Abstract
Background:
Task-based functional neuroimaging studies of schizophrenia have not yet replicated the increased coordinated hyperactivity in speech-related brain regions that is reported with symptom-capture and resting-state studies of hallucinations. This may be due to suboptimal selection of cognitive tasks.
Methods:
In the current study, we used a task that allowed experimental manipulation of control over verbal material and compared brain activity between 23 schizophrenia patients (10 hallucinators, 13 nonhallucinators), 22 psychiatric (bipolar), and 27 healthy controls. Two conditions were presented, one involving inner verbal thought (in which control over verbal material was required) and another involving speech perception (SP; in which control verbal material was not required).
Results:
A functional connectivity analysis resulted in a left-dominant temporal-frontal network that included speech-related auditory and motor regions and showed hypercoupling in past-week hallucinating schizophrenia patients (relative to nonhallucinating patients) during SP only.
Conclusions:
These findings replicate our previous work showing generalized speech-related functional network hypercoupling in schizophrenia during inner verbal thought and SP, but extend them by suggesting that hypercoupling is related to past-week hallucination severity scores during SP only, when control over verbal material is not required. This result opens the possibility that practicing control over inner verbal thought processes may decrease the likelihood or severity of hallucinations.
Key words: schizophrenia, inner speech, speech perception, functional magnetic resonance imaging, functional connectivity
Introduction
Auditory verbal hallucinations (AVHs) are speech perceptions (SPs) that occur in the absence of an external stimulus. They are a predominant feature of schizophrenia and typically occur out of the control of the patient. Symptom-capture studies investigating the hallucinatory state have reported hyperactivity in a network of speech-related brain regions while patients are actively hallucinating (eg, primary and secondary auditory cortices, Broca’s area, frontal operculum, hippocampus, and parahippocampal region) relative to periods of no hallucinations.1–5 Resting-state studies have also reported increased activation6 and connectivity7 in frontotemporal regions in hallucinating compared to nonhallucinating schizophrenia patients and healthy controls.
Expansion of these symptom-capture and resting-state findings to task-based functional neuroimaging is important for identifying the cognitive functions underlying this increased activity/connectivity and thereby contributing to cognitive-based theories about the genesis of AVHs. However, task-based functional neuroimaging studies often do not report whether or not activity/connectivity is increased in patients experiencing hallucinations in the past week.8,9 This methodology leads to difficulties in determining whether differences are specifically related to the presence of AVHs, the diagnosis of schizophrenia, or to psychiatric disorders more generally (when comparisons are made to healthy control subjects only). In addition, the seminal work in this area has focused on inclusion of a willful inner speech (or auditory imagery) condition,8,10 but no hallucination-associated hyperactivity/hypercoupling has emerged under those conditions (although decreased activity has). It has been argued that inclusion of a willful auditory imagery condition diverges somewhat from the experience of hallucinating patients11 because when hallucinating patients are asked to imagine speech cast in another person’s voice or one of their previously heard “voices,” the patient will not experience the result as a hallucination.12 This is because only unbidden experiences can be interpreted as hallucinations.11 Thus, because AVHs occur out of the control of the patient, experimental manipulation of control over verbal material should be important for understanding the functional biology of hallucinations.
Such an experimental manipulation can be achieved in straightforward fashion through comparison of willful inner speech (ie, voluntary verbal thought generation; VTG) to SP conditions.13 Inner speech, also called silent speech, covert speech, or verbal thought, can be defined as silent speech production in one’s own mind.14,15 A subtype of inner speech is the deliberate generation of silent coherent verbal material, or verbal thought, which activates the so-called task-positive brain network.13,16 This type of voluntary inner speech is to be contrasted with the less willful “mind wandering,” in which verbal thoughts are also mentally expressed, but in a less deliberate fashion, and which activates brain regions within the task-negative (or default mode) network.16,17 In the present study, we use the term voluntary verbal thought generation to describe an intended conscious production of inner speech in response to a stimulus. During VTG, participants exert some degree of control over verbal material as they are required to mentally generate definitions of common words. SP, however, does not require control over verbal material, as participants simply listen to prerecorded definitions. A preliminary study by our group using this comparison revealed coordinated hyperactivity/hypercoupling (It is important to note that a clear distinction between coordinated hyperactivity and hypercoupling is not available with functional connectivity analyses. Brain regions with correlated and strong activations over time, which emerge on the same functional network [eg, as a result of singular value decomposition or component analysis], can be thereby considered coupled, and do so because they increase and reduce activation in synchrony [ie, in a coordinated fashion] over time. Highly coordinated and strong increases and decreases in activity lead to higher intercorrelations between regions and can be interpreted as coordinated hyperactivity and/or hypercoupling. Therefore, we use the 2 terms interchangeably here.) in a temporal-frontal network of speech-related auditory and motor regions for schizophrenia patients relative to healthy controls during both VTG and SP13; however, it was not possible to examine differences between hallucinating and nonhallucinating schizophrenia patients due to a small sample. The goal of the present study was to extend our previous work by investigating whether this hypercoupling is associated with hallucination ratings in schizophrenia patients and whether exertion of control over verbal material affects brain activity within this network in hallucinating patients.
In accordance with our past work, we expected that schizophrenia patients, irrespective of hallucination status, would demonstrate hypercoupling in a temporal-frontal network including auditory and motor regions. We further expected that hypercoupling in this functional network would be higher in hallucinating schizophrenia patients for the SP condition (in which there is assumed to be little control over verbal material) relative to nonhallucinating schizophrenia patients, psychiatric, and healthy controls.
Methods
Participants
Participants were 23 schizophrenia patients (10 hallucinators; H_SZ; 13 nonhallucinators; NH_SZ), 22 nonhallucinating bipolar patients (BPs), and 27 healthy controls (HCs), all of whom had been using English daily for at least the past 5 years and responded accurately to questions about the consent form designed to confirm their ability to read and understand English. Most were right handed18 (n = 65; left handed = 2 HCs; mixed = 1 HC, 1 NH_SZ, 3 BPs). Both in- and outpatients were included in the patient samples. Bipolar patients were selected as a psychiatric control group due to the similarities between people with a diagnosis of bipolar disorder and people with a diagnosis of schizophrenia with regard to cognitive, genetic, and environmental susceptibility factors.19 Therefore, any aspect of task performance attributable to these factors (or other overlapping characteristics, such as the stigmatization associated with mental illness) should be present for individuals within both groups. A hearing test was carried out on all but 1 participant using an audiometer (AMBCO 650AB, www.ambco.com) to ensure absence of hearing impairment. All participants provided written informed consent and met magnetic resonance imaging (MRI) compatibility criteria. The study was approved by both the University of British Columbia (UBC) and UBC Hospital Clinical Research Ethics Committees, and participants received financial compensation of $10 CAD per hour and reimbursement of travel costs for participation. Details regarding demographic variables can be found in the supplementary material.
Patients’ symptoms were assessed using the Signs and Symptoms of Psychotic Illness scale (SSPI20; see table 1 for means and group differences). The SSPI consists of 30 items and is criterion referenced, providing specific examples of behavior (over the past week) that correspond to severity levels for each item (eg, hallucinations: 0 = absent; 1 = vague descriptions of hallucinations; 2 = hallucinations that the patient accepts as arising from within his/her own mind; 3 = definite hallucinations occurring occasionally [eg, <once/day]; 4 = definite hallucinations that are frequent and/or influence observable behavior). For the following analyses, schizophrenia patients were included in the hallucinating and nonhallucinating subgroups based on their SSPI hallucinations score (hallucinating: 3 [n = 3] or 4 [n = 7]; nonhallucinating: 0 [n = 9], 1 [n = 2], or 2 [n = 2]). All hallucinating patients reported auditory hallucinations, and 6 patients reported multimodal hallucinations (tactile = 6; visual = 3; olfactory = 1). All schizophrenia patients but one had experienced hallucinations in the past. All bipolar patients scored 0 on hallucinations, with the exception of one who was rated 2 (visual hallucinations only).
Table 1.
Signs and Symptoms of Psychotic Illness Means and SDs (in Parentheses) for Patient Groups
| Variable | Bipolar (BP) | Schizophrenia— Nonhallucinating (NH_SZ) | Schizophrenia— Hallucinating (H_SZ) | |||
|---|---|---|---|---|---|---|
| Mean (SD) | Range | Mean (SD) | Range | Mean (SD) | Range | |
| Anxiety | 1.45 (1.22) | 0–4 | 1.08 (1.26) | 0–3 | 1.70 (0.82) | 1–3 |
| Depression | 1.14 (1.36) | 0–4 | 0.85 (1.21) | 0–3 | 1.60 (1.26) | 0–3 |
| Anhedonia | 1.09 (1.34) | 0–4 | 0.92 (0.95) | 0–3 | 1.30 (1.25) | 0–3 |
| Elated mood | 0.64 (1.00) | 0–3 | 0.54 (0.88) | 0–2 | 0.30 (0.67) | 0–2 |
| Insomnia | 1.18 (1.33) | 0–4 | 1.08 (1.44) | 0–4 | 0.70 (1.06) | 0–3 |
| Somatic complaints | 0.18 (0.39) | 0–1 | 0.15 (0.38) | 0–1 | 0.30 (0.95) | 0–3 |
| Delusionsa | 0.64 (1.22) | 0–4 | 1.46 (1.39) | 0–4 | 3.00 (0.82) | 2–4 |
| Hallucinationsa | 0.09 (0.43) | 0–2 | 0.46 (0.78) | 0–2 | 3.70 (0.48) | 3–4 |
| Attentional impairment | 1.41 (0.73) | 0–2 | 1.54 (0.88) | 0–3 | 1.40 (0.97) | 0–3 |
| Disorientation | 0 | 0 | 0.08 (0.28) | 0–1 | 0.20 (0.42) | 0–1 |
| Overactivity | 1.00 (0.87) | 0–2 | 1.15 (1.14) | 0–3 | 0.80 (1.03) | 0–3 |
| Underactivity | 0.86 (1.04) | 0–3 | 1.38 (1.12) | 0–3 | 1.60 (1.17) | 0–3 |
| Flattened affectb | 0.55 (0.91) | 0–3 | 1.54 (1.20) | 0–3 | 1.60 (0.97) | 0–3 |
| Inappropriate affect | 0 | 0 | 0.23 (0.83) | 0–3 | 0.10 (0.32) | 0–3 |
| Pressure of speech | 0.14 (0.35) | 0–1 | 0.15 (0.38) | 0–1 | 0.20 (0.63) | 0–2 |
| Poverty of speech | 0.14 (0.47) | 0–2 | 0.31 (0.48) | 0–1 | 0.50 (0.71) | 0–2 |
| Disordered form of thought | 0 | 0 | 0.46 (0.97) | 0–3 | 0.20 (0.63) | 0–2 |
| Peculiar behavior | 0.09 (0.29) | 0–1 | 0.15 (0.38) | 0–1 | 0.30 (0.95) | 0–3 |
| Irritability/hostility | 0.27 (0.46) | 0–1 | 0.46 (0.97) | 0–3 | 0.50 (0.71) | 0–2 |
| Impaired insightc | 0.67 (1.06) | 0–4 | 0.92 (1.38) | 0–4 | 1.90 (0.99) | 0–3 |
Note: aH_SZ > NH_SZ & BP, P < .01.
bBP < H_SZ & NH_SZ, P < .01.
cBP < H_SZ, P < .01.
Task
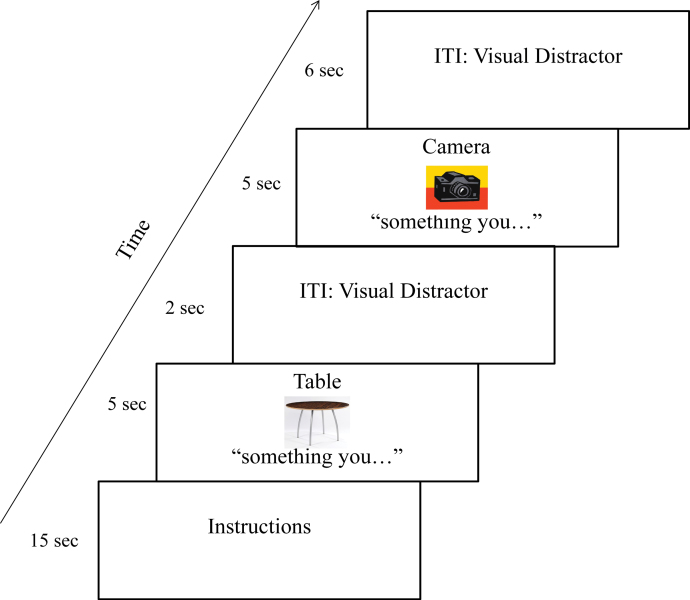
The task design employed here was nearly identical to that used in our previous study,13 with adjustments in stimulus timing, presentation, and the addition of a post-scan questionnaire to provide evidence that definitions were in fact generated. Briefly, participants were presented with a noun (object) and its corresponding image (eg, pillow) and instructed to either mentally generate (VTG) or to listen to (SP) a simple definition of the word (eg, “Something you rest your head on when sleeping”). The 2 experimental conditions were presented in blocks consisting of 15 trials each (30 trials total for each condition across 2 runs), with a 60-s rest break in between the 2 conditions. Stimuli were randomly assigned to each condition for each participant separately. The conditions were cued with the words “something you…” and “listen…” presented under the images in the VTG and SP conditions, respectively (see figure 1; see supplementary material for details on stimulus presentation and timing). Participants were administered a post-scan questionnaire where they were asked, for each trial, whether they generated a definition and, if so, what that definition was. Patients were also asked whether they experienced AVHs during functional MRI (fMRI) scanning; 1 schizophrenia patient reported auditory hallucinations during testing, and this occurred during both conditions.
Fig. 1.
Timeline of the experimental procedure. Participants were instructed to either mentally generate (voluntary verbal thought generation; VTG) or to listen to (speech perception; SP) a simple definition of a word (eg, “Something you rest your head on when sleeping” for the word “pillow”). The conditions were cued with the words “something you…” or “listen…” presented under the images for the VTG and SP conditions, respectively. The VTG condition is depicted here. For a color version, see this figure online.
Data Analysis
fMRI data analysis was carried out using constrained principal component analysis for fMRI (fMRI-CPCA; www.nitrc.org/projects/fmricpca) with orthogonal rotation.21–26 Details on image acquisition, image preprocessing, and data analysis procedures are presented in the supplementary material.
Results
Inspection of the scree plot27,28 suggested that 2 components should be extracted. Both components 1 and 2 showed a significant effect of peristimulus time, F(8,568) = 77.63, P < .001; F(8,568) = 95.78, P < .001, respectively, and visual inspection of the predictor weights confirmed a hemodynamic response (HDR) shape. The percentages of task-related variance accounted for by each rotated component were 17.91% and 7.97% for components 1 and 2, respectively.
Anatomical Descriptions
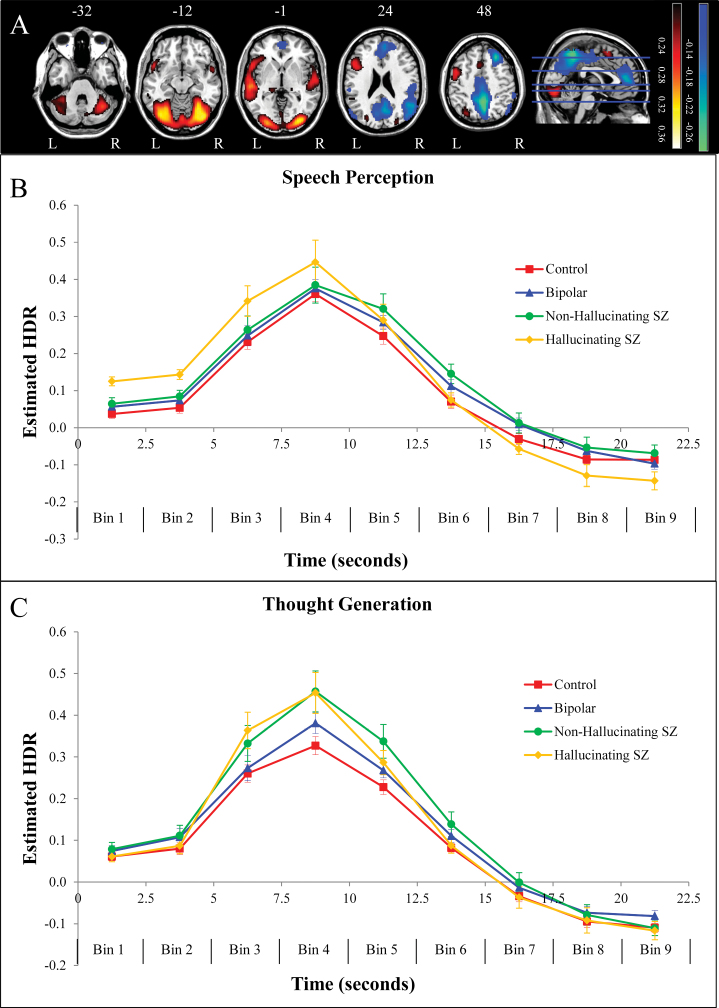
The brain regions associated with component 1 are displayed in figure 2A (top panel; red/yellow), with anatomical descriptions in supplementary table 2. Component 1 was characterized by a network of voxel clusters dominated by activations in regions involved in language production and comprehension including pars opercularis of the left inferior frontal gyrus (BA 44) and bilateral superior temporal gyri (STGs; BAs 21, 22), as well as activations within bilateral visual/fusiform regions (BAs 17, 18, 19, 37), and supplementary motor area (BA 6). The brain regions associated with component 2 are displayed in figure 2A (top panel; blue/green), with anatomical descriptions in supplementary table 3. Component 2 was characterized by a functional network involving increased activity in bilateral visual/fusiform regions (BAs 18, 19, 37) overlapping with those from component 1, and decreased activity in regions overlapping with the default mode or task-negative network,16,29 such as posterior cingulate cortex and precuneus (BA 23), medial prefrontal (BAs 9, 10), superior frontal (BA 8), and inferior parietal/lateral occipital cortex (BAs 39, 40), as well as decreased activity in other regions such as precentral gyrus (BA 6) and superior parietal cortex (BAs 2, 5, 7).
Fig. 2.
(A) Dominant 10% of component loadings for component 1 (red/yellow = positive loadings, threshold = 0.20, max = 0.37, no negative loadings passed threshold) and component 2 (blue/green = negative loadings, negative threshold = −0.14, min = −0.25). Component 2 positive loadings in the occipital regions overlapped with those from component 1 (see supplementary tables 2 and 3). Axial slices are located at Montreal Neurological Institute Z-axis coordinates −32, −12, −1, 24, 48. (B) Mean finite impulse response (FIR)-based predictor weights for speech perception, averaged over components and plotted as a function of peristimulus time. (C) Mean FIR-based predictor weights for voluntary verbal thought generation, averaged over components and plotted as a function of peristimulus time. Error bars are SEs. HDR, estimated hemodynamic response; L, left; R, right; SZ, schizophrenia. For a color version, see this figure online.
Relation to Experimental Conditions
A 2 × 9 × 2 × 4 mixed-model ANOVA revealed a significant Condition × Peristimulus Time × Group interaction, F(24,544) = 2.24, P < .001, 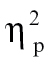 = 0.09, but no significant 4-way interaction (P > .8). This suggests that, with respect to understanding group differences, the HDR shape (indexed by peristimulus time) and condition must be taken into account, but components 1 and 2 can be combined, as is displayed in figure 2. In order to interpret this interaction, we examined group differences at each time bin for each condition separately, averaged over both components.
= 0.09, but no significant 4-way interaction (P > .8). This suggests that, with respect to understanding group differences, the HDR shape (indexed by peristimulus time) and condition must be taken into account, but components 1 and 2 can be combined, as is displayed in figure 2. In order to interpret this interaction, we examined group differences at each time bin for each condition separately, averaged over both components.
Observation of effect sizes in simple-simple main effects characterizing the significant Condition × Peristimulus Time × Group interaction demonstrated that, as is clear in figure 2B, the largest effects are observable when comparing hallucinating schizophrenia patients to the other 3 groups in SP: (1) relative to controls at time bin 1, F(1,68) = 17.84, P < .001, 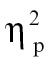 = 0.21, time bin 2, F(1,68) = 12.34, P < .005,
= 0.21, time bin 2, F(1,68) = 12.34, P < .005, 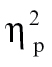 = 0.15, time bin 3, F(1,68) = 6.03, P < .05,
= 0.15, time bin 3, F(1,68) = 6.03, P < .05, 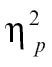 = 0.08, and time bin 9, F(1,68) = 4.77, P < .05,
= 0.08, and time bin 9, F(1,68) = 4.77, P < .05, 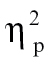 = 0.07; (2) relative to bipolar patients at time bin 1, F(1,68) = 10.14, P < .005,
= 0.07; (2) relative to bipolar patients at time bin 1, F(1,68) = 10.14, P < .005, 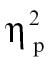 = 0.13, time bin 2, F(1,68) = 7.02, P < .05,
= 0.13, time bin 2, F(1,68) = 7.02, P < .05,  = 0.09, time bin 3, F(1,68) = 4.04, P < .05,
= 0.09, time bin 3, F(1,68) = 4.04, P < .05, 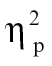 = 0.06, time bin 7, F(1,68) = 5.04, P < .05,
= 0.06, time bin 7, F(1,68) = 5.04, P < .05, 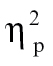 = 0.07, and time bin 8, F(1,68) = 4.93, P < .05,
= 0.07, and time bin 8, F(1,68) = 4.93, P < .05, 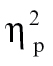 = 0.07; and (3) relative to nonhallucinating schizophrenia patients at time bin 1, F(1,68) = 6.54, P < .05,
= 0.07; and (3) relative to nonhallucinating schizophrenia patients at time bin 1, F(1,68) = 6.54, P < .05, 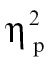 = 0.09, time bin 2, F(1,68) = 4.13, P = .05,
= 0.09, time bin 2, F(1,68) = 4.13, P = .05, 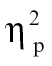 = 0.06, time bin 7, F(1,68) = 4.63, P < .05,
= 0.06, time bin 7, F(1,68) = 4.63, P < .05,  = 0.06, time bin 8, F(1,68) = 5.23, P < .05,
= 0.06, time bin 8, F(1,68) = 5.23, P < .05, 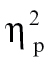 = 0.07, and time bin 9, F(1,68) = 6.39, P < .05,
= 0.07, and time bin 9, F(1,68) = 6.39, P < .05, 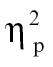 = 0.09. Nonhallucinating schizophrenia patients showed greater intensity relative to controls for time bin 6, F(1,68) = 6.53, P < .05,
= 0.09. Nonhallucinating schizophrenia patients showed greater intensity relative to controls for time bin 6, F(1,68) = 6.53, P < .05, 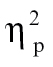 = 0.09, with no other group contrasts reaching significance for SP.
= 0.09, with no other group contrasts reaching significance for SP.
In addition, as is clear in figure 2C, the largest effects are observable when comparing schizophrenia patient groups to healthy and psychiatric controls in VTG: hallucinating schizophrenia patients demonstrated greater intensity relative to healthy controls at time bin 3, F(1,68) = 4.48, P < .05, 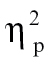 = 0.06, and time bin 4, F(1,68) = 6.60, P < .05,
= 0.06, and time bin 4, F(1,68) = 6.60, P < .05, = 0.09, and nonhallucinating schizophrenia patients demonstrated significantly greater intensity relative to controls at time bin 4, F(1,68) = 8.37, P < .01,
= 0.09, and nonhallucinating schizophrenia patients demonstrated significantly greater intensity relative to controls at time bin 4, F(1,68) = 8.37, P < .01, 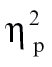 = 0.11, time bin 5, F(1,68) = 10.47, P < .005,
= 0.11, time bin 5, F(1,68) = 10.47, P < .005, 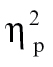 = 0.13, and time bin 6, F(1,68) = 5.33, P < .05,
= 0.13, and time bin 6, F(1,68) = 5.33, P < .05, 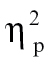 = 0.07, as well as relative to bipolar patients at time bin 5, F(1,68) = 3.92, P = .05,
= 0.07, as well as relative to bipolar patients at time bin 5, F(1,68) = 3.92, P = .05, 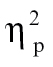 = 0.06. All increases in intensity can be interpreted as greater activation increases in red areas in figure 2A and greater activation decreases in blue areas in figure 2A.
= 0.06. All increases in intensity can be interpreted as greater activation increases in red areas in figure 2A and greater activation decreases in blue areas in figure 2A.
Correlation With Hallucinations
In order to examine associations with hallucinations, correlations were computed between the estimated HDR (ie, predictor weights) for each condition and the SSPI hallucinations item for schizophrenia patients. The estimated HDR was averaged across time bins 1, 2, and 3 for SP and time bins 3 and 4 for VTG, given that these were the time points on which hallucinating schizophrenia patients were distinguishable from controls, for each condition, respectively. The SSPI hallucinations score was significantly correlated with the estimated HDR in the SP condition, r(21) = .46, P < .05, and not in the VTG condition, r(21) = .11, P > .60; however, the difference between these correlations did not reach statistical significance, Z = 1.20, P < .24. Scatterplots for both of these correlations are presented in Supplementary figures 1 and 2 for SP and VTG, respectively. None of the remaining 19 SSPI categories were significantly related to the estimated HDR in either SP or VTG using a cutoff of significance of P = .01 in accordance with the exploratory nature of those correlations (see supplementary table 4 for the full list of correlations).
Discussion
Functional neuroimaging studies have reported hyperactivity in speech-related brain networks in hallucinating schizophrenia patients during the experience of hallucinations1–5 as well as at rest.6,7 However, task-based functional neuroimaging studies have not yet demonstrated that this increased activity/connectivity is associated with hallucinations in schizophrenia. In the current event-related fMRI study, we examined task-elicited activity during conditions requiring (VTG) or not requiring (SP) control over verbal material in schizophrenia patients with and without hallucinations, bipolar patients, and healthy controls. Functional connectivity analysis revealed a left-dominant temporal-frontal network including speech-related auditory and motor regions, which showed hypercoupling in hallucinating schizophrenia patients relative to all other groups during SP. In addition, this hypercoupling was higher in both hallucinating and nonhallucinating schizophrenia patients relative to controls and bipolar patients during VTG. These findings replicate our previous work showing generalized speech-related functional network hyperactivity in schizophrenia during inner verbal thought and SP,13 but extend them by suggesting that hypercoupling is related to hallucination scores only during SP, when control processes are not required.
The finding of hypercoupling in hallucinating schizophrenia patients when control processes are not required fits with the observation that AVHs occur out of the control of the patient.30 The involvement of the STG provides further evidence that bottom-up cognitive processes contribute to hallucinations, in accordance with a number of neurocognitive accounts of AVHs.11,31–33 However, the results also suggest that top-down influences may play an important role because the expectation of control over verbal material in VTG negated the hallucination-specific hypercoupling observed in SP. The nature of the interplay between top-down and bottom-up influences seems fertile ground for future research on AVHs, and has already been considered by other accounts as efference copy,11,33 or expectations, hypervigilance, imagination/fantasy, and memories/trauma.34 Another possible top-down influence on hallucinations is the cognitive biases underlying delusions, such as hypersalience of a match between evidence (increased vividness of perceptual qualities) and a self-selected hypothesis (“I will hear voices”).35–37
The hypercoupling observed in both hallucinating and nonhallucinating schizophrenia patients in VTG can be explained by the reduced cognitive efficiency account of schizophrenia as a diagnostic category.38 Assuming that the requirement for cognitive control in VTG requires more cognitive capacity than in SP, it is important to note that people with schizophrenia are known to demonstrate reduced efficiency in functional networks, whereby, relative to healthy controls, they must devote more cognitive resources to perform a moderately demanding task.22,38,39 Therefore, increased engagement of these functional networks would be expected regardless of hallucination severity because inefficiency is thought to be diagnosis based and not symptom based.22,38,39
Interestingly, the current results provide evidence that the hypercoupling for hallucinating schizophrenia patients relative to the other groups in SP was present during task-off periods, namely, in the period between 0 and 5 s poststimulus, when the HDR would not have had sufficient time to peak in response to task demands. Although brain activity during task-off periods reflects a wide range of cognitive processes,40,41 this hypercoupling in hallucinating schizophrenia patients in the current study was observed in the same network involved in the task-on period, suggesting that this particular task-off activity engages the same networks as SP. Note that an HDR shape with a sharp peak would not be expected during task-off periods (as it is for task-on periods) because the cognitive processes occurring during task-off periods do not have consistent timing. This suggests that for hallucinating patients during the off-task period of the SP block (1) a functional network that includes speech-related auditory and motor regions is more active, which has been suggested elsewhere for auditory cortex42 and (2) the deactivation of the default-mode network normally associated with task-related activity is already pronounced, as has been suggested for schizophrenia patients.43 Importantly, this effect was not present during VTG, which differed from the SP condition in that it involved the added expectation of exerting control over verbal material. This suggests that, for schizophrenia patients with hallucinations, the expectation of exerting cognitive control attenuated the abnormalities found during task-off periods; namely, it attenuated both exaggerated activation of temporal-frontal regions and exaggerated reduction of default-mode regions. From this, we can speculate that expecting to control inner verbal thought processes may reduce hypercoupling in the speech-related functional network and reduce the likelihood of hallucinations. The suggestion that control processes and hallucinations are incompatible has already been proposed by the breakaway speech/unbidden thoughts account of hallucinations.11, 31 ,32
It has been previously stated that AVHs may be attributable to frontotemporal disconnection,10,44,45 possibly resulting from a breakaway SP network. 31 , 32 However, with the current set of results, we provide evidence for hypercoupling in a left-dominant temporal-frontal network associated with AVHs during SP. The participants with the highest estimated HDR peaks (suggesting hypercoupling) were the schizophrenia patients experiencing the most severe hallucinations in the previous week. Additional evidence for coordinated hyperactivity/hypercoupling (as well as other connectivity concepts) may be achieved by combining structural measures of connectivity with functional measures, such as measures of white matter integrity (eg, diffusion tensor imaging; DTI). For example, DTI studies have supported the notion of increased connectivity between language and auditory processing regions in patients with AVHs but have also provided evidence for frontotemporal disconnection. 46–48
Limitations of this study include an absence of direct quantification of trial-by-trial task engagement. However, given that all group differences involved increased activity for schizophrenia patients relative to controls, it is unlikely that the results were influenced by patients being disengaged from the task. It was also not possible to discount the influence of cognitive processes occurring between the offset of the auditory stimulus and the onset of the inter-trial interval (ITI) in SP, which lasted just over 2 s; however, given the similarities between the shape of the estimated HDRs in SP and VTG, it is unlikely that cognitive processes during this period affected the current results. In addition, an alternative interpretation of the absence of an association with hallucinations during VTG is a noisier signal in that condition because the thought processes in VTG have more variable timing than the perceptual processes in SP. Another limitation is that, to the extent the cognitive processes studied here are affected by antipsychotic medication, the current results could be confounded by medication use, as dosage was not available for all participants. Finally, it was not possible in the current study to determine whether the hypercoupling observed during SP in hallucinating schizophrenia patients is specific to speech or is a more general effect. If hyperactivity in this network was specific to AVHs, one would not expect to see similar hyperactivity during a nonspeech auditory task. Further research will be needed in order to investigate these alternative possibilities.
Conclusions
The goal of the present study was to determine whether hallucination-associated task-based hypercoupling in a speech-related auditory-motor network depends on the engagement of control processes. Schizophrenia patients demonstrated hypercoupling in a left-dominant temporal-frontal network involving auditory-motor brain regions under conditions both requiring (VTG) and not requiring (SP) control over verbal material. Importantly, this effect was associated with hallucination ratings only for SP, when control processes were not engaged, suggesting that the expectation of exerting cognitive control led to a correction of hypercoupling in recently hallucinating patients. This result opens the possibility that practicing control over inner verbal thought processes may decrease the likelihood or severity of hallucinations, a finding that may be an important consideration for cognitive behavioral therapy for voice-hearing.49–51
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
T.S.W. was supported by a scholar award from the Michael Smith Foundation for Health Research (CI-SCH-00073) and a New Investigator Award from the Canadian Institutes of Health Research (MMS8770). Operating costs were supported by a grant to T.S.W. from the Mind Foundation of British Columbia.
Supplementary Material
Acknowledgments
The authors acknowledge the University of British Columbia MRI Research Centre and thank John Paiement for assistance with fMRI-CPCA computer programming. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Jardri R, Pouchet A, Pins D, Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am J Psychiatry. 2011;168:73–81. [DOI] [PubMed] [Google Scholar]
- 2. Suzuki M, Yuasa S, Minabe Y, Murata M, Kurachi M. Left superior temporal blood flow increases in schizophrenic and schizophreniform patients with auditory hallucination: a longitudinal case study using 123I-IMP SPECT. Eur Arch Psychiatry Clin Neurosci. 1993;242:257–261. [DOI] [PubMed] [Google Scholar]
- 3. Sommer IE, Diederen KM, Blom JD, et al. Auditory verbal hallucinations predominantly activate the right inferior frontal area. Brain. 2008;131:3169–3177. [DOI] [PubMed] [Google Scholar]
- 4. van de Ven VG, Formisano E, Röder CH, et al. The spatiotemporal pattern of auditory cortical responses during verbal hallucinations. NeuroImage. 2005;27:644–655. [DOI] [PubMed] [Google Scholar]
- 5. Dierks T, Linden DE, Jandl M, et al. Activation of Heschl’s gyrus during auditory hallucinations. Neuron. 1999;22:615–621. [DOI] [PubMed] [Google Scholar]
- 6. Wolf ND, Grön G, Sambataro F, et al. Magnetic resonance perfusion imaging of auditory verbal hallucinations in patients with schizophrenia. Schizophr Res. 2012;134:285–287. [DOI] [PubMed] [Google Scholar]
- 7. Hoffman RE, Fernandez T, Pittman B, Hampson M. Elevated functional connectivity along a corticostriatal loop and the mechanism of auditory/verbal hallucinations in patients with schizophrenia. Biol Psychiatry. 2011;69:407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shergill SS, Bullmore E, Simmons A, Murray R, McGuire P. Functional anatomy of auditory verbal imagery in schizophrenic patients with auditory hallucinations. Am J Psychiatry. 2000;157:1691–1693. [DOI] [PubMed] [Google Scholar]
- 9. Plaze M, Bartrés-Faz D, Martinot JL, et al. Left superior temporal gyrus activation during sentence perception negatively correlates with auditory hallucination severity in schizophrenia patients. Schizophr Res. 2006;87:109–115. [DOI] [PubMed] [Google Scholar]
- 10. McGuire PK, Silbersweig DA, Wright I, et al. Abnormal monitoring of inner speech: a physiological basis for auditory hallucinations. Lancet. 1995;346:596–600. [DOI] [PubMed] [Google Scholar]
- 11. Ford J, Hoffman R. Functional brain imaging of auditory hallucinations: from self-monitoring deficits to co-opted neural resources. In: Jardri R, Cachia A, Thomas P, Pins D, eds. The Neuroscience of Hallucinations. New York, NY: Springer; 2013:359–373. [Google Scholar]
- 12. Hoffman RE. Verbal hallucinations and language production processes in schizophrenia. Behav Brain Sci. 1986;9:503–517. [Google Scholar]
- 13. Rapin LA, Dohen M, Lœvenbruck H, Whitman JC, Metzak PD, Woodward TS. Hyperintensity of functional networks involving voice-selective cortical regions during silent thought in schizophrenia. Psychiatry Res. 2012;202:110–117. [DOI] [PubMed] [Google Scholar]
- 14. Morin A. Possible links between self-awareness and inner speech theoretical background, underlying mechanisms, and empirical evidence. J Consci Stud. 2005;12:115–134. [Google Scholar]
- 15. Perrone-Bertolotti M, Rapin L, Lachaux JP, Baciu M, Lœvenbruck H. What is that little voice inside my head? Inner speech phenomenology, its role in cognitive performance, and its relation to self-monitoring. Behav Brain Res. 2014;261:220–239. [DOI] [PubMed] [Google Scholar]
- 16. Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mason MF, Norton MI, Van Horn JD, Wegner DM, Grafton ST, Macrae CN. Wandering minds: the default network and stimulus-independent thought. Science. 2007;315:393–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Annett M. A classification of hand preference by association analysis. Br J Psychol (London, England: 1953). 1970;61:303–321. [DOI] [PubMed] [Google Scholar]
- 19. Barrett SL, Mulholland CC, Cooper SJ, Rushe TM. Patterns of neurocognitive impairment in first-episode bipolar disorder and schizophrenia. Br J Psychiatry. 2009;195:67–72. [DOI] [PubMed] [Google Scholar]
- 20. Liddle PF, Ngan ET, Duffield G, Kho K, Warren AJ. Signs and Symptoms of Psychotic Illness (SSPI): a rating scale. Br J Psychiatry. 2002;180:45–50. [DOI] [PubMed] [Google Scholar]
- 21. Metzak P, Feredoes E, Takane Y, et al. Constrained principal component analysis reveals functionally connected load-dependent networks involved in multiple stages of working memory. Hum Brain Mapp. 2011;32:856–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Metzak PD, Riley JD, Wang L, Whitman JC, Ngan ET, Woodward TS. Decreased efficiency of task-positive and task-negative networks during working memory in schizophrenia. Schizophr Bull. 2012;38:803–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Woodward TS, Cairo TA, Ruff CC, Takane Y, Hunter MA, Ngan ET. Functional connectivity reveals load dependent neural systems underlying encoding and maintenance in verbal working memory. Neuroscience. 2006;139:317–325. [DOI] [PubMed] [Google Scholar]
- 24. Woodward TS, Feredoes E, Metzak PD, Takane Y, Manoach DS. Epoch-specific functional networks involved in working memory. Neuroimage. 2013;65:529–539. [DOI] [PubMed] [Google Scholar]
- 25. Takane Y, Hunter MA. Constrained principal component analysis: a comprehensive theory. Appl Algebra Eng Commun Comput. 2001;12:391–419. [Google Scholar]
- 26. Takane Y, Shibayama T. Principal component analysis with external information on both subjects and variables. Psychometrika. 1991;56:97–120. [Google Scholar]
- 27. Cattell RB. The scree test for the number of factors. Multivar Behav Res. 1966;1:245–276. [DOI] [PubMed] [Google Scholar]
- 28. Cattell RB, Vogelmann S. A comprehensive trial of the scree and kg criteria for determining the number of factors. Multivar Behav Res. 1977;12:289–325. [DOI] [PubMed] [Google Scholar]
- 29. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. [DOI] [PubMed] [Google Scholar]
- 30. Hoffman RE, Varanko M, Gilmore J, Mishara AL. Experiential features used by patients with schizophrenia to differentiate ‘voices’ from ordinary verbal thought. Psychol Med. 2008;38:1167–1176. [DOI] [PubMed] [Google Scholar]
- 31. Hoffman RE. New methods for studying hallucinated ‘voices’ in schizophrenia. Acta Psychiatr Scand Suppl. 1999;395:89–94. [DOI] [PubMed] [Google Scholar]
- 32. David AS. The neuropsychological origin of auditory hallucinations. In: David AS, Cutting JC, eds. The Neuropsychology of Schizophrenia. Hillsdale, NJ: Lawrence Erlbaum; 1994:269–313. [Google Scholar]
- 33. Allen P, Larøi F, McGuire PK, Aleman A. The hallucinating brain: a review of structural and functional neuroimaging studies of hallucinations. Neurosci Biobehav Rev. 2008;32:175–191. [DOI] [PubMed] [Google Scholar]
- 34. Waters F, Allen P, Aleman A, et al. Auditory hallucinations in schizophrenia and nonschizophrenia populations: a review and integrated model of cognitive mechanisms. Schizophr Bull. 2012;38:683–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Balzan R, Delfabbro P, Galletly C, Woodward T. Reasoning heuristics across the psychosis continuum: the contribution of hypersalient evidence-hypothesis matches. Cogn Neuropsychiatry. 2012;17:431–450. [DOI] [PubMed] [Google Scholar]
- 36. Speechley WJ, Whitman JC, Woodward TS. The contribution of hypersalience to the “jumping to conclusions” bias associated with delusions in schizophrenia. J Psychiatry Neurosci. 2010;35:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Whitman JC, Menon M, Kuo SS, Woodward TS. Bias in favour of self-selected hypotheses is associated with delusion severity in schizophrenia. Cogn Neuropsychiatry. 2013;18:376–389. [DOI] [PubMed] [Google Scholar]
- 38. Callicott JH, Mattay VS, Verchinski BA, Marenco S, Egan MF, Weinberger DR. Complexity of prefrontal cortical dysfunction in schizophrenia: more than up or down. Am J Psychiatry. 2003;160:2209–2215. [DOI] [PubMed] [Google Scholar]
- 39. Cairo TA, Woodward TS, Ngan ET. Decreased encoding efficiency in schizophrenia. Biol Psychiatry. 2006;59:740–746. [DOI] [PubMed] [Google Scholar]
- 40. Stark CE, Squire LR. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci U S A. 2001;98:12760–12766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL. Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage. 2000;11:735–759. [DOI] [PubMed] [Google Scholar]
- 42. Northoff G, Qin P. How can the brain’s resting state activity generate hallucinations? A ‘resting state hypothesis’ of auditory verbal hallucinations. Schizophr Res. 2011;127:202–214. [DOI] [PubMed] [Google Scholar]
- 43. Garrity AG, Pearlson GD, McKiernan K, Lloyd D, Kiehl KA, Calhoun VD. Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry. 2007;164:450–457. [DOI] [PubMed] [Google Scholar]
- 44. Frith C. The Cognitive Neuropsychology of Schizophrenia. Hillsdale, NJ: Lawrence Erlbaum; 1992. [Google Scholar]
- 45. McGuire PK, Silbersweig DA, Wright I, Murray RM, Frackowiak RS, Frith CD. The neural correlates of inner speech and auditory verbal imagery in schizophrenia: relationship to auditory verbal hallucinations. Br J Psychiatry. 1996;169:148–159. [DOI] [PubMed] [Google Scholar]
- 46. Shergill SS, Kanaan RA, Chitnis XA, et al. A diffusion tensor imaging study of fasciculi in schizophrenia. Am J Psychiatry. 2007;164:467–473. [DOI] [PubMed] [Google Scholar]
- 47. Lee K, Yoshida T, Kubicki M, et al. Increased diffusivity in superior temporal gyrus in patients with schizophrenia: a Diffusion Tensor Imaging study. Schizophr Res. 2009;108:33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hubl D, Koenig T, Strik W, et al. Pathways that make voices: white matter changes in auditory hallucinations. Arch Gen Psychiatry. 2004;61:658–668. [DOI] [PubMed] [Google Scholar]
- 49. Beck AT, Rector NA. Cognitive therapy of schizophrenia: a new therapy for the new millennium. Am J Psychother. 2000;54:291–300. [DOI] [PubMed] [Google Scholar]
- 50. Chadwick P, Birchwood M. The omnipotence of voices. A cognitive approach to auditory hallucinations. Br J Psychiatry. 1994;164:190–201. [DOI] [PubMed] [Google Scholar]
- 51. Morrison AP, Renton JC. Cognitive therapy for auditory hallucinations: a theory-based approach. Cogn Behav Pract. 2001;8:147–160. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.