Abstract
Background:
Schizophrenia is a neurodevelopmental disorder with altered expression of GABA-related genes in the prefrontal cortex (PFC). However, whether these gene expression abnormalities reflect disturbances in postnatal developmental processes before clinical onset or arise as a consequence of clinical illness remains unclear.
Methods:
Expression levels for 7 GABA-related transcripts (vesicular GABA transporter [vGAT], GABA membrane transporter [GAT1], GABAA receptor subunit α1 [GABRA1] [novel in human and monkey cohorts], glutamic acid decarboxylase 67 [GAD67], parvalbumin, calretinin, and somatostatin [previously reported in human cohort, but not in monkey cohort]) were quantified in the PFC from 42 matched pairs of schizophrenia and comparison subjects and from 49 rhesus monkeys ranging in age from 1 week postnatal to adulthood.
Results:
Levels of vGAT and GABRA1, but not of GAT1, messenger RNAs (mRNAs) were lower in the PFC of the schizophrenia subjects. As previously reported, levels of GAD67, parvalbumin, and somatostatin, but not of calretinin, mRNAs were also lower in these subjects. Neither illness duration nor age accounted for the levels of the transcripts with altered expression in schizophrenia. In monkey PFC, developmental changes in expression levels of many of these transcripts were in the opposite direction of the changes observed in schizophrenia. For example, mRNA levels for vGAT, GABRA1, GAD67, and parvalbumin all increased with age.
Conclusions:
Together with published reports, these findings support the interpretation that the altered expression of GABA-related transcripts in schizophrenia reflects a blunting of normal postnatal development changes, but they cannot exclude a decline during the early stages of clinical illness.
Key words: schizophrenia, prefrontal cortex, neurodevelopment, parvalbumin, GABA
Introduction
Deficits in certain cognitive processes, such as working memory, are common in individuals with schizophrenia and have been attributed to dysfunction of the prefrontal cortex (PFC).1 This dysfunction appears to reflect, at least in part, alterations in molecular markers of GABA neurotransmission.2,3 For example, multiple postmortem studies of schizophrenia subjects have documented lower messenger RNA (mRNA) levels of the principal enzyme responsible for cortical GABA synthesis, the 67-kDa isoform of glutamic acid decarboxylase 67 (GAD67).4–10 Although less well studied, mRNA levels of the presynaptic GABA membrane transporter (GAT1), which is responsible for the reuptake of extracellular GABA, have also been reported to be lower,11 whereas mRNA levels of the vesicular GABA transporter (vGAT), which loads GABA into presynaptic vesicles, have been reported to be unchanged12 in the PFC of schizophrenia subjects. On the postsynaptic side, mRNA levels of 2 of the most common ionotropic GABAA receptor subunits, α1 and α2 (GABRA1 and 2), appear to be lower and higher, respectively, in some13–15 but not all7 studies of schizophrenia.
These alterations may be more common in particular subsets of cortical GABA neurons. For example, parvalbumin mRNA, which is expressed in ~25% of PFC GABA neurons, is lower in schizophrenia.16–19 Parvalbumin-containing neurons also exhibit lower expression of GAD67 mRNA18 and contain lower levels of GAD67 protein in their axon terminals.20 Importantly, these differences appear to reflect disease-related reductions in gene expression and not a deficit in the number of parvalbumin-containing neurons.4,18,21 A separate subset (~25%) of cortical GABA neurons that express somatostatin also show lower levels of the mRNA for this neuropeptide in subjects with schizophrenia.16,17,19,22,23 In contrast, studies have reported that mRNA and protein levels for calretinin, which is expressed in >40% of PFC GABA neurons that contain neither parvalbumin nor somatostatin, are not lower in the PFC of individuals with schizophrenia,18,19,23–28 but see reference.17
Understanding the potential contributions of alterations in GABA-related transcripts (defined here as gene products that regulate GABA neurotransmission or are selectively expressed by subsets of GABA neurons) to working memory impairments in schizophrenia requires knowledge of whether they represent causes, consequences, or confounds of the underlying disease process.29 The findings cited above do not appear to be confounds due to either a nonspecific downregulation or general degradation of cortical mRNAs, because, different GABA-related transcripts are lower, higher, or unchanged in the illness.2,7,15,17,18,23 Other findings suggest that the observed transcript alterations are not likely to be attributable to confounding factors such as medications or substance use.15,18,20,22
Given that altered levels of GABA-related transcripts were observed in postmortem studies of schizophrenia subjects with varying illness duration, they could reflect the consequences of illness chronicity. If so, then the magnitude of the GABA-related transcript alterations would be expected to co-vary with illness duration. Alternatively, these alterations could be part of a causal developmental pathway leading to PFC dysfunction and working memory impairments in schizophrenia.8,30,31 For example, working memory performance and the associated activation of the PFC undergo a protracted maturation with adult levels of performance not achieved until late adolescence.32 Interestingly, a recent longitudinal prospective cohort study reported that in individuals who were later diagnosed with schizophrenia working memory function did not differ from comparison subjects at age 7, but then failed to improve with age at the normal rate.33 Thus, disturbances in the developmental trajectories of GABA-related transcripts prior to the clinical onset of schizophrenia could be causal factors contributing to working memory impairments in the illness.34
In order to shed light on the “chronic illness consequence” vs “developmental cause” explanations of altered GABA-related gene expression in schizophrenia, we conducted 2 studies. First, we quantified mRNA levels of vGAT, GABRA1, GAT1, and the other GABA-related transcripts in the PFC from 42 matched pairs of schizophrenia and comparison subjects, and evaluated these data as a function of illness duration. The other GABA-related transcripts were quantified in previous studies and included GAD67, parvalbumin, calretinin, and somatostatin.19,20 Second, because it is not possible to assess the developmental trajectories of GABA-related transcripts in subjects with schizophrenia, we quantified the expression of these same transcripts in the PFC from 49 rhesus monkeys ranging in age from 1 week postnatal to adulthood. We then compared the developmental trajectory of each transcript with its expression status in schizophrenia to examine whether a disturbance during development could be a plausible explanation for the pattern of GABA-related transcript alterations seen in the illness. While this approach has been recently explored by other groups using a developmental human cohort,7,8,17,35,36 rhesus monkey studies provide the advantage of less between-subject variance due to factors other than age.
Methods
Human Studies
Brain specimens (n = 84) were obtained during autopsies conducted at the Allegheny County Office of the Medical Examiner (Pittsburgh, PA) after consent was obtained from the next-of-kin. Consensus Diagnostic and Statistical Manual of Mental Disorders-IV-Text Revision diagnoses for each subject were made using structured interviews with family members and review of medical records19; the absence of a psychiatric diagnosis was confirmed in healthy comparison subjects using the same approach. To reduce the effects of biological variance and control for experimental variance, subjects with schizophrenia or schizoaffective disorder (n = 42) were matched individually to one healthy comparison subject for gender, and as closely as possible for age, and samples from both subjects in a pair were processed together throughout all stages of the study. The mean age, postmortem interval, brain pH, RNA integrity number (RIN), and tissue storage time did not differ between subject groups (supplementary tables T1 and T2). The University of Pittsburgh’s Committee for the Oversight of Research Involving the Dead and Institutional Review Board for Biomedical Research approved all procedures.
Frozen tissue blocks from each subject were confirmed to contain PFC area 9 using Nissl-stained tissue sections cut on a cryostat at 40 μm thickness.5 Gray matter from adjacent sections was separately collected into a tube containing TRIzol reagent using a method that ensured minimal white matter contamination and excellent RNA preservation.19
Levels of vGAT, GAT1, and GABRA1 mRNAs were quantified by real-time quantitative PCR (qPCR) using previously described methods19 (see supplementary methods for details). The geometric mean of 3 normalizers (β-actin, cyclophilin-A, and glyceraldehyde 3-phosphate dehydrogenase [GAPDH]) were used and these Ct values did not differ between the schizophrenia and comparison subject groups as reported previously.19,20 The results of similar studies for GAD67, parvalbumin, calretinin, and somatostatin mRNAs in the same cohort of subjects have been recently reported.19,20
Monkey Studies
Forty-nine rhesus monkeys (Macacca mulatta) ranging in age from postnatal 1 week to 11.5 years were used (supplementary tables T3 and T4). Animals were housed according to age as previously described.37 All housing and experimental procedures were conducted in accordance with the guidelines of the US Department of Agriculture and the NIH Guide for the Care of Animals, and with approval from the University of Pittsburgh’s Institutional Animal Care and Use Committee.
Twenty-one animals were perfused transcardially with ice-cold artificial cerebrospinal fluid following deep anesthesia induced with ketamine and pentobarbital; in 5 of these animals, a small tissue block from the left principal sulcus had been surgically excised for in vitro electrophysiology studies 2–4 weeks prior to perfusion. The remaining 28 animals were experimentally naive. After deep anesthesia with ketamine and pentobarbital was induced, the brains were removed. For all animals, the right hemisphere of each brain was blocked coronally and each block was frozen and stored at −80°C.
Frontal pole sections (40 µm) composed entirely of gray matter were collected into tubes containing TRIzol reagent in a manner that ensured excellent RNA preservation.38 Area 10 was used due to the availability of tissue from that cortical region for each animal. qPCR was conducted as described for the human study with the following differences. Primer sets were designed against the macaque sequences of the same 7 target genes used in the human studies, as well as two reference genes, β-actin and cyclophilin-A, which exhibited stable relative expression levels across the postnatal developmental ages studied (F 7,41 = 1.594; P = .16 in the present study, and as previously reported).39 GAPDH was not used as a reference gene because its mRNA levels appeared to be unstable across early postnatal development.39
Statistical Analyses
Two ANCOVA models were used to examine the effect of diagnosis on vGAT, GABRA1, and GAT1 mRNA levels. The unpaired ANCOVA model included mRNA level as the dependent variable, diagnostic group as the main effect, and any relevant covariates that were significantly related to mRNA level. Since subjects were also paired to account for the parallel processing of tissue samples from a pair and to balance diagnostic groups for sex and age, a second paired ANCOVA model including subject pair as a blocking factor was also employed. In building the analytical models, graphics were used to assess model fit and to assure the absence of outliers. The statistical results (both test statistics and P values with degrees of freedom varying depending on the numbers of covariates used) are reported for both models.
Because the monkey data suggested that log(age) might perhaps provide a better fit to the monkey data, the human model was also reanalyzed using log(age) instead of age. Results of the 2 models did not differ, with P values being very similar; thus, subject log(age) results for humans are not presented.
To explore whether the alterations in GABA-related transcript levels in schizophrenia subjects were a consequence of the disease process, the correlations between illness duration (the difference between age at clinical onset and age at death) and the within-pair percent difference (schizophrenia subject minus comparison subject) in expression of each mRNA transcript were computed and Bonferroni-Holm adjusted P values are reported. In addition, the age by diagnosis interactions were assessed in both ANCOVA models.
The relationship of the within-subject pair differences for transcript levels to each of the following potential confounds were examined by chi-square tests: diagnosis of schizoaffective disorder, death by suicide, substance use diagnosis at time of death, or antidepressant, antipsychotic, or benzodiazepine use at time of death. The substance use and medication data were obtained from toxicology studies at time of death and records of prescriptions at time of death. Subjects with negative toxicology and a reported history of medication nonadherence were considered as not taking medications at time of death, regardless of active prescriptions. In addition, independent living status at the time of death and the Hollingshead Two-Factor Index of Social Position were used as surrogate measures for cognitive functioning (supplementary table T2). Independent samples 2-tailed t tests were used to examine the relationship between transcript expression level and independent living status at the time of death, while Pearson’s correlation coefficient was used to determine the relationship between transcript expression and Hollingshead Two-Factor Index of Social Position.
For the monkey studies, the correlations between age and each mRNA level were determined using Pearson’s correlation coefficient (with Bonferroni-Holm adjusted P values). A second analysis used an ANCOVA model in which animals were placed into 1 of 4 age groups based on existing data regarding probable inflection points in the maturation of primate PFC circuitry. Such inflection points have been best studied for the postnatal development trajectory of excitatory synapse density, which exhibits 4 distinct phases in both macaque and human PFC.40–43 Therefore, each animal in the present study was assigned to 1 of the following 4 age groups: (1) perinatal, 0.25–1 month of age, within the period of a rapid increase in excitatory synaptic density, (2) prepubertal, 3–9 months, within the period when the density of excitatory synapses is at a plateau, (3) juvenile, 15–37 months, within the period of excitatory synapse pruning and (4) postpubertal, 42–138 months, during the period when the density of excitatory synapses is at stable adult levels. We use the term developmental trajectory to characterize the pattern of change in a transcript level over postnatal development from 1 week after birth to midlife, which corresponds to the age range of the monkey cohort. The ANCOVA model for gene expression used age group as a factor. Levels of vGAT, GAT1, and calretinin mRNAs significantly differed (all F 1,44 > 7.95, all P < .01) as a function of tissue storage time, which was included as a covariate in the ANCOVA analyses, as well as any conditional analysis, for these transcripts. Pairwise comparison tests with α = 0.05 were conducted for post hoc comparisons between age groups. Bonferroni-Holm adjusted P values were computed to control for multiple comparison tests among mRNAs.
Results
GABA-Related Transcript Expression in Schizophrenia
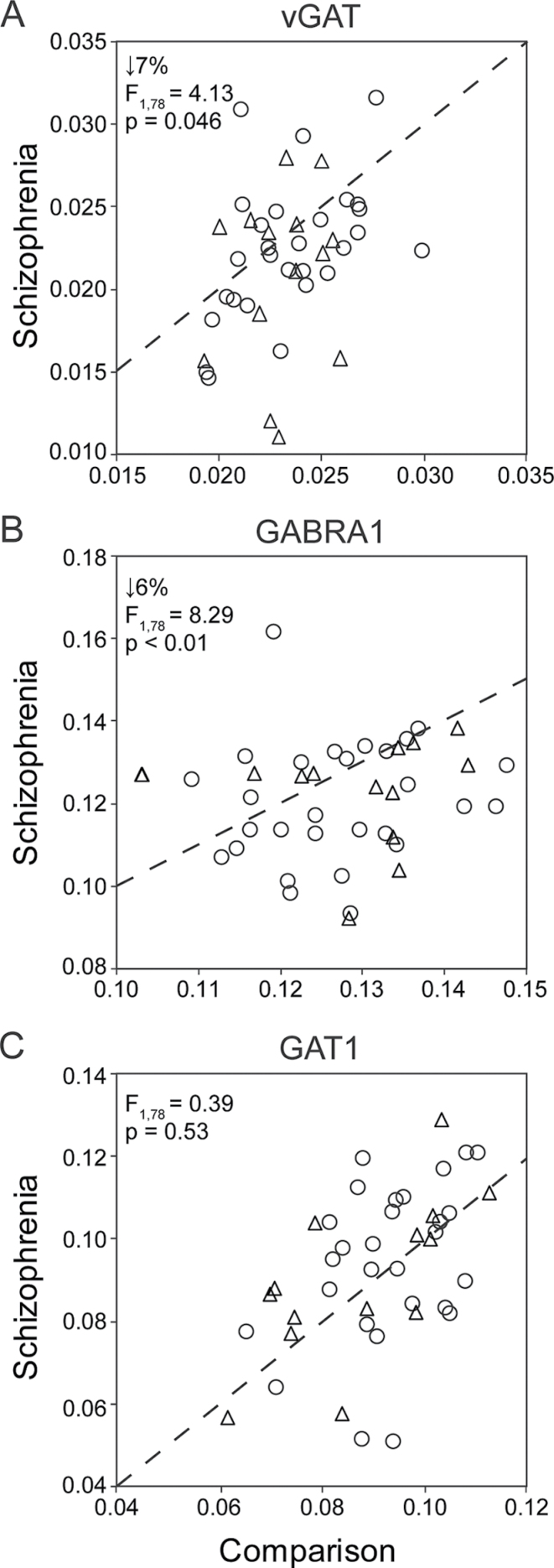
In PFC area 9, mean levels of vGAT (figure 1A; unpaired: F 1,78 = 4.13, P = .046; paired: F 1,40 = 4.76, P = .04) and GABRA1 (figure 1B; unpaired: F 1,79 = 8.39, P < .01; paired: F 1,41 = 8.33, P < .01) mRNAs were modestly but significantly lower in the subjects with schizophrenia. In contrast, GAT1 mRNA levels did not differ (figure 1C; unpaired: F 1,78 = 0.39, P = .53; paired: F 1,40 = 0.72, P = .40) between groups. Levels of vGAT (unpaired: F 1,76 = 6.94, P = .01) and GAT1 (unpaired: F 1,76 = 9.07, P < .01) mRNAs were negatively associated with tissue storage time. Therefore, tissue storage time was included in the paired and unpaired statistical models for vGAT and GAT1. Other covariates including pH and RIN were not related to vGAT, GABRA1, or GAT1 mRNA levels. In the subjects with schizophrenia, levels of vGAT, GABRA1 and GAT1 mRNAs also did not differ as a function of diagnosis of schizoaffective disorder, death by suicide, substance use diagnosis at time of death, or antidepressant, antipsychotic, or benzodiazepine use at time of death (all |t| < 1.96, P > .05).
Fig. 1.
Vesicular GABA transporter (vGAT) (A), GABAA receptor subunit α1 (GABRA1) (B), and GABA membrane transporter (GAT1) (C) messenger RNA levels in prefrontal cortex from schizophrenia and comparison subjects. Scatter plots show the transcript levels for each matched pair of a comparison and either a schizophrenia (open circles) or schizoaffective disorder subject (open triangles). Values below the dashed unity line reflect pairs in which transcript levels are lower in the schizophrenia (or schizoaffective) subject relative to the comparison subject.
In this same cohort of 42 subject pairs, using the same qPCR method, we recently reported that mRNA levels for GAD67, parvalbumin, and somatostatin were lower, and mRNA levels for calretinin were higher, in the subjects with schizophrenia.19,20 Transcript expression levels for vGAT, GABRA1, GAT1, GAD67, parvalbumin, calretinin, or somatostatin were not significantly related to independent living status at time of death (all |t| < 0.423; all P > .69) or Hollingshead Two-Factor Index of Social Position (all |r| < .27; all P > .08).
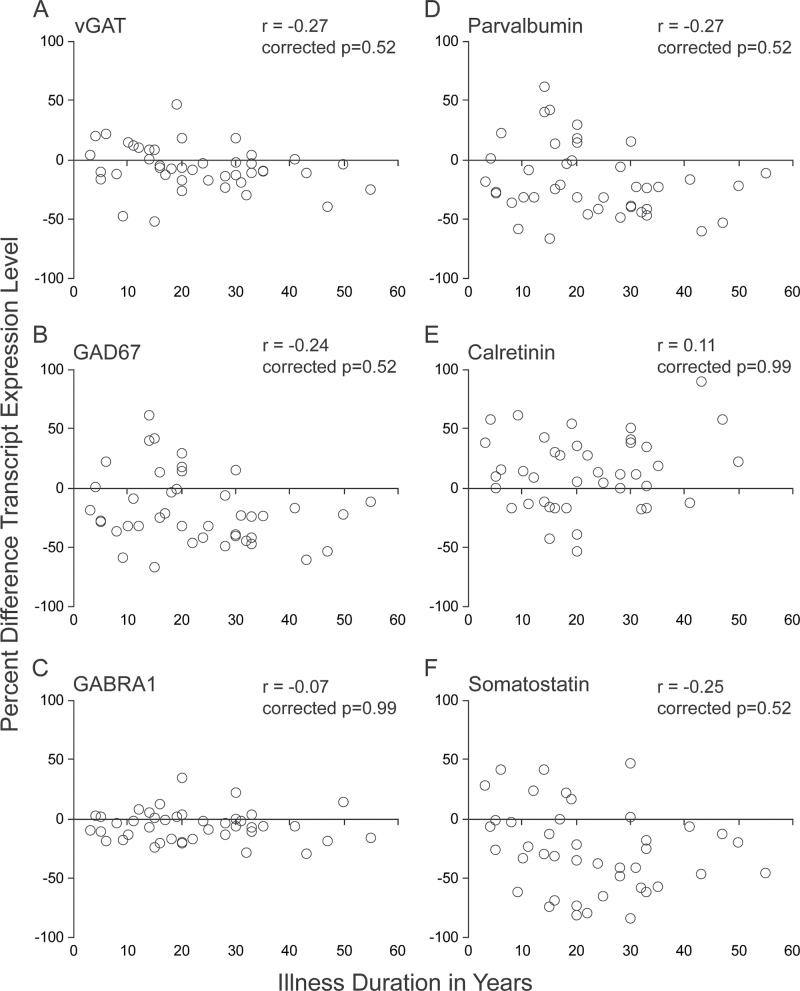
To determine if levels of the 6 GABA-related transcripts altered in schizophrenia (vGAT, GABRA1, GAD67, parvalbumin, calretinin, and somatostatin) were correlated with illness duration, the within-pair percent difference in transcript expression was plotted against illness duration for each schizophrenia subject. The percent differences of the expression levels of all transcripts did not significantly correlate with illness duration (all |r| < .27, Bonferroni-Holm corrected P > .52) (figure 2). In addition, the percent differences of the expression levels for these same transcripts did not significantly correlate with age at illness onset (all |r| < .25, Bonferroni-Holm corrected P > .69).
Fig. 2.
Within-pair percent difference (schizophrenia minus comparison subject) in prefrontal cortex transcript levels vs illness duration in schizophrenia subjects. Pearson’s correlation coefficient (r) was not statistically significant for any of the GABA-related messenger RNA levels examined that were altered in schizophrenia (A–F). Bonferroni-Holm corrected P values are shown for each transcript. For each panel, each matched pair of schizophrenia and comparison subjects is shown as an open black circle.
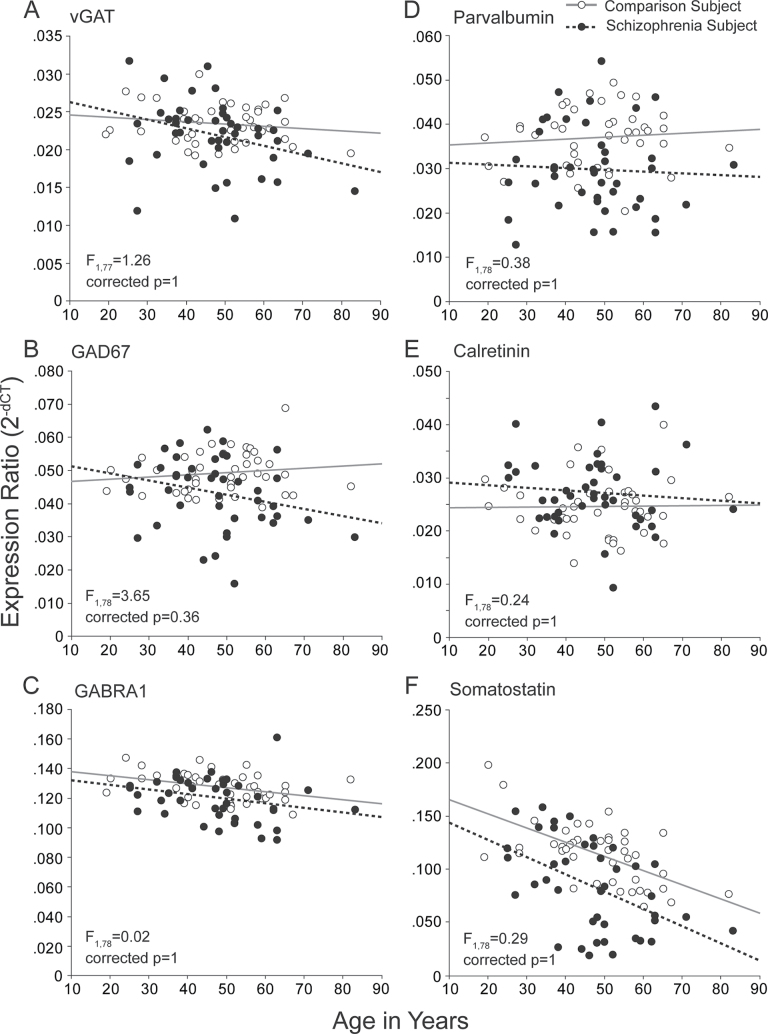
We further examined if any of the 6 GABA-related transcripts altered in schizophrenia exhibited an age-related effect that differed between diagnostic groups. There was no significant age by diagnosis interaction on mRNA levels for any of these GABA-related markers (nominal P values: vGAT: F 1,77 = 1.26, P = .26; GAD67: F 1,78 = 3.65, P = .06; GABRA1: F 1,78 = 0.02, P = .88; parvalbumin: F 1,78 = 0.38, P = .54; calretinin: F 1,78 = 0.24, P = .63; somatostatin: F 1,78 = 0.29, P = .59; all Bonferroni-Holm corrected P > .36) (figure 3).
Fig. 3.
Interaction of age by diagnosis on GABA-related messenger RNA levels in the prefrontal cortex of schizophrenia subjects. For each panel, comparison subjects are shown in open circles and schizophrenia subjects in filled circles. Gray solid and black dashed lines indicate lines of best fit for comparison and schizophrenia subjects, respectively. F- and Bonferroni-Holm adjusted P values represent the age by diagnosis interaction statistics.
Together, these findings suggest that changes in GABA-related transcripts in schizophrenia are not a consequence of illness chronicity.
Postnatal Trajectories of GABA-Related Transcripts in Monkey PFC
To investigate whether the pattern of altered cortical GABA-related mRNA levels in schizophrenia could be consistent with developmental abnormalities occurring before clinical illness onset, we determined the normative postnatal developmental trajectories of these GABA-related transcripts in monkey PFC. Due to the significant effect of tissue storage time found in the human studies, preliminary analysis was done in the monkey studies and showed that tissue storage time was a significant covariate for the vGAT, GAT1, and calretinin mRNAs. Thus, the following analyses for these 3 transcripts were adjusted for tissue storage time.
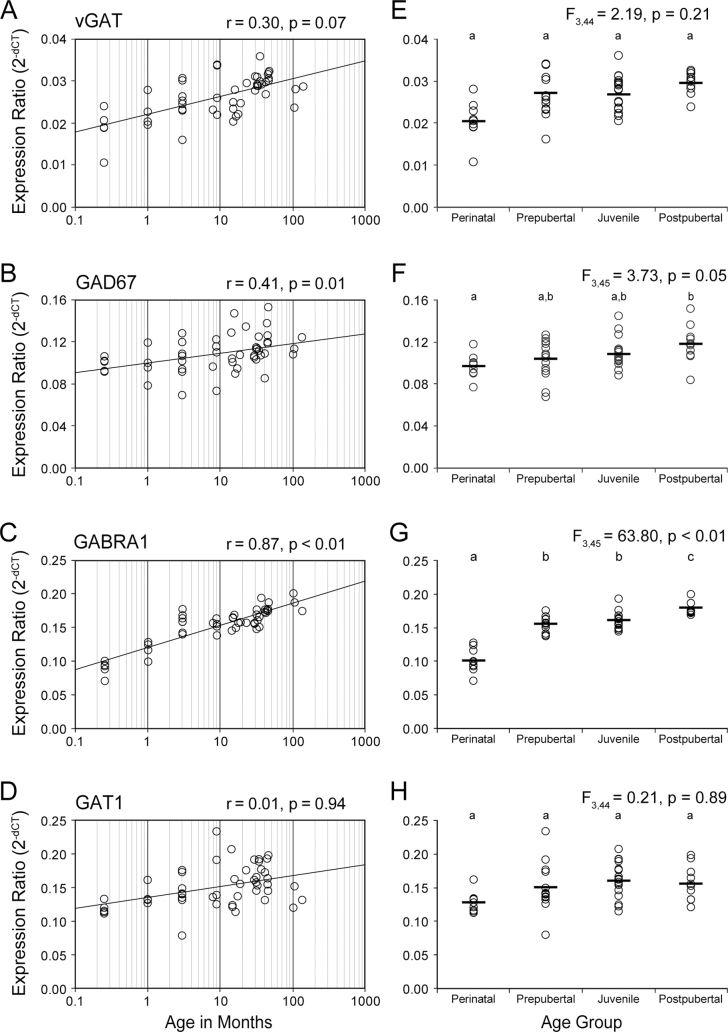
For the 4 markers of GABA neurotransmission, log(age) was significantly positively correlated with mRNA levels for GABRA1 (r = .87, P < .0001, Bonferroni-Holm corrected P < .001) and GAD67 (r = .41, P = .004, Bonferroni-Holm corrected P = .01), but not with vGAT (partial r = .30, P = .04, Bonferroni-Holm corrected P = .07) or GAT1 (partial r = .01, P = .94) mRNAs (figure 4A–D). When analyzed by age group, GAD67 (F 3,45 = 3.73, P = .02, Bonferroni-Holm corrected P = .05) and GABRA1 (F 3,45 = 63.8, P < .0001, Bonferroni-Holm corrected P < .001) significantly differed across the 4 age groups. The mean mRNA levels were higher in the postpubertal relative to perinatal group for vGAT (+16%, P = .09, Bonferroni-Holm corrected P = .17), GAD67 (+21%, P = .006, Bonferroni-Holm corrected P = .02), and GABRA1 (+78%, P < .0001, Bonferroni-Holm corrected P < .001) (figure 4E–H).
Fig. 4.
Postnatal developmental trajectories of transcripts regulating GABA neurotransmission in monkey prefrontal cortex. For panels A–D, the black line indicates least squares line of best fit; and Pearson’s correlation coefficient (r) and corresponding Bonferroni-Holm adjusted P value are indicated for each panel. For panels E–H, the black bars indicate group means. Age groups that do not share the same letters are significantly different (Bonferroni-Holm adjusted P < .05).
For the 3 markers of GABA neuron subpopulations, mRNA levels were positively correlated with log(age) for parvalbumin (r = .63, P < .0001, Bonferroni-Holm corrected P < .001), negatively correlated with log(age) for somatostatin (r = −.55, P < .0001, Bonferroni-Holm corrected P < .001), and marginally negatively correlated with log(age) for calretinin (partial r = −.25, P = .08) (figure 5A–C). When analyzed by age group, parvalbumin (F 3,45 = 27.02, P < .0001, Bonferroni-Holm corrected P < .001) and somatostatin (F 3,45 = 13.47, P < .0001, Bonferroni-Holm corrected P < .001) significantly differed across the 4 age groups. Mean levels in the postpubertal group were significantly higher than in the perinatal group for parvalbumin (+787%, P < .0001, Bonferroni-Holm corrected P < .001), lower for somatostatin (−33%, P < .0001, Bonferroni-Holm corrected P < .001), and marginally lower for calretinin (−23%, P = .06, Bonferroni-Holm corrected P = .06) (figure 5D–F).
Fig. 5.
Postnatal developmental trajectories of GABA neuronal population markers in monkey prefrontal cortex. For panels A–C, the black line indicates least squares line of best fit; and Pearson’s correlation coefficient (r) and corresponding Bonferroni-Holm adjusted P value are indicated for each panel on the left. For panels D–F, the black bars indicate group means. Age groups that do not share the same letters are significantly different (Bonferroni-Holm adjusted P < .05).
Given that a number of environmental exposures from birth through adolescence are associated with increased risk for schizophrenia,44 and may have a particularly strong influence on neural circuits during sensitive periods of cortical development,45 we determined when during postnatal development transitions in the expression levels of GABA-related mRNAs might occur. From the perinatal to prepubertal period, parvalbumin (+578%, P < .0001, Bonferroni-Holm corrected P < .001) and GABRA1 (+54%, P < .0001, Bonferroni-Holm corrected P < .001) mRNA levels significantly increased, while vGAT (+10%, P = .19) mRNA levels marginally increased (supplementary table T5). GABRA1 mRNA expression also increased significantly from the juvenile to postpubertal period (+12%, P = .001, Bonferroni-Holm corrected P = 0.003), albeit more modestly than the earlier rise. Although GAD67 mRNA levels increased progressively from the perinatal to postpubertal period, there were no observable inflection points between adjacent age groups. Somatostatin mRNA levels decreased significantly from the perinatal to prepubertal and prepubertal to juvenile periods (−17%, P = .007, Bonferroni-Holm corrected P = .01 and −18%, P = .005, Bonferroni-Holm corrected P = .014, respectively). Calretinin mRNA levels decreased significantly (−23%, P = .02, Bonferroni-Holm corrected P = .03) and parvalbumin mRNA levels increased significantly (+28%, P = .04, Bonferroni-Holm corrected P = .048) from the prepubertal to juvenile periods. Taken together, the majority of the prominent GABA-related transcript expression changes occurred before the juvenile age period in monkey PFC.
Discussion
We report modest but significantly lower tissue levels of vGAT and GABRA1 mRNAs in the PFC of this 42 pair cohort of schizophrenia subjects. Given these and other recently reported GABA-related transcript abnormalities from the same subject cohort,19,20 we sought to determine whether altered expression of GABA-related mRNAs in schizophrenia could reflect the consequences of illness chronicity. Our analyses suggest that duration of clinical illness does not account for the observed alterations in GABA-related gene expression. To the extent that alterations in GABA neurotransmission contribute to cognitive impairments in schizophrenia, our findings are consistent with reports that cognitive deficits in schizophrenia are not progressive after illness onset.46,47
To explore an alternative possibility that alterations in cortical GABA-related transcripts in schizophrenia might reflect developmental disturbances, we determined the postnatal developmental trajectories of GABA-related transcripts in the PFC from healthy rhesus monkeys. When comparing the mRNA levels between the first week of life and after puberty, the levels of GAD67, GABRA1, and parvalbumin mRNAs significantly increased, vGAT mRNA levels marginally increased, somatostatin mRNA levels significantly decreased, calretinin mRNA levels marginally decreased, and GAT1 mRNA levels remained unchanged. These developmental patterns generally matched those previously reported in human PFC.7,17,48 For example, microarray49 and PCR8 studies (available online at http://www.libd.org/braincloud) using PFC tissue from a human developmental cohort reported developmental trajectories for vGAT, GAD67, GABRA1, parvalbumin, and somatostatin that generally matched those reported here in macaque PFC.
Interestingly, for most transcripts the change in expression level with development was in the opposite direction of the difference observed between schizophrenia and comparison subjects. For example, levels of vGAT, GAD67, GABRA1, and parvalbumin mRNAs all increased during postnatal development, whereas subjects with schizophrenia showed lower expression of these transcripts. In contrast, calretinin mRNA levels decreased significantly during postnatal development in human PFC17 and were recently reported to be higher in subjects with schizophrenia.19 Finally, transcripts, such as GAT1, that did not change in expression level across postnatal development were not altered in schizophrenia. Together, these comparisons suggest the hypothesis that the altered expression of GABA-related transcripts in schizophrenia reflects blunted or incomplete developmental trajectories, resulting in a failure to achieve normal, mature mRNA levels.
This idea of an incomplete maturation of cortical GABA-related transcripts in schizophrenia is supported by previous findings in the literature. For example, the GABAA receptor α2 subunit declines across postnatal development in monkey and human PFC7,50 and is higher in the PFC of schizophrenia subjects,14 and the GABAA receptor δ subunit in the PFC increases across postnatal development and is lower in schizophrenia.51 In addition, the µ-opioid receptor, which is expressed by parvalbumin neurons52–54 and regulates GABA release,55,56 declines with postnatal development and is higher in schizophrenia.39 Furthermore, expression patterns of alternatively spliced transcripts of the GAD1 gene are also consistent with this hypothesis; GAD67 mRNA increases and GAD25 mRNA decreases normally during early postnatal development of human PFC, but in schizophrenia subjects, the relative levels of GAD67 and GAD25 mRNAs were lower and higher, respectively, than in matched comparison subjects.8
However, not all GABA-related transcripts with altered expression in schizophrenia fit the pattern predicted by the incomplete maturation hypothesis. For example, somatostatin mRNA levels are lower in the PFC of subjects with schizophrenia,17,22 and in accordance with the incomplete maturation hypothesis would be expected to increase in transcript expression during postnatal development. However, this transcript undergoes a pronounced decline with age that begins during early postnatal development and persists throughout adulthood both in macaques and humans.17,22 The fact that cortical somatostatin mRNA levels are also lower in other psychiatric disorders57 suggests that the altered expression of this transcript in schizophrenia may be driven by factors that are common to these disease processes and that could obscure the influence of early developmental events. Given the heterogeneity in disease course in schizophrenia, along with recent findings that a subset of individuals with schizophrenia show marked alterations in the expression of GABA-related transcripts,19 not all individuals with schizophrenia would be expected to show the molecular signatures of incomplete maturation.
The timing of the largest developmental changes in expression levels differed across GABA-related transcripts (supplementary table T5). These differences may reflect transcript-specific sensitive periods during development, and reveal which transcripts might be most expected to be affected by adverse environmental events that are associated with an increased risk of schizophrenia. For example, risk factors such as birth complications and urbanicity44,58 may be particularly likely to affect parvalbumin, GABRA1, and somatostatin expression since these transcripts change substantially during the perinatal to prepubertal periods. Similarly, risk factors occurring later in development, such as cannabis use during the early juvenile period,59 may predominantly impact the developmental trajectories of parvalbumin, calretinin, and somatostatin, which exhibit significant changes during the prepubertal to juvenile periods.
Such effects of environmental events on the specific GABA-related transcripts are likely to have a broader impact as GABA neurons are involved in shaping the maturation of cortical circuits during these same developmental periods.45,60 For example, the timing of the critical period for the development of binocular vision can be manipulated by the enhancement or reduction of GABA transmission in visual cortex,45 especially via GABRA1-containing neurons61 postsynaptic to parvalbumin-containing GABA neurons.62 Thus, the substantial increases in GABRA1 and parvalbumin mRNA levels in the primate PFC during postnatal development suggest that parvalbumin-containing inputs to GABRA1-containing postsynaptic structures may be especially susceptible to environmental exposures during development.
It is important to note that not all of the findings of the present study are in agreement with some reports in the literature. For example, recent postmortem studies7,63 using a different subject cohort failed to find the significantly lower levels of vGAT and GABRA1 mRNAs observed in the present study. This discrepancy could reflect differences in subject composition of the cohorts because we recently reported that altered expression of GABA-related transcripts is particularly marked in a subset of individuals with schizophrenia.19
In concert, our findings suggest that altered expression of certain GABA-related transcripts in the PFC of subjects with schizophrenia is not the consequence of cumulative illness effects and may be due to blunted or incomplete developmental trajectories of these transcripts. However, these data cannot rule out a decline in GABA-related transcripts during the early stages of clinical illness after onset of psychosis. These findings may provide clues as to what molecular systems might be targeted for preemptive interventions in at-risk individuals, and when such interventions might be most effective.
Supplementary Material
Supplementary material is available at http://schizophre niabulletin.oxfordjournals.org.
Funding
National Institutes of Mental Health (MH093079 to G.D.H., MH051234, MH084053 to D.A.L., MH084016 to D.W.V.).
Supplementary Material
Acknowledgments
We are grateful to Mary Brady and Elizabeth Sengupta for their technical expertise. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Mental Health or the National Institutes of Health. D.A.L. currently receives investigator-initiated research support from Bristol-Myers Squibb and Pfizer and in 2010–2012 served as a consultant in the areas of target identification and validation and new compound development to Bristol-Myers Squibb and Concert Pharmaceuticals. A.R.S. is a statistical consultant to Janssen Pharmaceutical Research and Development LLC, a Janssen Pharmaceutical Company. All other authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Barch DM, Ceaser A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci. 2012;16:27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Benes FM, Berretta S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. Neuropsychopharmacology. 2001;25:1–27. [DOI] [PubMed] [Google Scholar]
- 3. Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. [DOI] [PubMed] [Google Scholar]
- 4. Akbarian S, Kim JJ, Potkin SG., et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. [DOI] [PubMed] [Google Scholar]
- 5. Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. [DOI] [PubMed] [Google Scholar]
- 6. Guidotti A, Auta J, Davis JM., et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069. [DOI] [PubMed] [Google Scholar]
- 7. Duncan CE, Webster MJ, Rothmond DA, Bahn S, Elashoff M, Shannon Weickert C. Prefrontal GABA(A) receptor alpha-subunit expression in normal postnatal human development and schizophrenia. J Psychiatr Res. 2010;44:673–681. [DOI] [PubMed] [Google Scholar]
- 8. Hyde TM, Lipska BK, Ali T., et al. Expression of GABA signaling molecules KCC2, NKCC1, and GAD1 in cortical development and schizophrenia. J Neurosci. 2011;31:11088–11095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vawter MP, Crook JM, Hyde TM., et al. Microarray analysis of gene expression in the prefrontal cortex in schizophrenia: a preliminary study. Schizophr Res. 2002;58:11–20. [DOI] [PubMed] [Google Scholar]
- 10. Straub RE, Lipska BK, Egan MF., et al. Allelic variation in GAD1 (GAD67) is associated with schizophrenia and influences cortical function and gene expression. Mol Psychiatry. 2007;12:854–869. [DOI] [PubMed] [Google Scholar]
- 11. Volk D, Austin M, Pierri J, Sampson A, Lewis D. GABA transporter-1 mRNA in the prefrontal cortex in schizophrenia: decreased expression in a subset of neurons. Am J Psychiatry. 2001;158:256–265. [DOI] [PubMed] [Google Scholar]
- 12. Fung SJ, Webster MJ, Weickert CS, Weicker CS. Expression of VGluT1 and VGAT mRNAs in human dorsolateral prefrontal cortex during development and in schizophrenia. Brain Res. 2011;1388:22–31. [DOI] [PubMed] [Google Scholar]
- 13. Akbarian S, Huntsman MM, Kim JJ., et al. GABAA receptor subunit gene expression in human prefrontal cortex: comparison of schizophrenics and controls. Cereb Cortex. 1995;5:550–560. [DOI] [PubMed] [Google Scholar]
- 14. Beneyto M, Abbott A, Hashimoto T, Lewis DA. Lamina-specific alterations in cortical GABA(A) receptor subunit expression in schizophrenia. Cereb Cortex. 2011;21:999–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Volk DW, Pierri JN, Fritschy JM, Auh S, Sampson AR, Lewis DA. Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cereb Cortex. 2002;12:1063–1070. [DOI] [PubMed] [Google Scholar]
- 16. Mellios N, Huang HS, Baker SP, Galdzicka M, Ginns E, Akbarian S. Molecular determinants of dysregulated GABAergic gene expression in the prefrontal cortex of subjects with schizophrenia. Biol Psychiatry. 2009;65:1006–1014. [DOI] [PubMed] [Google Scholar]
- 17. Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, Weickert CS. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry. 2010;167:1479–1488. [DOI] [PubMed] [Google Scholar]
- 18. Hashimoto T, Volk DW, Eggan SM., et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Volk DW, Matsubara T, Li S., et al. Deficits in transcriptional regulators of cortical parvalbumin neurons in schizophrenia. Am J Psychiatry. 2012;169:1082–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Curley AA, Arion D, Volk DW., et al. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features. Am J Psychiatry. 2011;168:921–929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Woo TU, Miller JL, Lewis DA. Schizophrenia and the parvalbumin-containing class of cortical local circuit neurons. Am J Psychiatry. 1997;154:1013–1015. [DOI] [PubMed] [Google Scholar]
- 22. Morris HM, Hashimoto T, Lewis DA. Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cereb Cortex. 2008;18:1575–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hashimoto T, Arion D, Unger T., et al. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13:147–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Reynolds GP, Abdul-Monim Z, Neill JC, Zhang ZJ. Calcium binding protein markers of GABA deficits in schizophrenia–postmortem studies and animal models. Neurotox Res. 2004;6:57–61. [DOI] [PubMed] [Google Scholar]
- 25. Sakai T, Oshima A, Nozaki Y., et al. Changes in density of calcium-binding-protein-immunoreactive GABAergic neurons in prefrontal cortex in schizophrenia and bipolar disorder. Neuropathology. 2008;28:143–150. [DOI] [PubMed] [Google Scholar]
- 26. Beasley CL, Cotter DR, Everall IP. Density and distribution of white matter neurons in schizophrenia, bipolar disorder and major depressive disorder: no evidence for abnormalities of neuronal migration. Mol Psychiatry. 2002;7:564–570. [DOI] [PubMed] [Google Scholar]
- 27. Cotter D, Landau S, Beasley C., et al. The density and spatial distribution of GABAergic neurons, labelled using calcium binding proteins, in the anterior cingulate cortex in major depressive disorder, bipolar disorder, and schizophrenia. Biol Psychiatry. 2002;51:377–386. [DOI] [PubMed] [Google Scholar]
- 28. Tooney PA, Chahl LA. Neurons expressing calcium-binding proteins in the prefrontal cortex in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:273–278. [DOI] [PubMed] [Google Scholar]
- 29. Lewis DA, González-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141–165. [DOI] [PubMed] [Google Scholar]
- 30. Weinberger DR. From neuropathology to neurodevelopment. Lancet. 1995;346:552–557. [DOI] [PubMed] [Google Scholar]
- 31. Beneyto M, Lewis DA. Insights into the neurodevelopmental origin of schizophrenia from postmortem studies of prefrontal cortical circuitry. Int J Dev Neurosci. 2011;29:295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Luna B, Garver KE, Urban TA, Lazar NA, Sweeney JA. Maturation of cognitive processes from late childhood to adulthood. Child Dev. 2004;75:1357–1372. [DOI] [PubMed] [Google Scholar]
- 33. Reichenberg A, Caspi A, Harrington H., et al. Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. Am J Psychiatry. 2010;167:160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hoftman GD, Lewis DA. Postnatal developmental trajectories of neural circuits in the primate prefrontal cortex: identifying sensitive periods for vulnerability to schizophrenia. Schizophr Bull. 2011;37:493–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Catts VS, Fung SJ, Long LE., et al. Rethinking schizophrenia in the context of normal neurodevelopment. Front Cell Neurosci. 2013;7:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Webster MJ, Elashoff M, Weickert CS. Molecular evidence that cortical synaptic growth predominates during the first decade of life in humans. Int J Dev Neurosci. 2011;29:225–236. [DOI] [PubMed] [Google Scholar]
- 37. Erickson SL, Lewis DA. Postnatal development of parvalbumin- and GABA transporter-immunoreactive axon terminals in monkey prefrontal cortex. J Comp Neurol. 2002;448:186–202. [DOI] [PubMed] [Google Scholar]
- 38. Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008;165:479–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Volk DW, Radchenkova PV, Walker EM, Sengupta EJ, Lewis DA. Cortical opioid markers in schizophrenia and across postnatal development. Cereb Cortex. 2012;22:1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex. 1994;4:78–96. [DOI] [PubMed] [Google Scholar]
- 41. Huttenlocher PR. Synaptic density in human frontal cortex - developmental changes and effects of aging. Brain Res. 1979;163:195–205. [DOI] [PubMed] [Google Scholar]
- 42. Petanjek Z, Judaš M, Šimic G., et al. Extraordinary neoteny of synaptic spines in the human prefrontal cortex. Proc Natl Acad Sci USA. 2011;108:13281–13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Anderson SA, Classey JD, Condé F, Lund JS, Lewis DA. Synchronous development of pyramidal neuron dendritic spines and parvalbumin-immunoreactive chandelier neuron axon terminals in layer III of monkey prefrontal cortex. Neuroscience. 1995;67:7–22. [DOI] [PubMed] [Google Scholar]
- 44. van Os J, Kenis G, Rutten BP. The environment and schizophrenia. Nature. 2010;468:203–212. [DOI] [PubMed] [Google Scholar]
- 45. Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. [DOI] [PubMed] [Google Scholar]
- 46. Lewandowski KE, Cohen BM, Ongur D. Evolution of neuropsychological dysfunction during the course of schizophrenia and bipolar disorder. Psychol Med. 2011;41:225–241. [DOI] [PubMed] [Google Scholar]
- 47. Zipursky RB, Reilly TJ, Murray RM. The myth of schizophrenia as a progressive brain disease. Schizophr Bull. 2013;39:1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Huang HS, Matevossian A, Whittle C., et al. Prefrontal dysfunction in schizophrenia involves mixed-lineage leukemia 1-regulated histone methylation at GABAergic gene promoters. J Neurosci. 2007;27:11254–11262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Colantuoni C, Lipska BK, Ye T., et al. Temporal dynamics and genetic control of transcription in the human prefrontal cortex. Nature. 2011;478:519–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hashimoto T, Nguyen QL, Rotaru D., et al. Protracted developmental trajectories of GABAA receptor alpha1 and alpha2 subunit expression in primate prefrontal cortex. Biol Psychiatry. 2009;65:1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Maldonado-Avilés JG, Curley AA, Hashimoto T., et al. Altered markers of tonic inhibition in the dorsolateral prefrontal cortex of subjects with schizophrenia. Am J Psychiatry. 2009;166:450–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Drake CT, Milner TA. Mu opioid receptors are in somatodendritic and axonal compartments of GABAergic neurons in rat hippocampal formation. Brain Res. 1999;849:203–215. [DOI] [PubMed] [Google Scholar]
- 53. Drake CT, Milner TA. Mu opioid receptors are in discrete hippocampal interneuron subpopulations. Hippocampus. 2002;12:119–136. [DOI] [PubMed] [Google Scholar]
- 54. Stumm RK, Zhou C, Schulz S, Höllt V. Neuronal types expressing mu- and delta-opioid receptor mRNA in the rat hippocampal formation. J Comp Neurol. 2004;469:107–118. [DOI] [PubMed] [Google Scholar]
- 55. Drasbek KR, Hoestgaard-Jensen K, Jensen K. Modulation of extrasynaptic THIP conductances by GABAA-receptor modulators in mouse neocortex. J Neurophysiol. 2007;97:2293–2300. [DOI] [PubMed] [Google Scholar]
- 56. Drasbek KR, Jensen K. THIP, a hypnotic and antinociceptive drug, enhances an extrasynaptic GABAA receptor-mediated conductance in mouse neocortex. Cereb Cortex. 2006;16:1134–1141. [DOI] [PubMed] [Google Scholar]
- 57. Sibille E, Morris HM, Kota RS, Lewis DA. GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. Int J Neuropsychopharmacol. 2011;14:721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Lewis DA, Levitt P. Schizophrenia as a disorder of neurodevelopment. Annu Rev Neurosci. 2002;25:409–432. [DOI] [PubMed] [Google Scholar]
- 59. Moore TH, Zammit S, Lingford-Hughes A., et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370:319–328. [DOI] [PubMed] [Google Scholar]
- 60. Le Magueresse C, Monyer H. GABAergic interneurons shape the functional maturation of the cortex. Neuron. 2013;77:388–405. [DOI] [PubMed] [Google Scholar]
- 61. Fagiolini M, Fritschy JM, Löw K, Möhler H, Rudolph U, Hensch TK. Specific GABAA circuits for visual cortical plasticity. Science. 2004;303:1681–1683. [DOI] [PubMed] [Google Scholar]
- 62. Nusser Z, Sieghart W, Benke D, Fritschy JM, Somogyi P. Differential synaptic localization of two major gamma-aminobutyric acid type A receptor alpha subunits on hippocampal pyramidal cells. Proc Natl Acad Sci USA. 1996;93:11939–11944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Fung SJ, Sivagnanasundaram S, Weickert CS. Lack of change in markers of presynaptic terminal abundance alongside subtle reductions in markers of presynaptic terminal plasticity in prefrontal cortex of schizophrenia patients. Biol Psychiatry. 2011;69:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.