Abstract
Background
Protocadherin8 has been demonstrated to play critical roles in initiation and progression of several human cancers. It is frequently inactivated by promoter methylation in cancers and may be used as a potential biomarker. However, the methylation status of protocadherin8 and its clinical significance in prostate cancer remains largely unknown. The purpose of this study was to evaluate the clinical significance of protocadherin8 methylation in early-stage prostate cancer.
Material/Methods
The promoter methylation status of protocadherin8 in 162 prostate cancer tissues and 47 normal prostate tissues was examined using methylation-specific PCR (MSP). Subsequently, the relationships between protocadherin8 methylation and clinicopathological features of prostate cancer patients and biochemical recurrence-free survival of patients were analyzed.
Results
We found that protocadherin8 methylation occurred frequently in prostate cancer tissues but not in normal prostate tissues. Moreover, protocadherin8 methylation was significantly associated with advanced pathologic stage, higher level of preoperative prostate specific antigen (PSA), higher Gleason score, positive lymph node metastasis, and biochemical recurrence. In addition, patients with protocadherin8 methylated have shorter biochemical recurrence-free survival time than patients without. Multivariate Cox regression analysis revealed that protocadherin8 methylation was an independent predictor of biochemical recurrence-free survival in prostate cancer patients.
Conclusions
Promoter methylation of protocadherin8 is a frequent event in prostate cancer, and might be used as an independent prognostic factor for biochemical recurrence-free survival in patients with prostate cancer.
MeSH Keywords: Biological Markers, DNA Methylation, Prostatic Neoplasms
Background
Prostate cancer is a common malignancy and a main cause of cancer-related death in men [1,2]. In 2014 in the United States, an estimated 233 000 new cases will be diagnosed and 29 480 men will die of the disease [1]. In recent years, with the widely adopted prostate-specific antigen (PSA)-based screening and biopsy program, the majority of prostate cancer can be detected in the early stage and can be treated by radical prostatectomy [3]. However, approximately 30% of prostate cancer patients will experience recurrence after the initial surgery and many of them will develop metastatic disease [4–7]. Prostate cancer is a heterogeneous disease in the clinical course. In some cases, the disease progresses relatively slow, but other cases grow aggressively and rapidly metastasize to other part of the body [8,9]. Currently, the major challenge in prostate cancer management is to predict the outcome of the disease accurately at the time of diagnosis, to identify who will need active treatment [10,11]. Clinical stage, PSA, and Gleason score are currently commonly used as prognostic indicators, but the accuracy is limited. Thus, novel markers are needed to improve the accuracy of prognosis [12,13].
It is well known that the initiation and progression of prostate cancer results from the accumulation of genetic and epigenetic changes [13]. Epigenetic changes are characteristic of nearly all human tumors, including prostate cancer, and include changes in DNA methylation, microRNAs, and histone modifications [14]. Identification of epigenetic changes involved in the initiation and progression of prostate cancer will identify novel diagnostic and prognostic biomarkers and therapeutic targets. Although the exact mechanism of how these epigenetic changes arise in prostate cancer is not well understood, the fact that they occur much more frequently than genetic changes may make them useful as potential biomarkers [14]. DNA methylation is the best studied epigenetic modification in prostate cancers, which occurs at CpG islands in the promoter region of a number of genes and cause transcriptional silencing of gene expression [15,16]. Promoter hypermethylation of critical genes could be useful biomarkers and therapeutic targets for prostate cancer.
The protocadherin8 gene is located on human chromosome 13q14.3; the gen product contains 6 extracellular cadherin domains, a transmembrane domain, and a cytoplasmic domain [17,18]. Protocadherin8 plays crucial roles in cell adhesion, signal transduction, proliferation, migration, and invasion [19–24]. A growing number of studies have demonstrated that protocadherin8 functions as a tumor suppressor in human cancers [19–24]. Moreover, it is frequently inactivated by promoter methylation in bladder cancer, renal cell carcinoma, nasopharyngeal carcinoma, gastric cancer and breast cancer, and is associated with poor prognosis [19–24]. However, the methylation status and its clinical significance in prostate cancer remain unclear.
In the current study, we analyzed the methylation status of protocadherin8 in localized prostate cancer tissues using methylation-specific PCR (MSP). MSP is a major technique for detecting gene methylation and can provide specific and sensitive results [13]. We evaluated the relationship between protocadherin8 and main clinicopathological characteristics of prostate cancer. We also evaluated its association with biochemical recurrence-free survival of patients with prostate cancer in order to analyze its potential as a biomarker in this disease.
Material and Methods
Patients and tissue samples
A total of 162 prostate cancer tissues were obtained from patients with early-stage prostate cancer during radical retropubic prostatectomy at the Third Hospital of Hebei Medical University between 1999 and 2008. Prostate tissues obtained from 47 patients with benign prostatic hyperplasia (BPH) during transurethral resection of prostate at the same time in the same hospital were used as normal controls. These tissues were examined pathologically to exclude the possibility of incidental tumors. None of the patients with prostate cancer received any form of anti-tumor therapy before surgery, and none received adjuvant therapy before recurrence. The samples were flash-frozen in liquid nitrogen at the time of collection and stored at −80°C until used. Biochemical recurrence was defined as the period between radical prostatectomy and the measurement of 2 successive values of serum PSA level ≥0.2 ng/ml [25,26]. The common clinicopathologic parameters were recorded, including preoperative serum PSA, pathological stage, seminal vesicle invasion, Gleason score, lymph node status, margin status, and follow-up data. The patients were followed up at intervals, ranging from 15 months to 60 months. This study was performed according to the Declaration of Helsinki and was approved by the ethics committee of the Third Hospital of Hebei Medical University (No. 19981101THM007). Written informed consent was obtained from each participant. The clinicopathological features of patients with prostate cancer are shown in Table 1.
Table 1.
The clinicopathologic features of patients with prostate cancer (n=162).
| Features | Variables | No. (%) |
|---|---|---|
| Age (years) | <70 | 87 (53.7) |
| ≥70 | 75 (46.3) | |
| Pathological stage | pT2 | 142 (87.6) |
| pT3 | 20 (12.4) | |
| Preoperative PSA | <10 | 75 (46.3) |
| ≥10 | 87 (53.7) | |
| Gleason score | <7 | 69 (42.6) |
| 7 | 48 (29.6) | |
| >7 | 45 (27.8) | |
| Surgical margin status | Positive | 13 (8.0) |
| Negative | 149 (92.0) | |
| Lymph node metastasis | Presence | 15 (9.3) |
| Absence | 147 (90.7) | |
| Biochemical recurrence | Yes | 46 (28.4) |
| No | 116 (71.6) |
Genomic DNA extraction, bisulfite treatment and MSP
Genomic DNA was isolated from frozen tissues using the DNeasy Tissue Kit (Qiagen, Valencia, CA). The isolated DNA was modified with bisulfite using EpiTect Bisulfite Kit (Qiagen, Valencia, CA) and standard protocol as described previously [5,11]. Bisulfite modified DNA was used for MSP with primers specific for either methylated or unmethylated DNA. The primers were used as previously reported [19,20]. Methylated: forward 5′-CGGTTATTGGTTATTCGGTTCC-3′ and reverse 5′-ACGAACTCTAAAAACGCGCG-3′. Unmethylated: forward 5′-GGTGGTTATTGGTTATTTGGTTT-3′ and reverse 5′-CCAACAAACTCTAAAAACACACA-3′. Amplifications were carried out using the following profile: 1 cycle of 95°C for 5 min, 40 cycles of 95°C for 30 s, 60°C for 30 s, and 72°C for 30 s, and 1 cycle of 72°C for 5 min. In vitro methylated DNA and unmethylated DNA (New England Biolabs, Beverly, MA, USA) was used as methylation and unmethylation positive control, and water blanks were included with each assay as previously reported [19,20]. MSP products were separated in 2% agarose gel, stained with ethidium bromide, and visualized under ultraviolet illumination for analysis. Samples were scored as methylation negative when bands were present only in the unmethylated DNA lane and as methylation positive when methylated alleles were present in the methylated DNA lane [19,20].
Statistical analysis
Statistical analysis was done using SAS version 8.0 (SAS Institute, Cary, N.C., USA) software for Windows. The difference of protocadherin8 methylation status between prostate cancer tissues and controls were evaluated using Fisher’s exact test. The associations between protocadherin8 methylation and clinicopathologic parameters were evaluated by chi-square test. For biochemical recurrence-free survival analysis, Kaplan-Meier survival analysis was used and the differences in survival were analyzed using the log-rank test. Univariate and multivariate Cox proportional hazard models were used to evaluate the prognostic effect of protocadherin8 methylation in prostate cancer. A 2-sided p value <0.05 was considered statistically significant [11,19,20,26].
Results
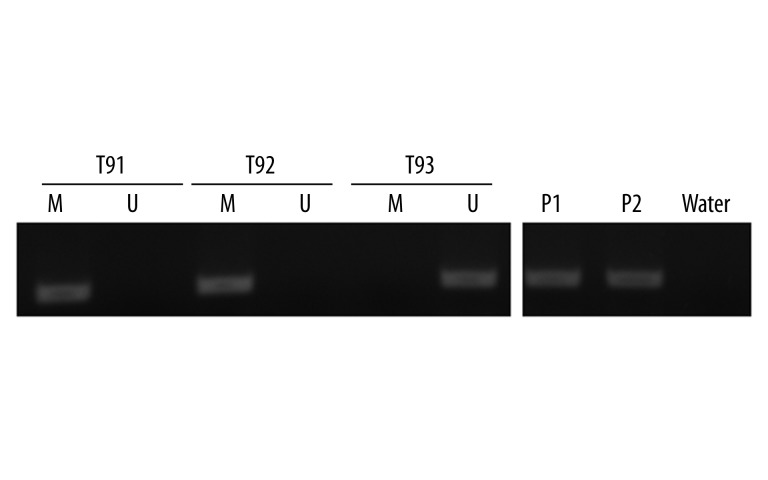
In the current study, the methylation status of protocadherin8 in 162 prostate cancer tissues and 47 controls was detected by MSP. Protocadherin8 methylation was detected in 76 (46.9%) prostate cancer cases (Figure 1). However, no protocadherin8 methylation was found in the controls, and the difference between prostate cancer cases and controls was statistically significant (P<0.0001).
Figure 1.
Representative MSP results for protocadherin8 methylation in tumor samples of patients with prostate cancer. T: tumor sample; M: methylated; U: unmethylated; P1: methylation positive control; P2: unmethylation positive control. T91 and T92 exhibited protocadherin8 methylation, T93 exhibited protocadherin8 unmethylation.
Subsequently, we linked the methylation status of protocadherin8 to clinicopathologic parameters in prostate cancer cases to elucidate its clinical significance. The associations between protocadherin8 methylation and clinicopathologic characteristics are summarized in Table 2. Protocadherin8 methylation was significantly associated with advanced pathologic stage (P=0.0122), higher level of preoperative PSA (P=0.0004), higher Gleason score, (P=0.0187), positive lymph node metastasis (P=0.0314), and biochemical recurrence (P<0.0001). However, it was not significantly associated with age, surgical margin status, or seminal vesicle invasion.
Table 2.
The associations between protocadherin8 promoter methylation and clinicopathologic features of patients with prostate cancer (n=162).
| Features | Variables | No. | M (%) | U (%) | P |
|---|---|---|---|---|---|
| Age | <70 | 87 | 41 (47.1) | 46 (52.9) | 0.6531 |
| ≥70 | 75 | 38 (50.7) | 37 (49.3) | ||
| Stage | pT2 | 142 | 64 (45.1) | 78 (54.9) | 0.0122 |
| pT3 | 20 | 15 (75.0) | 5 (25.0) | ||
| Gleason score | <7 | 69 | 25 (36.2) | 44 (63.8) | 0.0187 |
| ≥7 | 93 | 51 (54.8) | 42 (45.2) | ||
| PSA | <10 | 75 | 24 (32.0) | 51 (68.0) | 0.0004 |
| ≥10 | 87 | 52 (59.8) | 35 (40.2) | ||
| Surgical margin status | Positive | 13 | 7 (53.8) | 6 (46.2) | 0.6015 |
| Negative | 149 | 69 (46.3) | 80 (53.7) | ||
| Lymph node metastasis | Presence | 15 | 11 (73.3) | 4 (26.7) | 0.0314 |
| Absence | 147 | 65 (44.2) | 82 (55.8) | ||
| BCR | Yes | 46 | 37 (80.4) | 9 (19.6) | <0.0001 |
| No | 116 | 39 (33.6) | 77 (66.4) |
M – methylation; U – unmethylation; BCR – biochemical recurrence.
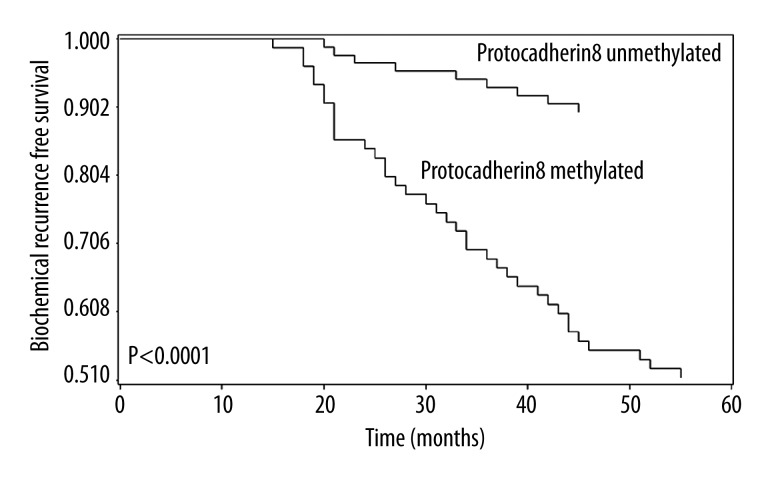
Next, we examined the biochemical recurrence-free survival of patients with prostate cancer according to protocadherin8 methylation status to elucidate its prognostic value. Interestingly, patients with protocadherin8 methylated had worse prognosis than patients without (P<0.0001, Figure 2). To further determine the associations between biochemical recurrence-free survival and potential risk factors, univariate and multivariate Cox regression model analysis was performed. Univariate Cox regression analysis first showed that Gleason score, preoperative PSA level, pathologic stage, and protocadherin8 promoter methylation were risk factors for poor biochemical recurrence-free survival. These risk factors were entered into multivariate Cox regression analysis, revealing that protocadherin8 methylation and Gleason score were independent prognostic risk factors for biochemical recurrence-free survival of patients with prostate cancer. These results are summarized in Table 3.
Figure 2.
Correlations between protocadherin8 methylation and biochemical recurrence-free survival in patients with prostate cancer. Patients with protocadherin8 methylated showed significant shorter biochemical recurrence-free survival time than patients with protocadherin8 unmethylated (log-rank test, P<0.0001).
Table 3.
Univariate and multivariate Cox regression analyses of biochemical recurrence free survival in prostate cancer patients (n=162).
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |
| Age | 1.037 | 0.893–1.392 | 0.8743 | |||
| Protocadherin8 methylation | 3.427 | 1.536–7.641 | <0.0001 | 3.417 | 1.457–6.693 | <0.0001 |
| Gleason score | 2.981 | 1.254–9.771 | <0.0001 | 2.315 | 1.246–7.942 | 0.0021 |
| Stage | 1.725 | 1.067–6.859 | 0.0147 | 1.326 | 0.809–3.168 | 0.0617 |
| Surgical margin status | 1.346 | 0.924–4.285 | 0.0773 | |||
| Lymph node metastasis | 1.142 | 0.773–5.744 | 0.6024 | |||
| PSA | 2.544 | 1.213–6.631 | 0.0083 | 1.436 | 0.852–4.742 | 0.0523 |
HR – Hazard Ratio.
Discussion
Prostate cancer is a major public health problem worldwide. It is a heterogeneous-multifocal disease with prognosis difficult to predict [27,28]. It is of great important to identify novel prognostic and predictive markers to understand this multifaceted disease process and to identify which patients need more aggressive treatment after initial curative surgery [6,7,14–16]. DNA methylation of CpG islands within the promoter region of genes is an alternative mechanism of gene silence to genetic changes and is frequently involved in the development and progression of many types of human cancers, including prostate cancer [3,4]. Aberrant promoter methylation of some genes that are normally unmethylated may be used as potential molecular markers for the diagnosis, surveillance, and prognosis in prostate cancer.
Protocadherin8 is a tumor suppressor gene and is frequently silenced by aberrant promoter methylation in several human cancers. To date, the association between promoter methylation of protocadherin8 and the prognosis of prostate cancer has not been reported. This is the first study to investigate the prognostic value of protocadherin8 methylation in prostate cancer based on a relatively large number of clinical samples. In the current study, we analyzed the methylation status of protocadherin8 in 162 prostate cancer tissues and 47 normal prostate tissues using MSP. We demonstrated that protocadherin8 methylation appeared more frequently in prostate cancer tissues than in normal prostate tissues. Moreover, when we analyzed the association between protocadherin8 methylation and traditional clinicopathologic features in prostate cancer, we found that protocadherin8 methylation was significantly correlated with advanced pathologic stage, higher level of preoperative PSA, higher Gleason score, positive lymph node metastasis, and the occurrence of biochemical recurrence. These results suggest that protocadherin8 methylation plays an import role in the progression of prostate cancer and may be associated with the poor prognosis. To verify this hypothesis, Kaplan-Meier survival analysis was performed, showing that the biochemical recurrence-free survival time of patients with protocadherin8 methylated was significantly shorter than patients without. Moreover, univariate and multivariate Cox regression analysis demonstrated that protocadherin8 methylation is an independent predictor of the prognosis of prostate cancer. These results suggest that the detection of protocadherin8 methylation in tumor samples after surgery may help identify the patients prone to recurrence and could be a novel predictor for disease course in prostate cancer patients.
Our present data demonstrated that aberrant promoter methylation of protocadherin8 occurred frequently in prostate cancer, but not in normal prostate tissues. This result indicates that protocadherin8 methylation is tumor-specific and may be an early event in prostate cancer. This result is consistent with previous studies on other tumors [22,24]. In addition, the clinical significance of protocadherin8 methylation in prostate cancer is similar to findings of studies on other cancers [19–21]. Our findings further verified the possibility of using protocadherin8 methylation as a potential biomarker in prostate cancer. However, the causes of protocadherin8 methylation in prostate cancer are largely unknown. Studies are needed to investigate the cause of protocadherin8 methylation and to discover how to reverse the methylation status of protocadherin8 in prostate cancer.
Conclusions
Promoter methylation of protocadherin8 is a frequent event in prostate cancer, and is associated with traditional risk factors of poor prognosis and shorter biochemical recurrence-free survival time. The present results indicate that protocadherin8 methylation is an independent prognostic factor for biochemical recurrence-free survival in prostate cancer patients. Our findings suggest that protocadherin8 methylation might be a useful prognostic biomarker and a potential therapeutic target for prostate cancer.
Footnotes
Conflicts of interest
None.
Source of support: This study was supported by the Scientific Research Project of the Department of Health of Heilongjiang Province (No. 2009-328)
References
- 1.Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. Cancer J Clin. 2014;64(1):11–30. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Keane FK, Chen MH, Zhang D, et al. The likelihood of death from prostate cancer in men with favorable or unfavorable intermediate-risk disease. Cancer. 2014;120(12):1787–93. doi: 10.1002/cncr.28609. [DOI] [PubMed] [Google Scholar]
- 3.Chao C, Chi M, Preciado M, et al. Methylation markers for prostate cancer prognosis: a systematic review. Cancer Causes Control. 2013;24(9):1615–41. doi: 10.1007/s10552-013-0249-2. [DOI] [PubMed] [Google Scholar]
- 4.Daniunaite K, Jarmalaite S, Kalinauskaite N, et al. Prognostic Value of RASSF1 Promoter Methylation In Prostate Cancer. J Urol. 2014 doi: 10.1016/j.juro.2014.06.075. S0022-5347(14)03913-5. [DOI] [PubMed] [Google Scholar]
- 5.Lin YL, Xie PG, Wang L, et al. Aberrant methylation of protocadherin 17 and its clinical significance in patients with prostate cancer after radical prostatectomy. Med Sci Monit. 2014;20:1376–82. doi: 10.12659/MSM.891247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Efthimiou I, Skrepetis K, Bournia E. Single foci prostate cancer: current diagnosis and management. Curr Urol. 2013;7(1):1–6. doi: 10.1159/000343544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ragsdale JW, Halstater B, Martinez-Bianchi V. Prostate cancer screening. Prim Care. 2014;41(2):355–70. doi: 10.1016/j.pop.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 8.Heidenreich A, Bastian PJ, Bellmunt J, et al. EAU guidelines on prostate cancer. part 1: screening, diagnosis, and local treatment with curative intent-update 2013. Eur Urol. 2014;65(1):124–37. doi: 10.1016/j.eururo.2013.09.046. [DOI] [PubMed] [Google Scholar]
- 9.Ling XH, Han ZD, Xia D, et al. MicroRNA-30c serves as an independent biochemical recurrence predictor and potential tumor suppressor for prostate cancer. Mol Biol Rep. 2014;41(5):2779–88. doi: 10.1007/s11033-014-3132-7. [DOI] [PubMed] [Google Scholar]
- 10.Zhang H, Cheng S, Wang A, et al. Expression of RABEX-5 and its clinical significance in prostate cancer. J Exp Clin Cancer Res. 2014;33:31. doi: 10.1186/1756-9966-33-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Xie PG, Lin YL, et al. Aberrant methylation of PCDH10 predicts worse biochemical recurrence-free survival in patients with prostate cancer after radical prostatectomy. Med Sci Monit. 2014;20:1363–68. doi: 10.12659/MSM.891241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castelli T, Cimino S, Magno C, et al. Molecular markers for prostatic cancer. Front Biosci (Elite Ed) 2010;2:641–56. doi: 10.2741/e120. [DOI] [PubMed] [Google Scholar]
- 13.Park JY. Promoter hypermethylation in prostate cancer. Cancer Control. 2010;17(4):245–55. doi: 10.1177/107327481001700405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chiam K, Ricciardelli C, Bianco-Miotto T. Epigenetic biomarkers in prostate cancer: Current and future uses. Cancer Lett. 2014;342(2):248–56. doi: 10.1016/j.canlet.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 15.Van der Heijden AG. The role of methylation in urological tumours. Arch Esp Urol. 2013;66(5):432–39. [PubMed] [Google Scholar]
- 16.Goering W, Kloth M, Schulz WA. DNA methylation changes in prostate cancer. Methods Mol Biol. 2012;863:47–66. doi: 10.1007/978-1-61779-612-8_4. [DOI] [PubMed] [Google Scholar]
- 17.Strehl S, Glatt K, Liu QM, et al. Characterization of two novel protocadherins (PCDH8 and PCDH9) localized on human chromosome 13 and mouse chromosome 14. Genomics. 1998;53(1):81–89. doi: 10.1006/geno.1998.5467. [DOI] [PubMed] [Google Scholar]
- 18.Kim SY, Yasuda S, Tanaka H, et al. Non-clustered protocadherin. Cell Adh Migr. 2011;5(2):97–105. doi: 10.4161/cam.5.2.14374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin YL, Wang YL, Ma JG, et al. Clinical significance of protocadherin 8 (PCDH8) promoter methylation in non-muscle invasive bladder cancer. J Exp Clin Cancer Res. 2014;33:68. doi: 10.1186/s13046-014-0068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin YL, Ma JH, Luo XL, et al. Clinical significance of protocadherin-8 (PCDH8) promoter methylation in bladder cancer. J Int Med Res. 2013;41(1):48–54. doi: 10.1177/0300060513475571. [DOI] [PubMed] [Google Scholar]
- 21.Zhang D, Zhao W, Liao X, et al. Frequent silencing of protocadherin 8 by promoter methylation, a candidate tumor suppressor for human gastric cancer. Oncol Rep. 2012;28(5):1785–91. doi: 10.3892/or.2012.1997. [DOI] [PubMed] [Google Scholar]
- 22.He D, Zeng Q, Ren G, et al. Protocadherin8 is a functional tumor suppressor frequently inactivated by promoter methylation in nasopharyngeal carcinoma. Eur J Cancer Prev. 2012;21(6):569–75. doi: 10.1097/CEJ.0b013e328350b097. [DOI] [PubMed] [Google Scholar]
- 23.Morris MR, Ricketts CJ, Gentle D, et al. Genome-wide methylation analysis identifies epigenetically inactivated candidate tumour suppressor genes in renal cell carcinoma. Oncogene. 2011;30(12):1390–401. doi: 10.1038/onc.2010.525. [DOI] [PubMed] [Google Scholar]
- 24.Yu JS, Koujak S, Nagase S, et al. PCDH8, the human homolog of PAPC, is a candidate tumor suppressor of breast cancer. Oncogene. 2008;27(34):4657–65. doi: 10.1038/onc.2008.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haldrup C, Mundbjerg K, Vestergaard EM, et al. DNA methylation signatures for prediction of biochemical recurrence after radical prostatectomy of clinically localized prostate cancer. J Clin Oncol. 2013;31(26):3250–58. doi: 10.1200/JCO.2012.47.1847. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H, Qi C, Wang A, et al. Prognostication of prostate cancer based on NUCB2 protein assessment: NUCB2 in prostate cancer. J Exp Clin Cancer Res. 2013;32:77. doi: 10.1186/1756-9966-32-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stein M, Goodin S, Doyle-Lindrud S, et al. Transdermal estradiol in castrate and chemotherapy resistant prostate cancer. Med Sci Monit. 2012;18(4):CR260–64. doi: 10.12659/MSM.882626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bryniarski P, Paradysz A, Fryczkowski M. PSA mass as a marker of prostate cancer progression after radical prostatectomy. Med Sci Monit. 2011;17(2):CR104–9. doi: 10.12659/MSM.881395. [DOI] [PMC free article] [PubMed] [Google Scholar]