mooseker MS, Snyder M. Compartmentalization of the cell cortex by septins is required for maintenance of cell polarity in yeast. Mol Cell. 2000;5:841–851. doi: 10.1016/s1097-2765(00)80324-x. [DOI] [PubMed] [Google Scholar]
- 9.Takizawa PA, DeRisi JL, Wilhelm JE, Vale RD. Plasma membrane compartmentalization in yeast by messenger RNA transport and a septin diffusion barrier. Science. 2000;290:341–344. doi: 10.1126/science.290.5490.341. [DOI] [PubMed] [Google Scholar]
- 10.Reiter JF, Blacque OE, Leroux MR. The base of the cilium: roles for transition fibres and the transition zone in ciliary formation, maintenance and compartmentalization. EMBO Rep. 2012;13:608–618. doi: 10.1038/embor.2012.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma S, Quintana A, Findlay GM, Mettlen M, Baust B, Jain M, Nilsson R, Rao A, Hogan PG. An siRNA screen for NFAT activation identifies septins as coordinators of store-operated Ca2+ entry. Nature. 2013;499:238–242. doi: 10.1038/nature12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilden JK, Peck S, Chen YC, Krummel MF. The septin cytoskeleton facilitates membrane retraction during motility and blebbing. J Cell Biol. 2012;196:103–114. doi: 10.1083/jcb.201105127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Warenda AJ, Konopka JB. Septin function in Candida albicans morphogenesis. Mol Biol Cell. 2002;13:2732–2746. doi: 10.1091/mbc.E02-01-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douglas LM, Alvarez FJ, McCreary C, Konopka JB. Septin function in yeast model systems and pathogenic fungi. Eukaryot Cell. 2005;4:1503–1512. doi: 10.1128/EC.4.9.1503-1512.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.John CM, Hite RK, Weirich CS, Fitzgerald DJ, Jawhari H, Faty M, Schlapfer D, Kroschewski R, Winkler FK, Walz T, et al. The Caenorhabditis elegans septin complex is nonpolar. EMBO J. 2007;26:3296–3307. doi: 10.1038/sj.emboj.7601775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pan F, Malmberg RL, Momany M. Analysis of septins across kingdoms reveals orthology and new motifs. BMC Evol Biol. 2007;7:103. doi: 10.1186/1471-2148-7-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nishihama R, Onishi M, Pringle JR. New insights into the phylogenetic distribution and evolutionary origins of the septins. Biol Chem. 2011;392:681–687. doi: 10.1515/BC.2011.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sirajuddin M, Farkasovsky M, Hauer F, Kuhlmann D, Macara IG, Weyand M, Stark H, Wittinghofer A. Structural insight into filament formation by mammalian septins. Nature. 2007;449:311–315. doi: 10.1038/nature06052. [DOI] [PubMed] [Google Scholar]
- 19.Field CM, al-Awar O, Rosenblatt J, Wong ML, Alberts B, Mitchison TJ. A purified Drosophila septin complex forms filaments and exhibits GTPase activity. J Cell Biol. 1996;133:605–616. doi: 10.1083/jcb.133.3.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frazier JA, Wong ML, Longtine MS, Pringle JR, Mann M, Mitchison TJ, Field C. Polymerization of purified yeast septins: evidence that organized filament arrays may not be required for septin function. J Cell Biol. 1998;143:737–749. doi: 10.1083/jcb.143.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bertin A, McMurray MA, Grob P, Park SS, Garcia G, 3rd, Patanwala I, Ng HL, Alber T, Thorner J, Nogales E. Saccharomyces cerevisiae septins: supramolecular organization of heterooligomers and the mechanism of filament assembly. Proc Natl Acad Sci U S A. 2008;105:8274–8279. doi: 10.1073/pnas.0803330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bridges AA, Zhang H, Mehta SB, Occhipinti P, Tani T, Gladfelter AS. Septin assemblies form by diffusion-driven annealing on membranes. Proc Natl Acad Sci U S A. 2014 doi: 10.1073/pnas.1314138111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodal AA, Kozubowski L, Goode BL, Drubin DG, Hartwig JH. Actin and septin ultrastructures at the budding yeast cell cortex. Mol Biol Cell. 2005;16:372–384. doi: 10.1091/mbc.E04-08-0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeMay BS, Bai X, Howard L, Occhipinti P, Meseroll RA, Spiliotis ET, Oldenbourg R, Gladfelter AS. Septin filaments exhibit a dynamic, paired organization that is conserved from yeast to mammals. J Cell Biol. 2011;193:1065–1081. doi: 10.1083/jcb.201012143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeMay BS, Noda N, Gladfelter AS, Oldenbourg R. Rapid and quantitative imaging of excitation polarized fluorescence reveals ordered septin dynamics in live yeast. Biophys J. 2011;101:985–994. doi: 10.1016/j.bpj.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vrabioiu AM, Mitchison TJ. Structural insights into yeast septin organization from polarized fluorescence microscopy. Nature. 2006;443:466–469. doi: 10.1038/nature05109. [DOI] [PubMed] [Google Scholar]
- 27.Dobbelaere J, Gentry MS, Hallberg RL, Barral Y. Phosphorylation-dependent regulation of septin dynamics during the cell cycle. Dev Cell. 2003;4:345–357. doi: 10.1016/s1534-5807(03)00061-3. [DOI] [PubMed] [Google Scholar]
- 28.Sudbery PE. The germ tubes of Candida albicans hyphae and pseudohyphae show different patterns of septin ring localization. Mol Microbiol. 2001;41:19–31. doi: 10.1046/j.1365-2958.2001.02459.x. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Novo A, Correa-Bordes J, Labrador L, Sanchez M, Vazquez de Aldana CR, Jimenez J. Sep7 is essential to modify septin ring dynamics and inhibit cell separation during Candida albicans hyphal growth. Mol Biol Cell. 2008;19:1509–1518. doi: 10.1091/mbc.E07-09-0876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alvarez-Tabares I, Perez-Martin J. Septins from the phytopathogenic fungus Ustilago maydis are required for proper morphogenesis but dispensable for virulence. PLoS One. 2010;5:e12933. doi: 10.1371/journal.pone.0012933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boyce KJ, Chang H, D'Souza CA, Kronstad JW. An Ustilago maydis septin is required for filamentous growth in culture and for full symptom development on maize. Eukaryot Cell. 2005;4:2044–2056. doi: 10.1128/EC.4.12.2044-2056.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bai X, Bowen JR, Knox TK, Zhou K, Pendziwiat M, Kuhlenbaumer G, Sindelar CV, Spiliotis ET. Novel septin 9 repeat motifs altered in neuralgic amyotrophy bind and bundle microtubules. J Cell Biol. 2013 doi: 10.1083/jcb.201308068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spiliotis ET. Regulation of microtubule organization and functions by septin GTPases. Cytoskeleton (Hoboken) 2010;67:339–345. doi: 10.1002/cm.20448. [DOI] [PubMed] [Google Scholar]
- 34.Warenda AJ, Kauffman S, Sherrill TP, Becker JM, Konopka JB. Candida albicans septin mutants are defective for invasive growth and virulence. Infect Immun. 2003;71:4045–4051. doi: 10.1128/IAI.71.7.4045-4051.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Juvvadi PR, Fortwendel JR, Rogg LE, Steinbach WJ. Differential localization patterns of septins during growth of the human fungal pathogen Aspergillus fumigatus reveal novel functions. Biochemical and Biophysical Research Communications. 2011;405:238–243. doi: 10.1016/j.bbrc.2011.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hernandez-Rodriguez Y, Hastings S, Momany M. The septin AspB in Aspergillus nidulans forms bars and filaments and plays roles in growth emergence and conidiation. Eukaryot Cell. 2012;11:311–323. doi: 10.1128/EC.05164-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim HB, Haarer BK, Pringle JR. Cellular Morphogenesis in the Saccharomyces-Cerevisiae Cell-Cycle - Localization of the Cdc3 Gene-Product and the Timing of Events at the Budding Site. Journal of Cell Biology. 1991;112:535–544. doi: 10.1083/jcb.112.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Longtine MS, Theesfeld CL, McMillan JN, Weaver E, Pringle JR, Lew DJ. Septin-dependent assembly of a cell cycle-regulatory module in Saccharomyces cerevisiae. Mol Cell Biol. 2000;20:4049–4061. doi: 10.1128/mcb.20.11.4049-4061.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.King K, Kang H, Jin M, Lew DJ. Feedback control of Swe1p degradation in the yeast morphogenesis checkpoint. Mol Biol Cell. 2013;24:914–922. doi: 10.1091/mbc.E12-11-0812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.King K, Jin M, Lew D. Roles of Hsl1p and Hsl7p in Swe1p degradation: beyond septin tethering. Eukaryot Cell. 2012;11:1496–1502. doi: 10.1128/EC.00196-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lew DJ. The morphogenesis checkpoint: how yeast cells watch their figures. Curr Opin Cell Biol. 2003;15:648–653. doi: 10.1016/j.ceb.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Okada S, Leda M, Hanna J, Savage NS, Bi E, Goryachev AB. Daughter cell identity emerges from the interplay of Cdc42, septins, and exocytosis. Dev Cell. 2013;26:148–161. doi: 10.1016/j.devcel.2013.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gladfelter AS, Kozubowski L, Zyla TR, Lew DJ. Interplay between septin organization, cell cycle and cell shape in yeast. J Cell Sci. 2005;118:1617–1628. doi: 10.1242/jcs.02286. [DOI] [PubMed] [Google Scholar]
- *44.Li L, Zhang C, Konopka JB. A Candida albicans temperature-sensitive cdc12-6 mutant identifies roles for septins in selection of sites of germ tube formation and hyphal morphogenesis. Eukaryot Cell. 2012;11:1210–1218. doi: 10.1128/EC.00216-12. For the first time, a Cdc12 temperature sensitive mutant analogous to the heavily studied S. cerevisiae allele was generated to better describe the role of this essential septin in hyphal morphology and virulence. Cells containing the mutant allele display strong morphology and polarity defects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kozubowski L, Heitman J. Septins enforce morphogenetic events during sexual reproduction and contribute to virulence of Cryptococcus neoformans. Mol Microbiol. 2010;75:658–675. doi: 10.1111/j.1365-2958.2009.06983.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sinha I, Wang YM, Philp R, Li CR, Yap WH, Wang Y. Cyclin-dependent kinases control septin phosphorylation in Candida albicans hyphal development. Dev Cell. 2007;13:421–432. doi: 10.1016/j.devcel.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 47.Marty AJ, Gauthier GM. Blastomyces dermatitidis septins CDC3, CDC10, and CDC12 impact the morphology of yeast and hyphae, but are not required for the phase transition. Med Mycol. 2013;51:93–102. doi: 10.3109/13693786.2012.699685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **48.Baumann S, Konig J, Koepke J, Feldbrugge M. Endosomal transport of septin mRNA and protein indicates local translation on endosomes and is required for correct septin filamentation. EMBO Rep. 2014;15:94–102. doi: 10.1002/embr.201338037. In this work, authors demonstrate for the first time that septins are translated on endosomes being shuttled to growing cell tips in the highly polarized phytopathogen U. maydis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *49.Dagdas YF, Yoshino K, Dagdas G, Ryder LS, Bielska E, Steinberg G, Talbot NJ. Septin-mediated plant cell invasion by the rice blast fungus, Magnaporthe oryzae. Science. 2012;336:1590–1595. doi: 10.1126/science.1222934. This work showed that septins in M. oryzae assemble into a ring structure required for the formation of invasive pegs involved in breaking the rice cuticle. In the absence of septins, M. oryzae are unable to cause rice blast disease. [DOI] [PubMed] [Google Scholar]
- 50.Mostowy S, Bonazzi M, Hamon MA, Tham TN, Mallet A, Lelek M, Gouin E, Demangel C, Brosch R, Zimmer C, et al. Entrapment of intracytosolic bacteria by septin cage-like structures. Cell Host Microbe. 2010;8:433–444. doi: 10.1016/j.chom.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 51.Mostowy S, Danckaert A, Tham TN, Machu C, Guadagnini S, Pizarro-Cerda J, Cossart P. Septin 11 restricts InlB-mediated invasion by Listeria. J Biol Chem. 2009;284:11613–11621. doi: 10.1074/jbc.M900231200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mostowy S, Nam Tham T, Danckaert A, Guadagnini S, Boisson-Dupuis S, Pizarro-Cerda J, Cossart P. Septins regulate bacterial entry into host cells. PLoS One. 2009;4:e4196. doi: 10.1371/journal.pone.0004196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **53.Phan QT, Eng DK, Mostowy S, Park H, Cossart P, Filler SG. Role of endothelial cell septin 7 in the endocytosis of Candida albicans. MBio. 2013;4:e00542–00513. doi: 10.1128/mBio.00542-13. Authors demonstrate a role for human Sept7 in the endocytosis of C. albicans by endothelial cells. This is the first time host septins have been implicated in the entry of fungal pathogens. [DOI] [PMC free article] [PubMed] [Google Scholar]