Abstract
Objective:
Cognitive deficits that characterize schizophrenia are present in the prodrome, worsen with illness onset, and predict functional outcome. Cognitive dysfunction is thus a critical target for early intervention in young individuals with recent onset schizophrenia.
Method:
This 2-site double-blind randomized controlled trial investigated cognitive training of auditory processing/verbal learning in 86 subjects with recent onset schizophrenia (mean age of 21 years). Subjects were given laptop computers to take home and were asked to perform 40 hours of training or 40 hours of commercial computer games over 8 weeks. We examined cognitive measures recommended by the Measurement and Treatment Research to Improve Cognition in Schizophrenia initiative (MATRICS), symptoms, and functioning. We also assessed baseline reward anticipation to index motivational system functioning and measured changes in auditory processing speed after 20 hours of training to assess target engagement.
Results:
Auditory training subjects demonstrated significant improvements in global cognition, verbal memory, and problem solving compared with those of computer games control subjects. Both groups showed a slight but significant decrease in symptoms and no change in functional outcome measures. Training-induced cognitive gains at posttraining showed significant associations with reward anticipation at baseline and with improvement in auditory processing speed at 20 hours.
Conclusion:
Neuroscience-informed cognitive training via laptop computer represents a promising treatment approach for cognitive dysfunction in early schizophrenia. An individual’s baseline motivational system functioning (reward anticipation), and ability to engage in auditory processing speed improvement, may represent important predictors of treatment outcome. Future studies must investigate whether cognitive training improves functioning and how best to integrate it into critical psychosocial interventions.
Key words: cognitive remediation, cognitive training, motivation, first-episode schizophrenia, early psychosis
Introduction
Early intervention in schizophrenia is a critical treatment goal, and cognitive dysfunction is arguably a key treatment target.1,2 Cognitive deficits are present during the prodrome and worsen with the onset of psychosis.3,4 Importantly, processing speed and verbal memory at first episode predict community functioning 7 years later.5
Two prior randomized controlled trials (RCTs) of cognitive remediation have been conducted in early schizophrenia—one using paper-and-pencil methods6 and one using a 2-year combination of computerized remediation plus social skills groups7—with respective effect sizes of 0.13 and 0.60 on global cognition.8 In both studies, active and control groups differed in the number of hours of clinician contact and/or received different adjunctive psychotherapies, and some assessments were conducted by staff not blind to group assignment, making it difficult to determine the active ingredients of treatment response.
To address these issues, we performed a 2-site double-blind RCT comparing approximately 40 hours of computerized auditory processing and verbal learning training to 40 hours of commercial computer games (CG) in adolescents and young adults who were within 5 years of psychosis onset (mean illness duration less than 2 years). The cognitive training was derived from basic research on neuroplasticity and shows demonstrable effects in frontotemporal networks in adults with persistent schizophrenia.9–12 A heavy schedule of auditory perceptual training is embedded within increasingly complex auditory/verbal working memory exercises in order to improve the temporally detailed resolution of auditory cortical representations and downstream working memory processes. In a prior double-blind RCT, adults with persistent schizophrenia (average age of 40 years), showed significant gains in verbal learning/memory and global cognition after 50 hours of training compared with commercial CG played for 50 hours. Gains were accompanied by adaptive neurobiological changes not seen in the control group.9–13 Four other studies have investigated this form of training in schizophrenia, but methodologies and results have been inconsistent across studies.14–17 While some of the findings have been promising, the discrepancies indicate the need for additional research.
In this study, we investigated the effects of this form of auditory training (AT) in young individuals after illness onset, delivered at home on a portable computer rather than in a laboratory or clinical setting, in keeping with a stigma-free and recovery-oriented treatment model. Participants were loaned laptop computers with the necessary software and participated in the intervention on their own schedule, with participation verified by the software. We hypothesized that participants would show significant improvement in verbal learning/memory and general cognition, consistent with our findings in adults with persistent illness. We also examined two behavioral predictors of training response—baseline motivational system functioning (reward anticipation) and auditory system target engagement. Because anticipatory reward processing and motivation are known to enhance learning and memory performance,18–20 we hypothesized that self-ratings of higher reward anticipation at baseline would predict better response to training (larger cognitive gains). We additionally hypothesized that individuals who showed early evidence of improved auditory processing speed within the training exercises—ie, successful engagement with the auditory system training target—would show greater cognitive gains (see also Murthy et al16).
Method
Participants
Eighty-six subjects completed the study protocol in our university-based early psychosis clinics at University of California, San Francisco and University of California, Davis (ClinicalTrials.gov NCT00694889). Subjects were recruited via presentations/flyers and through clinician referrals, and they met the following inclusion/exclusion criteria: (1) dagnosis of schizophrenia, schizophreniform, or schizoaffective disorder; (2) onset of first psychotic episode within past 5 years; (2) good general physical health; (3) age 14–30 years; (4) fluent and proficient in English; (5) intelligence quotient (IQ) ≥ 70; (6) no neurological disorder; and (7) no substance dependence in past year. All subjects had achieved outpatient status for at least 3 months and, among participants taking psychiatric medications (N = 81), were on a stable dose for at least one month prior to participation. Five participants did not take psychiatric medications.
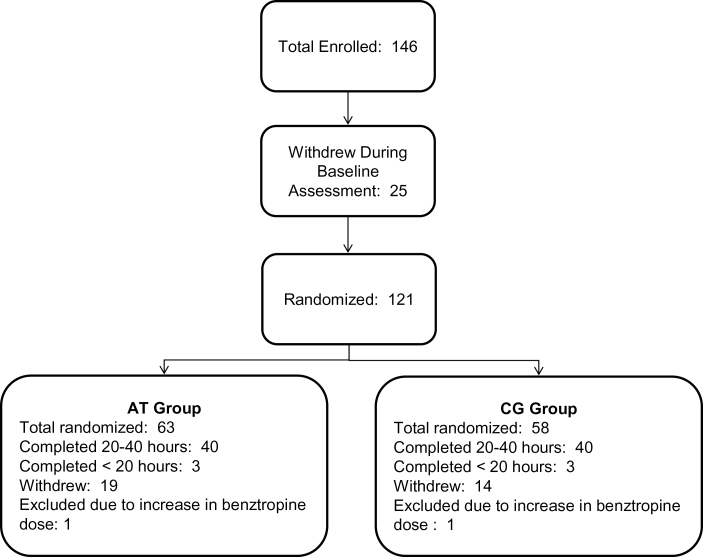
Participants aged 18 and older gave written informed consent, while those younger than age 18 provided assent, with written parental/legal guardian consent. Baseline assessments were conducted prior to randomization. Subjects were stratified by IQ, gender, and symptom severity and randomly assigned to AT or to the CG control condition (CONSORT diagram in figure 1). Subjects were loaned laptop computers and participated in the intervention at home, except for 1 training subject who preferred to participate in the laboratory. Subjects were asked to participate for 40 hours (1 h/d, 5 d/wk, for 8 wk), followed by posttraining assessments.
Fig. 1.
Consort diagram of subjects with recent onset schizophrenia who received computerized auditory training (AT) and patients who played computer games (CG).
Participants were contacted 1–2 times per week by telephone to discuss progress. Coaching was provided if a participant indicated difficulty in completing the recommended number of hours/week (eg, goal-setting; discussion of scheduling; setting an alarm, and using reminders). At a “check-in” appointment after every 10 sessions completed, the same coaching was provided and participants were paid $5 for each completed hour, $20 for every 10 sessions, and $30 after 40 hours, as well as $20 per assessment appointment. Mean training time was 34.65 hours (SD = 9.82) across both groups (table 1). While in the trial, participants received treatment by outside providers or clinic personnel not involved in the study (psychoeducation, psychotherapy, adjustments in medications as clinically indicated). Demographic characteristics and medications are presented in tables 1 and 2.
Table 1.
Baseline Characteristics of Subjects With Recent Onset Schizophrenia Who Received Computerized Auditory Training (AT) and Subjects Who Played Computer Games (CG)
| AT (N = 43) | CG (N = 43) | t | df | p | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| Male/femalea | 31/12 | 33/10 | |||||
| Age (range 16–30) | 21.70 | 3.26 | 20.74 | 3.37 | −1.34 | 84 | .19 |
| Education | 12.88 | 1.60 | 12.86 | 2.10 | −0.06 | 84 | .95 |
| WASI IQb | 102.63 | 12.12 | 100.67 | 15.06 | −0.66 | 84 | .51 |
| PANSS totalc | 57.95 | 12.72 | 59.60 | 14.33 | 0.55 | 80 | .58 |
| Strauss Carpenter | 7.83 | 2.34 | 8.00 | 2.76 | 0.31 | 80 | .76 |
| Global functioning role | 4.79 | 2.47 | 4.71 | 2.14 | −0.16 | 80 | .88 |
| Global functioning social | 5.72 | 1.30 | 5.74 | 1.43 | 0.07 | 80 | .95 |
| Hours of training | 32.93 | 10.45 | 36.37 | 8.94 | 1.64 | 84 | .10 |
| Months of illness (range 1–60) | 18.87 | 15.64 | 20.26 | 17.45 | 0.39 | 84 | .70 |
aChi square (1, N = 86) = 0.24, P = .62.
bWechsler Abbreviated Scale of Intelligence (WASI).
cPositive and Negative Syndrome Scale.
Table 2.
Medication Regimens of Study Participants
| AT (N = 43) | CG (N = 43) | Total | Test Statistic | P Value | |
|---|---|---|---|---|---|
| Antipsychotic medicationa | |||||
| First generation (N) | 1 | 1 | 2 | X 2(1) = 0.00 | 1.00 |
| Second generation (N) | 37 | 34 | 71 | X 2(1) = 0.73 | .39 |
| Multiple (N) | 2 | 3 | 5 | X 2(1) = 0.21 | .64 |
| No antipsychotic (N) | 3 | 5 | 8 | X 2(1) = 0.55 | .46 |
| Other psychiatric medication | |||||
| Antidepressants or mood stabilizers (N) | 8 | 12 | 20 | X 2(1) = 1.04 | .31 |
| Benzodiazepines (N) | 5 | 9 | 14 | X 2(1) = 1.37 | .24 |
| Other medication measures | |||||
| Chlorpromazine equivalentsb | 235.47 (155.96) | 256.36 (136.75) | t (75) = 0.62 | .53 | |
| Changes in medication while in study | 12 | 13 | X 2(1) = 0.06 | .81 | |
Note: AT, auditory training group; CG, computer games control group.
aFirst generation antipsychotic medication = halperidol, perphenazine, thiothixene, trifluoperazine;
Second generation antipsychotic medication = aripiprazole, clozapine, olanzapine, quetiapine, risperidone, ziprasidone.
bMean and SD of chlorpromazine equivalents.21
Cognitive Training Program and CG Control Condition
The cognitive training program was provided by Posit Science Corporation and has been described previously.9 It consists of computerized exercises designed to improve speed and accuracy of auditory information processing while engaging auditory and verbal working memory. This training approach is based on evidence that schizophrenia is characterized by widespread disturbances in frontotemporal neural systems subserving auditory processing and verbal memory.22,23 Exercises continuously adjust difficulty level to maintain an 80%–85% correct performance rate in order to engage the user in a dense reward schedule and drive successful learning. Correct trials are rewarded with points and animations. In each session, a participant works with 4 of 6 exercises for 15 minutes per exercise. Compliance is monitored by electronic data upload.
The CG control condition allows for maintenance of a double-blind trial design and controls for effects of computer exposure, contact with research personnel, monetary payments, and nonspecific engagement of attention, executive functions, and motivation. Control subjects rotated through a series of 16 different commercially available games (supplementary table 1) for the same number of hours as training subjects, playing 4–5 games on any given day.
Assessment Procedures
All assessment staff were blind to group assignment. Cognitive assessment staff were trained and monitored at each site on manualized assessment procedures by the same senior researcher (M.F.) to ensure cross-site consistency (the Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) battery showed an intraclass correlation of 0.88 in a multisite RCT).24 Clinical assessment staff were trained and observed by expert clinical supervisors at each site (R.L.L., J.D.R., and T.N.). Subject eligibility was determined in regular reliability rounds. Interrater reliability was calculated from staff ratings of training tapes, with an average intraclass correlation across sites of 0.83 for symptom ratings and an average kappa value of 0.95 for diagnostic agreement.
Eligibility diagnoses were determined using the Structured Clinical Interview for DSM-IV. Symptoms and functioning were assessed with the Positive and Negative Syndrome Scale25 (PANSS), Strauss Carpenter Outcome Scale (3 clinician-rated items for number of weekly social contacts, days hospitalized, and proportion of time engaged in work/school),26 and Global Functioning: Role and Social Scales (each containing a single clinician-rated item ranging from 1–10 developed for the late adolescent/young adult early psychosis population).27 An abbreviated battery of MATRICS-recommended measures28 was administered (table 3). The Tower Test from the Delis-Kaplan Executive Function System (D-KEFS)29 was used in place of the Neuropsychological Assessment Battery (NAB) Mazes. Raw scores were converted to z scores using age-appropriate normative data provided in testing manuals and age-appropriate, published normative data for Trails A,30 Category Fluency,31 and the Brief Visuospatial Memory Test-Revised (BVMT-R).32 A 6-point Likert scale was used to assess level of enjoyment.
Table 3.
Scores on Cognitive Domains, Symptoms and Functional Outcomes Before and After Intervention for Subjects With Recent Onset Schizophrenia Who Received Computerized Auditory Training (AT) and Subjects Who Played Computer Games (CG)
| Outcome Measuresa | AT (N = 43) | CG (N = 43) | F b | P | Effect Size | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Post | Baseline | Post | ||||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| Global cognition | −0.86 | 0.73 | −0.46 | 0.73 | −0.92 | 0.89 | −0.87 | 1.00 | 11.07 | <0.01 | 0.73 |
| Speed of processing | −0.66 | 0.76 | −0.27 | 0.80 | −0.63 | 0.79 | −0.55 | 1.11 | 2.50 | 0.12 | 0.33 |
| Working memory | −0.36 | 0.78 | −0.12 | 1.01 | −0.62 | 1.13 | −0.41 | 1.00 | 0.01 | 0.92 | 0.04 |
| Verbal learning | −1.45 | 1.41 | −1.29 | 1.38 | −1.55 | 1.33 | −1.88 | 1.80 | 2.45 | 0.12 | 0.43 |
| Verbal memory | −1.68 | 1.54 | −1.10 | 1.32 | −1.46 | 1.51 | −1.79 | 1.78 | 9.35 | <0.01 | 0.69 |
| Visual learning | −1.16 | 1.62 | −0.68 | 1.46 | −1.09 | 1.51 | −1.04 | 1.66 | 3.27 | 0.07 | 0.33 |
| Visual memory | −1.06 | 1.78 | −0.60 | 1.43 | −1.31 | 1.86 | −1.07 | 1.84 | 0.66 | 0.42 | 0.14 |
| Problem solving | −0.36 | 0.76 | 0.29 | 0.86 | −0.38 | 0.81 | −0.12 | 1.00 | 4.83 | 0.03 | 0.46 |
| PANSS totalc | 57.95 | 12.72 | 53.56 | 12.48 | 59.60 | 14.33 | 55.86 | 14.18 | 0.45 | 0.51 | 0.06 |
| Strauss Carpenter | 7.83 | 2.34 | 8.13 | 2.27 | 8.00 | 2.76 | 8.55 | 2.65 | 0.28 | 0.60 | −0.10 |
| Global functioning role | 4.79 | 2.47 | 4.79 | 2.63 | 4.71 | 2.14 | 4.71 | 2.43 | 0.01 | 0.91 | 0.00 |
| Global functioning social | 5.72 | 1.30 | 5.79 | 1.15 | 5.74 | 1.43 | 5.95 | 1.62 | 0.38 | 0.54 | −0.11 |
aCognitive measures were transformed to z scores using normative data of healthy samples. Global cognition (average z score across all measures); speed of processing (Trail Making Test Part A; category fluency animal naming); working memory (letter-number span; WMS-III spatial span); verbal learning and verbal memory (HVLT-R immediate and delayed recall); visual learning and visual memory (BVMT-R immediate and delayed recall); problem solving (D-KEFS Tower Test). Symptoms (PANSS) and functional outcome measures (Strauss Carpenter; global functioning role and social) are clinician ratings.
bRepeated measures ANCOVA, condition-by-time interaction, controlling for site, age, and hours of training.
c Positive and Negative Syndrome Scale.
All primary outcome measures were distinct and independent from tasks practiced during training. Alternate forms of the Hopkins Verbal Learning Test-Revised (HVLT-R) and BVMT-R were administered and counterbalanced at baseline and posttraining.
Baseline Reward Anticipation
Reward anticipation was measured using the Temporal Experience of Pleasure Scale, which assesses anticipatory and consummatory pleasure (see Gard et al33 for psychometric properties). Eighteen items are rated on a scale of 1 (very false for me) to 6 (very true for me). An example of an anticipatory item is “When something exciting is coming up in my life, I really look forward to it.” Accumulated evidence indicates that these are distinct neurobehavioral processes34; anticipatory pleasure appears closely linked to motivation and goal-directed behavior. Because prior research indicates that schizophrenia patients have a deficit in anticipatory pleasure and because successful learning requires intact brain motivational systems,18–20,35 we hypothesized that individuals with higher reward anticipation at baseline would show greater cognitive gains after training.
Target Engagement: Gains in Auditory Processing Speed
Subjects’ ability to demonstrate target engagement—ie, to show improvement in auditory cortical processing efficiency—was monitored at baseline and after 20 hours of training using auditory processing speed. This measure consists of a time-order judgment of a sequence of 2 frequency-modulated tones and is considered a measure of successive signal interference/forward and backward masking. Performance threshold was determined using a dual staircase method based on the ZEST algorithm36 that adaptively modifies the interstimulus interval (ISI) between tones and the tone duration, which is held equal to the ISI. The resulting score was the number of milliseconds of ISI (and tone duration) at which the subject correctly performed 66% of trials, allowing for a precise measure of psychophysical threshold under moderate perceptual challenge, as outlined in prior research on reliable threshold assessments in auditory processing.37
Planned Analyses
We performed data analysis on all subjects completing baseline and posttraining assessments (N = 86), regardless of hours of intervention (see figure 1), and an intent-to-treat analysis using last observation carried forward on all randomized subjects (N = 121). Based on our previous finding that medication-induced anticholinergic burden adversely affects training response,38 we excluded from analysis 2 subjects (1 in each condition) who were prescribed an increased dose of benztropine mesylate while in the study. All variables were screened and normally distributed after winsorising of outlying values.
Chi square was used to test for group differences in attrition rate. Groups were compared on change in cognitive measures, symptoms, and functional outcome ratings using repeated measures ANCOVA, controlling for site, age, and hours of training. Effect sizes (Cohen’s d) were computed using the mean change scores of the AT and CG groups (posttraining minus baseline) and the change score SDs of each group. All measures are listed in table 3. Paired sample’s t-test tested for significant gains in auditory processing speed. Pearson’s correlations tested whether baseline reward anticipation and improvement in auditory processing speed were associated with gains in global cognition.
Results
Adherence to Cognitive Training via Laptop
Nineteen out of 63 (30%) AT subjects withdrew from the study compared with 14 of 58 (24%) CG subjects, a nonsignificant difference, X 2 (1, N = 121) = 0.55, P = .46. Mean enjoyment ratings were 3.78 (SD = 0.90, range = 1.83–6) in AT subjects, and 3.88 (SD = 1.05, range = 2–6) in CG subjects, a nonsignificant difference, t (70) = 0.45, P = .66; rating of 4 = “slightly enjoyed” on a scale of 1 (“extremely disliked”) to 6 (“extremely enjoyed”).
We compared study completers with those who withdrew and found no significant differences in demographic variables, baseline symptoms and functioning, or reward anticipation (supplementary table 2). All baseline cognitive differences were nonsignificant with the exception of working memory. Study completers showed lower baseline working memory (M = −0.49, SD = 0.98) relative to participants who withdrew (M = −0.10, SD = 0.60), t (117) = 2.62, P = 0.01.
Cognitive Outcome Measures
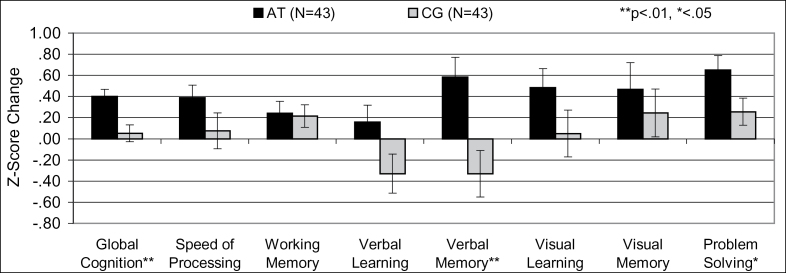
There were no differences between groups in baseline symptom severity, functioning, cognitive measures, or medication regimens (table 2). A multivariate ANCOVA on cognitive change across all cognitive measures showed a significant effect of condition (F = 2.62, df = 1, 75, P = 0.02). Repeated measures ANCOVA revealed significant condition-by-time interactions for global cognition, verbal memory, and problem solving and a difference at trend level in visual learning (table 3, figure 2). The results were the same with and without covarying for site, age, and hours of training.
Fig. 2.
Change in cognitive performance in subjects with recent onset schizophrenia after computerized auditory training (AT) or computer games (CG). Verbal memory post hoc tests: gain in AT subjects P = .003, decline in CG subjects P = .14.
An intent-to-treat analysis revealed the same significant condition-by-time interactions in global cognition (mean z score change AT = 0.28, SD = 0.40, CG = 0.04, SD = 0.45, F = 10.48, df = 1, 114, P < .01), verbal memory (mean z score change AT = 0.41, SD = 1.04, CG = −0.25, SD = 1.25, F = 9.29, df = 1, 114, P < .01), problem solving (mean z score change AT = 0.45, SD = 0.80, CG = 0.19, SD = 0.73, F = 4.47, df = 1, 114, P < .04), and a difference at trend level in visual learning (mean z score change AT = 0.34, SD = 1.00, CG = 0.04, SD = 1.25, F = 3.04, df = 1, 114, P < .08).
Cognitive Effect Sizes of the Intervention
In study completers, strong positive effects for training were found in global cognition, verbal memory, and problem solving (table 3). Effect sizes were comparable with our findings in middle-aged adult subjects who were more cognitively impaired at baseline.9 In all randomized subjects, effect sizes were in the moderate range for global cognition (d = 0.56) and verbal memory (d = 0.57), and in the small range for problem solving (d = 0.34).
Post hoc analyses of verbal memory revealed that the increase in AT subjects was significant, t (42) = 3.19, P = .003, while the decline in the computer CG group approached trend level, t (42) = 1.51, P = .14. A similar nonsignificant decline in verbal learning and memory was shown in middle-aged CG subjects with persistent schizophrenia.9
Symptom and Functional Outcome Measures
Four subjects (3 AT, 1 CG) did not complete all clinical measures at both time points. There were no significant condition-by-time interactions on the PANSS total or subscales, Strauss Carpenter Scale, or Global Functioning Role and Social scales. There was a main effect of time in the PANSS Total and General Psychopathology Subscales. Both groups showed a small decrease in PANSS Total (Mean Rating Change = −4.05, SD = 11.47, F = 6.23, df = 1, 81, P = .02), PANSS General Psychopathology (Mean Rating Change = -2.83, SD = 5.88, F = 7.57, df = 1, 81, P < .01), and no significant change in PANSS Positive or Negative Symptoms. On measures of functional outcome, all main effects of time were nonsignificant. The results remained the same with and without imputation (last observation carried forward/backward) of the 4 subjects’ missing values.
The intent-to-treat analysis revealed the same findings: a main effect of time in the PANSS Total (Mean Rating Change = −2.79, SD = 9.69, F = 4.84, df = 1, 114, P = .03) and General Psychopathology Subscales (Mean Rating Change = −1.95, SD = 9.69, F = 5.04, df = 1, 114, P = .03), and no significant main effects of time or condition-by-time interactions in PANSS Positive or Negative Symptoms, or functional outcome measures.
Baseline Reward Anticipation and Cognitive Gains
The reward anticipation measure was available on 24 AT and 23 CG subjects. In the AT group, baseline reward anticipation was significantly associated with gains in global cognition (r = 0.52, P < .01) and verbal memory (r = 0.51, P = .01) and remained significant controlling for hours of training (supplementary figure 1). These associations were not significant in the CG group. (−0.20 < r < 0.09, 0.36 < P < .68).
Target Engagement and Cognitive Gains
The auditory processing speed measure was available at the 2 time points on 30 of 40 AT subjects. There was a highly significant decrease from baseline (Mean = 98.07ms, SD = 42.86) to 20 hours (Mean = 63.20ms, SD = 37.45), t (29) = 5.07, P < .001), indicating that subjects became more efficient at rapid processing of successive auditory stimuli (target engagement). This improvement was significantly associated with gains in global cognition (r = −0.47, P < .01) (supplementary figure 2).
Discussion
Young individuals with recent onset schizophrenia who completed up to 40 hours of cognitive training via laptop computer at home showed significant increases in global cognition, verbal memory, and problem solving, compared with individuals who played up to 40 hours of CG. Participants in both groups—the majority of whom were enrolled in university-based early psychosis clinics—showed small improvements in symptoms. These findings suggest that the cognitive deficits of schizophrenia can be addressed early in the course of illness using cognitive training delivered via a portable computing device. This is particularly important as emerging data show that current clinical interventions in early psychosis may not differentially impact outcome 5 years later.39 Symptom reduction and psychosocial support alone are probably not sufficient as treatment targets if our ultimate goal is to disrupt the deteriorating course of schizophrenia. We posit that cognition is a third critical factor that must be addressed vigorously and systematically in young individuals.
Young recent onset subjects showed a similar profile of training-induced cognitive improvement as was observed in our prior study of persistently ill adults9; similarly, young “computer games” subjects showed a decrease in HVLT performance approaching trend level. To the best of our knowledge, this is the first report of decreases in verbal memory after commercial CG in a young clinical group. In a sobering study with healthy school-age children, Dworak et al40 found a significant reduction in verbal memory after a single day of exposure to voluntary excessive computer game playing and television. We hypothesize that the nonspecific visuospatial processing from an intensive schedule of CG resulted in competitive interference for limited neural resources, causing worse performance on the HVLT. These findings require replication, as they have important implications for young individuals with schizophrenia.
We also examined 2 predictors of treatment response: reward anticipation (motivational system functioning) and auditory target engagement. We found that baseline ratings of reward anticipation were significantly associated with cognitive gains in AT subjects, but not in the CG control group, even after controlling for number of hours of training. This finding is consistent with growing knowledge on the role of interindividual differences in reward processing in successful learning and plasticity mechanisms.18–20,35 Indeed, Tas et al41 recently reported that motivation and metacognition predicted learning potential in people with remitted schizophrenia.
Additionally, we found that participants with the largest improvement in auditory processing speed after 20 hours of exposure to training showed the greatest gains in global cognition at posttraining. Improvement in auditory processing speed indicates successful target engagement of the auditory system with an improvement in cortical processing efficiency. This is consistent with our prior study,9 and with reports from Murthy et al16 and Surti et al,42 demonstrating a relationship between processing speed gains and generalization of training effects. The National Institute of Mental Health recommends incorporating measures of target engagement into treatment trials in order to provide very early signs of potential failure or success of an intervention. Our current findings suggest that target engagement may be measurable mid-way through treatment (and perhaps even earlier). Early gains in auditory processing speed likely serve as an indirect measure of training-induced plasticity and increased efficiency in the neural systems subserving auditory encoding. Experiments in our persistent illness subjects indicate that training induces an increase in evoked responses in auditory cortex, accompanied by changes in activation patterns in prefrontal cortex that are associated with cognitive improvement.11
To date, few variables reliably predict response to cognitive training in schizophrenia: not type of training nor participant characteristics.8 While our results require replication, they do suggest that— at least for the highly targeted “neural system” cognitive training that we are studying—the inherent “plasticity potential” of the brain as assessed via intact reward systems and engagement of plasticity mechanisms in neural system targets of interest may be much more important predictors of treatment response than participant demographics. Indeed, these data begin to open the door to the possibility of truly personalized medicine.
There are several limitations to our study. Foremost is the lack of immediate impact on everyday functioning, which likely reflects the short study duration, or perhaps the lack of social cognition training or psychosocial interventions that enhance generalization.7,43,44 We are currently investigating whether cognitive gains persist 6 months beyond the intervention and are associated with functional improvement, as previously observed in persistently ill adults.45 Second, subjects were drawn from 2 university clinics, were of average IQ, and are not representative of community settings. We do not know if this intervention would be as effective in individuals with lower IQ or under 16 years of age. Subjects were provided monetary compensation for participation in the trial, which limits our knowledge about acceptability and adherence in real-world settings where payment will not be provided. We did not place restrictions on medication regimens during study participation, and, while there is no evidence of significant differences between the two groups, we cannot rule out medication effects on the response to the intervention. We also did not control for at-home exposure to video games or other computerized activities, which may represent a confounding variable. Further, the lack of significant improvement in MATRICS processing speed and working memory raises important questions for the design of future cognitive training programs, the selection of key training targets, and the as-yet-unknown association linking gains across cognitive domains. Finally, while the attrition rate is similar to other behavioral treatment studies in recent onset schizophrenia,46 it is still quite large. Future efforts must focus on the pragmatic effectiveness of this intervention and on enhancing appeal and increasing participant adherence.
In sum, results indicate that a neuroscience-informed approach to cognitive training in young individuals with schizophrenia, using a portable computer, can generate significant improvements in cognition, a finding with promising implications for the long-term course of illness. Given limited mental health resources and the rapid expansion of portable digital technology, further well-controlled trials of cognitive training via mobile devices is an important area for future investigation. If our data are replicated, a number of critical questions will need to be addressed. What are the necessary and sufficient elements of training that generate optimal and enduring functional gains in young individuals? How do we design the intervention to be optimally motivating and rewarding? How should it best be disseminated in real-world treatment settings? And most importantly, how can it be personalized and combined with psychosocial treatments so that every young person with schizophrenia can look forward to a productive, stable, and maximally fulfilling life course?
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
The Stanley Medical Research Institute (06TAF-972); the Laszlo Tauber Foundation; the National Institute of Mental Health (5R01MH081051); the San Francisco Department of Veterans Affairs Medical Center.
Supplementary Material
Acknowledgments
The cognitive training software used in this study was supplied to the first author free of charge by Posit Science. Dr Vinogradov is a paid consultant to Brain Plasticity Inc. (now a division of Posit Science), a company with a commercial interest in the cognitive training software used in this study. None of the other authors have any financial interest in Brain Plasticity Inc. or Posit Science. Drs Fisher, Loewy, Carter, Ragland, Niendam, Schlosser, and Vinogradov and Ms Lee, Ms Pham, and Ms Miskovich report no conflicts of interest. Drs Loewy, Carter, Ragland, Niendam, Schlosser, and Vinogradov have received grants or research support from the National Institute of Mental Health. Dr Vinogradov serves on advisory boards for Genentech, Hoffman-LaRoche, and Envivo. Dr Carter has served on the advisory board of Merck, Lilly, Pfizer, Roche, and Servier and has received research funding from Glaxo Smith Kline. Dr Loewy has received research funding from Genentech. This article’s contents are solely the responsibility of the authors.
References
- 1. Insel TR. Rethinking schizophrenia. Nature. 2010;468:187–193. [DOI] [PubMed] [Google Scholar]
- 2. McGorry P. Transition to adulthood: the critical period for pre-emptive, disease-modifying care for schizophrenia and related disorders. Schizophr Bull. 2011;37:524–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jahshan C, Heaton RK, Golshan S, Cadenhead KS. Course of neurocognitive deficits in the prodrome and first episode of schizophrenia. Neuropsychology. 2010;24:109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23:315–336. [DOI] [PubMed] [Google Scholar]
- 5. Milev P, Ho BC, Arndt S, Andreasen NC. Predictive values of neurocognition and negative symptoms on functional outcome in schizophrenia: a longitudinal first-episode study with 7-year follow-up. Am J Psychiatry. 2005;162:495–506. [DOI] [PubMed] [Google Scholar]
- 6. Wykes T, Newton E, Landau S, Rice C, Thompson N, Frangou S. Cognitive remediation therapy (CRT) for young early onset patients with schizophrenia: an exploratory randomized controlled trial. Schizophr Res. 2007;94:221–230. [DOI] [PubMed] [Google Scholar]
- 7. Eack SM, Greenwald DP, Hogarty SS, et al. Cognitive enhancement therapy for early-course schizophrenia: effects of a two-year randomized controlled trial. Psychiatr Serv. 2009;60:1468–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. Am J Psychiatry. 2011;168:472–485. [DOI] [PubMed] [Google Scholar]
- 9. Fisher M, Holland C, Merzenich MM, Vinogradov S. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am J Psychiatry. 2009;166:805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Adcock RA, Dale C, Fisher M, et al. When top-down meets bottom-up: auditory training enhances verbal memory in schizophrenia. Schizophr Bull. 2009;35:1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dale CL, Findlay AM, Adcock RA, et al. Timing is everything: neural response dynamics during syllable processing and its relation to higher-order cognition in schizophrenia and healthy comparison subjects. Int J Psychophysiol. 2010;75:183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Subramaniam K, Luks TL, Fisher M, Simpson GV, Nagarajan S, Vinogradov S. Computerized cognitive training restores neural activity within the reality monitoring network in schizophrenia. Neuron. 2012;73:842–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vinogradov S, Fisher M, Holland C, Shelly W, Wolkowitz O, Mellon SH. Is serum brain-derived neurotrophic factor a biomarker for cognitive enhancement in schizophrenia? Biol Psychiatry. 2009;66:549–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Popov T, Jordanov T, Rockstroh B, Elbert T, Merzenich MM, Miller GA. Specific cognitive training normalizes auditory sensory gating in schizophrenia: a randomized trial. Biol Psychiatry. 2011;69:465–471. [DOI] [PubMed] [Google Scholar]
- 15. Keefe RS, Vinogradov S, Medalia A, et al. Feasibility and pilot efficacy results from the multisite Cognitive Remediation in the Schizophrenia Trials Network (CRSTN) randomized controlled trial. J Clin Psychiatry. 2012;73:1016–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Murthy NV, Mahncke H, Wexler BE, et al. Computerized cognitive remediation training for schizophrenia: an open label, multi-site, multinational methodology study. Schizophr Res. 2012;139:87–91. [DOI] [PubMed] [Google Scholar]
- 17. Rass O, Forsyth JK, Bolbecker AR, et al. Computer-assisted cognitive remediation for schizophrenia: a randomized single-blind pilot study. Schizophr Res. 2012;139:92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JD. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 2006;50:507–517. [DOI] [PubMed] [Google Scholar]
- 19. Meyer PJ, Lovic V, Saunders BT, et al. Quantifying individual variation in the propensity to attribute incentive salience to reward cues. PLoS One. 2012;7:e38987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Weis T, Brechmann A, Puschmann S, Thiel CM. Feedback that confirms reward expectation triggers auditory cortex activity. J Neurophysiol. 2013;110:1860–1868. [DOI] [PubMed] [Google Scholar]
- 21. Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ragland JD, Gur RC, Raz J, et al. Effect of schizophrenia on frontotemporal activity during word encoding and recognition: a PET cerebral blood flow study. Am J Psychiatry. 2001;158:1114–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kasai K, Nakagome K, Itoh K, et al. Impaired cortical network for preattentive detection of change in speech sounds in schizophrenia: a high-resolution event-related potential study. Am J Psychiatry. 2002;159:546–553. [DOI] [PubMed] [Google Scholar]
- 24. Keefe RS, Fox KH, Harvey PD, Cucchiaro J, Siu C, Loebel A. Characteristics of the MATRICS Consensus Cognitive Battery in a 29-site antipsychotic schizophrenia clinical trial. Schizophr Res. 2011;125:161–168. [DOI] [PubMed] [Google Scholar]
- 25. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 26. Strauss JS, Carpenter WT., Jr The prediction of outcome in schizophrenia. II. Relationships between predictor and outcome variables: a report from the WHO international pilot study of schizophrenia. Arch Gen Psychiatry. 1974;31:37–42. [DOI] [PubMed] [Google Scholar]
- 27. Cornblatt BA, Auther AM, Niendam T, et al. Preliminary findings for two new measures of social and role functioning in the prodromal phase of schizophrenia. Schizophr Bull. 2007;33:688–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry. 2008;165:203–213. [DOI] [PubMed] [Google Scholar]
- 29. Delis DC, Kramer JH, Kaplan E, Holdnack J. Reliability and validity of the Delis-Kaplan Executive Function System: an update. J Int Neuropsychol Soc. 2004;10:301–303. [DOI] [PubMed] [Google Scholar]
- 30. Strauss E, Sherman EMS, Spreen O. A Compendium of neuropsychological tests: Administration, norms, and commentary. 3rd ed. New York: Oxford University Press; 2006. [Google Scholar]
- 31. Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol. 1999;14:167–177. [PubMed] [Google Scholar]
- 32. Smerbeck AM, Parrish J, Yeh EA, et al. Regression-based pediatric norms for the brief visuospatial memory test: revised and the symbol digit modalities test. Clin Neuropsychol. 2011;25:402–412. [DOI] [PubMed] [Google Scholar]
- 33. Gard DE, Germans Gard M, Kring AM, John OP. Anticipatory and consummatory components of the experience of pleasure: a scale development study. J Res Person. 2006;40:1086–1102. [Google Scholar]
- 34. Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr Res. 2007;93:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Robinson TE, Flagel SB. Dissociating the predictive and incentive motivational properties of reward-related cues through the study of individual differences. Biol Psychiatry. 2009;65:869–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Watson AB, Pelli DG. QUEST: a Bayesian adaptive psychometric method. Percept Psychophys. 1983;33:113–120. [DOI] [PubMed] [Google Scholar]
- 37. Cacace AT, McFarland DJ. Factors Influencing Tests of Auditory Processing: A Perspective on Current Issues and Relevant Concerns. J Am Acad Audiol. 2013;24:572–589. [DOI] [PubMed] [Google Scholar]
- 38. Vinogradov S, Fisher M, Warm H, Holland C, Kirshner MA, Pollock BG. The cognitive cost of anticholinergic burden: decreased response to cognitive training in schizophrenia. Am J Psychiatry. 2009;166:1055–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bertelsen M, Jeppesen P, Petersen L, et al. Five-year follow-up of a randomized multicenter trial of intensive early intervention vs standard treatment for patients with a first episode of psychotic illness: the OPUS trial. Arch Gen Psychiatry. 2008;65:762–771. [DOI] [PubMed] [Google Scholar]
- 40. Dworak M, Schierl T, Bruns T, Strüder HK. Impact of singular excessive computer game and television exposure on sleep patterns and memory performance of school-aged children. Pediatrics. 2007;120:978–985. [DOI] [PubMed] [Google Scholar]
- 41. Tas C, Brown EC, Esen-Danaci A, Lysaker PH, Brüne M. Intrinsic motivation and metacognition as predictors of learning potential in patients with remitted schizophrenia. J Psychiatr Res. 2012;46:1086–1092. [DOI] [PubMed] [Google Scholar]
- 42. Surti TS, Corbera S, Bell MD, Wexler BE. Successful computer-based visual training specifically predicts visual memory enhancement over verbal memory improvement in schizophrenia. Schizophr Res. 2011;132:131–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bowie CR, McGurk SR, Mausbach B, Patterson TL, Harvey PD. Combined cognitive remediation and functional skills training for schizophrenia: effects on cognition, functional competence, and real-world behavior. Am J Psychiatry. 2012;169:710–718. [DOI] [PubMed] [Google Scholar]
- 44. Bartholomeusz CF, Allott K, Killackey E, Liu P, Wood SJ, Thompson A. Social cognition training as an intervention for improving functional outcome in first-episode psychosis: a feasibility study. Early Interv Psychiatry. 2013;7:421–426. [DOI] [PubMed] [Google Scholar]
- 45. Fisher M, Holland C, Subramaniam K, Vinogradov S. Neuroplasticity-based cognitive training in schizophrenia: an interim report on the effects 6 months later. Schizophr Bull. 2010;36:869–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Marshall M, Rathbone J. Early intervention for psychosis. Cochrane Database Syst Rev. 2011;15:CD004718. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.