Abstract
Impaired cognitive empathy (ie, understanding the emotional experiences of others) is associated with poor social functioning in schizophrenia. However, it is unclear whether the neural activity underlying cognitive empathy relates to social functioning. This study examined the neural activation supporting cognitive empathy performance and whether empathy-related activation during correctly performed trials was associated with self-reported cognitive empathy and measures of social functioning. Thirty schizophrenia outpatients and 24 controls completed a cognitive empathy paradigm during functional magnetic resonance imaging. Neural activity corresponding to correct judgments about the expected emotional expression in a social interaction was compared in schizophrenia subjects relative to control subjects. Participants also completed a self-report measure of empathy and 2 social functioning measures (social competence and social attainment). Schizophrenia subjects demonstrated significantly lower accuracy in task performance and were characterized by hypoactivation in empathy-related frontal, temporal, and parietal regions as well as hyperactivation in occipital regions compared with control subjects during accurate cognitive empathy trials. A cluster with peak activation in the supplementary motor area (SMA) extending to the anterior midcingulate cortex (aMCC) correlated with social competence and social attainment in schizophrenia subjects but not controls. These results suggest that neural correlates of cognitive empathy may be promising targets for interventions aiming to improve social functioning and that brain activation in the SMA/aMCC region could be used as a biomarker for monitoring treatment response.
Key words: empathy, social functioning, functional neuroimaging, schizophrenia
Introduction
Empathy can be defined as sharing and understanding the emotional experiences of others1 by cognitively processing the behaviors and cues displayed during social encounters.2 These empathic processes can be separated into “affective” and “cognitive” subcomponents2,3; sharing the emotions of others is critical to affective empathy, whereas understanding the emotional perspective of others and maintaining a distinction between one’s own feelings and the experiences of others is critical for cognitive empathy.4
Research suggests that affective empathy is generally supported by activation of the inferior frontal gyrus (IFG), anterior insula, anterior cingulate cortex, and supplementary motor area (SMA), while cognitive empathy is supported by activation of the IFG, anterior insula, and the anterior midcingulate cortex (aMCC).5,6 The activation of the IFG and anterior insula during cognitive empathy performance suggests that cognitive empathy may require affective processing.7,8 Cognitive empathy may also be supported by activation in regions associated with the mentalizing network such as the medial prefrontal cortex (mPFC),9–12 right temporoparietal junction (TPJ),12–14 and precuneus.9,15,16 These same regions subserve theory-of-mind processes (ie, mentally representing the nonemotional beliefs and intentions of others).17–19 Thus, cognitive empathy appears to use neural regions associated with both affective processing and theory-of-mind.3,20
Recent studies suggest that schizophrenia subjects demonstrate hypoactivation of brain regions related to empathy.21–24 Moreover, cognitive empathy is particularly impaired in schizophrenia subjects,25–27 while self-reported and performance-based measures of cognitive empathy explain unique variation in measures of social functioning after accounting for neurocognitive deficits and clinical symptoms.28,29 Hence, cognitive empathy may be a critical target for developing interventions aimed at improving social functioning in this population. For instance, a recent finding suggests that cognitive empathy in schizophrenia subjects may be enhanced with oxytocin treatment.30 Although multiple studies investigated the neural correlates associated with impaired cognitive empathy in schizophrenia, they have not yet examined whether these neural correlates might also be related to social functioning.
Schizophrenia subjects have been characterized by alterations in frontotemporoparietal brain activation when making judgments related to cognitive empathy.21–23 Some studies found that schizophrenia subjects had hypoactivation of the mPFC and right TPJ as compared with healthy subjects during cognitive empathy performance,22,31 while others found that schizophrenia subjects had hypoactivation of the IFG, mPFC, aMCC, and precuneus during cognitive empathy performance.21 Overall, the results suggest that schizophrenia subjects may have generalized hypoactivation across multiple regions involved in empathic decision making. Although some studies demonstrated that brain activation during social cognitive task performance (ie, facial affect perception, theory-of-mind) was associated with social functioning,32,33 the relationship between neural activation underlying cognitive empathy and social functioning among schizophrenia subjects has not been examined.
The current study used an event-related design with whole-brain analysis to examine the brain activation in schizophrenia subjects and healthy controls during a cognitive empathy paradigm and the associations between empathy-related brain activation, and measures of cognitive empathy and social functioning. Based on our review of the literature, we hypothesized that schizophrenia subjects would: (1) demonstrate poorer cognitive empathy and social functioning as compared with control subjects, (2) be characterized by hypoactivation in anterior insula, IFG, mPFC, aMCC, TPJ, and precuneus as compared with control subjects, and (3) exhibit brain-behavior correlations between empathy-related brain activation and measures of cognitive empathy and social functioning.
Methods
Subjects
Clinically stable schizophrenia subjects (n = 30) and healthy controls (n = 24), group-matched for age (18–50 years old), gender, race, parental socioeconomic status,34 and handedness (table 1), participated in the study, which was approved by the institutional review board at Northwestern University Feinberg School of Medicine. These subjects are a subset from a larger study on empathy among individuals with schizophrenia who returned to complete neuroimaging procedures.29 Schizophrenia subjects were recruited from advertisements at local outpatient treatment centers, surrounding neighborhoods, and the National Alliance for Mental Illness. Control subjects were recruited from neighborhoods near the service providers and via online advertisements. Subjects were excluded if they (1) met Diagnostic and Statistical Manual of Mental Disorders-4th Edition (DSM-IV) criteria for current substance abuse or dependence within the past 6 months, (2) had a severe medical disorder, (3) had a head injury with neurological sequelae, or (4) met criteria for mental retardation. Control subjects were further excluded if they had a lifetime history of any DSM-IV axis I disorder or a first-degree relative with a psychotic disorder. Written informed consent was obtained after a complete description of the study was provided to subjects.
Table 1.
Demographic and Clinical Characteristics of Study Groups
| Between-Group Differences, Mean (SD) | P Value | |||||
|---|---|---|---|---|---|---|
| Control Subjects (n = 24) | Schizophrenia Subjects (n = 30) | χ 2 Statistic | ||||
| N | % | N | % | |||
| Demographic | ||||||
| Gender (% male) | 14 | 58.3 | 18 | 60.0 | 0.1 | .90 |
| Race | ||||||
| Caucasian | 12 | 50.0 | 13 | 43.3 | 0.5 | .79 |
| African American | 9 | 37.5 | 14 | 46.7 | ||
| Other | 3 | 12.5 | 3 | 10.0 | ||
| Mean | SD | Mean | SD | F Statistic | ||
| Age (years) | 34.4 | 8.9 | 33.6 | 7.1 | 0.1 | .72 |
| Handedness | 0.9 | 0.3 | 0.9 | 0.4 | 0.5 | .57 |
| Mean parental SESa | 26.0 | 10.5 | 22.6 | 10.9 | 1.3 | .24 |
| Clinical | ||||||
| SAPS global ratings | ||||||
| Hallucinations | — | — | 2.8 | 2.1 | — | — |
| Delusions | — | — | 3.3 | 1.9 | — | — |
| Bizarre behavior | — | — | 1.7 | 1.9 | — | — |
| Positive formal thought disorder | — | — | 2.2 | 1.6 | — | — |
| SANS global ratings | ||||||
| Affective flattening | — | — | 3.2 | 1.6 | — | — |
| Alogia | — | — | 2.3 | 1.6 | — | — |
| Avolition | — | — | 3.4 | 1.5 | — | — |
| Anhedonia | — | — | 3.1 | 1.5 | — | — |
| Attention | — | — | 3.1 | 1.5 | — | — |
| Duration of illness (years) | — | — | 13.6 | 7.5 | — | — |
| Chlorpromazine equivalent | — | — | 362.8 | 237.7 | — | — |
Note: Parental socioeconomic status (SES), Scale for the assessment of positive symptoms (SAPS), Scale for the assessment of negative symptoms (SANS).
aCompleted by n = 23 control subjects and n = 29 schizophrenia subjects.
Demographic and Clinical Measures
Demographic and clinical measures were collected using the Structured Clinical Interview for DSM-IV (SCID),35 which was administered by Master- and PhD-level research staff who regularly participated in training and reliability sessions. Diagnosis was validated from a consensus between a semistructured psychiatrist interview and the SCID. Antipsychotic medication dosages were converted into chlorpromazine equivalents using a standardized method.36 Psychopathology was assessed in schizophrenia subjects using the global ratings from the Scale for the Assessment of Positive Symptoms37 and the Scale for the Assessment of Negative Symptoms.38 Self-reported empathy was assessed using the interpersonal reactivity index (IRI),39 which includes subscales for fantasy, perspective-taking, empathic concern, personal distress, and an IRI total score.
Social Functioning
Social functioning was assessed using validated measures of social competence and social attainment.40,41 Social competence was assessed using the social skills performance assessment, a video-recorded test comprised of 2 role-play scenes involving meeting a new neighbor and requesting help from a landlord.42 Each scene was rated on a 5-point scale across 8 criteria for the first scene and 9 criteria for the second scene42 and a final score was calculated by averaging the 2 role-play scores (intraclass correlation coefficient = 0.92 for 2 blinded raters on 25% of the videos). Social attainment was assessed using the total score from the Specific Levels of Functioning Scale, which measures interpersonal relationships, social acceptability, daily living activities, and work skills.43
Cognitive Empathy Task
Cognitive empathy was assessed using an emotional perspective-taking (EPT) event-related paradigm adapted from Derntl et al44 and administered while subjects underwent functional magnetic resonance imaging (fMRI). Participants completed 2 runs and each run consisted of 2 blocks of EPT trials and 2 blocks of control trials. The run order was counterbalanced across participants. In each trial, one of 70 static scenes of 2 people engaged in an emotional interaction with the face of one person masked was presented (see online supplementary figure 1). For EPT trials, subjects viewed the static scene and were then presented with 2 emotional (or neutral) faces from which they were instructed to choose the best option that characterized the emotion of the masked face (eg, fear, anger, sadness, disgust, or happiness).44 For control trials, subjects viewed the static scene and were asked to choose whether the masked face was on the left or right. The locations of the response labels were counterbalanced across trials (eg, “left” on right side of the screen, thus requiring a right button press). Stimuli were presented in blocks of 7 trials; each block was preceded by directions indicating whether the block included emotion or control trials. The face stimuli were taken from a standardized stimulus set.45 Static scenes were presented at fixed intervals of 4000 ms followed by a pseudorandom fixation period (0, 550, 1100, or 1650 ms) after which the response stimuli were presented for ≤4000 ms. Responses triggered progression to the next intertrial interval displaying a central cross-hair for 8–12 s. Accuracy and response times were recorded for each trial.
fMRI Acquisition and Processing
MR scanning was performed on a 3T TIM Trio system (Siemens Medical Systems) at Northwestern University’s Center for Translational Imaging. Anatomical MRI was collected using a high-resolution 3D T1-weighted MPRAGE sequence optimized for gray-white contrast (echo time (TE) = 3.16 ms, repetition time (TR) = 2400 ms, 1 × 1 × 1 mm voxels, time = 8.09min). During each functional run, 360 volumes were collected consisting of 40 contiguous axial images acquired parallel to the anterior-posterior commissure plane using an echo-planar sequence contrast (T2*) (TR = 2200 ms, TE = 20 ms, field of view (FOV) = 220 × 206 mm, flip = 80°, 1.7 × 1.7 × 3.0 mm voxels). Each run lasted 13:12min and the first 3 volumes of each run were excluded to account for nuclear magnetic resonance and eddy-current equilibrium.
Functional data preprocessing, individual subject, and group-level fMRI analyses were conducted using Analysis of Functional NeuroImages (AFNI).46 The data were slice-timing and motion corrected, volume registered, spatially smoothed to have a Gaussian blur of 8 mm and aligned to a common Talairach-Tournoux template. First-level modeling used multiple linear regression to model our stimuli of interest with the functional time series to yield least squares estimates of the linear regression coefficients and the T-statistics for significance of the coefficients using 3dDeconvolve within AFNI. A model hemodynamic response function and its temporal derivative modeled the onset of directions displayed prior to each block, the duration of scene presentation for correct and incorrect EPT and control trials separately, the trials in which a response was not made, and correct and incorrect responses. Timepoints with excessive motion (>1 mm) were not analyzed and no subject had >25% of their timepoints discarded. Groups did not differ with respect to the number of timepoints censored (T = 0.3, df = 52, P > .10).
Demographic and Behavioral Analyses
Group differences on demographic variables as well as measures of self-reported empathy, task accuracy and response time, and social functioning were evaluated with ANOVA for continuous variables and chi-square (χ 2) tests for categorical variables.
Group Analyses Using Functional Imaging
Mixed-effects meta-analysis, which uses group-level variation and a precision estimate of the effect of interest from individual participants,47 was used in the whole-brain analysis of the event-related paradigm to examine the main effect of group for the activation throughout the duration of the emotional scene displayed to participants. There were not enough incorrect trials to independently model the activation during the viewed scenes with an incorrect response and thus these trials were excluded from imaging analyses. Control trial data were not included in the imaging analyses due to the emotional nature of the control trial scenes. This approach is consistent with prior work using this same paradigm.21
The differential blood-oxygen-level-dependent (BOLD) response during correct EPT scenes was evaluated between schizophrenia subjects and control subjects via a whole-brain analysis. In prior studies, the differentiation between correctly and incorrectly performed trials is often not made which can complicate interpretation of differences in regional activation between groups who differ in performance. By analyzing only correctly performed trials, we can identify differences in regional activation between groups when they are engaged in the same behavior (ie, accurate empathic decision making). To correct for multiple comparisons, an alpha probability simulation was used to compute the probability that a random field of noise would produce a cluster of a given size. With an individual voxel threshold of P < .001 and a minimum cluster size of ≥65 voxels, the clusters of significant difference between the 2 groups are corrected for multiple comparisons to have a corrected P value of ≤.05.
Correlation Analyses
The correct EPT trial BOLD response from each functionally defined cluster (derived from between-group differences in brain activation) in regions hypothesized to be involved in cognitive empathy were used for subsequent correlation analysesincluding the anterior insula, IFG, SMA/aMCC, mPFC, right TPJ, and precuneus. We examined whether the mean activation across each cluster was associated with measures of empathy and social functioning using 2-tailed Pearson partial correlations that covaried for the number of censored timepoints and task response time. We applied a false discovery rate to adjust for multiple comparisons in these correlation analyses at a value of 0.05.48
Exploratory Analyses
We generated exploratory 2-tailed Pearson partial correlations (uncorrected with censored timepoints and task reaction time as covariates) between the clusters of brain activation in non-hypothesized neural regions and measures of empathy, social functioning, clinical symptoms, duration of illness, and medication use.
Results
Demographic, Empathy, and Social Functioning
Means and SDs using ANOVA and χ 2 analyses for demographic and clinical data can be found in table 1. Tests of between-group effects revealed schizophrenia subjects had significantly lower accuracy (F = 6.2, df = 1,52, P < .05) and slower response times (F = 3.5, df = 1,52, P = .07) at the trend level as compared with control subjects on EPT trials. Schizophrenia subjects did not differ from control subjects with respect to accuracy (F = 2.2, df = 1,52, P > .10) and response time (F = 2.8, df = 1,52, P > .10) on the control trials. Schizophrenia subjects scored lower than controls on measures of social competence (F = 18.8, df = 1,43, P < .001), social attainment (F = 17.2, df = 1,52, P < .001), IRI perspective taking (F = 9.3, df = 1,52, P < .01) and higher on the IRI personal distress (F = 17.3, df = 1,52, P < .001). Groups did not differ on measures of IRI fantasy and empathic concern. See table 2 for means and SDs for empathy and social functioning data.
Table 2.
Mean (SD) of Empathy and Social Functioning Measures
| Between-Group Differences | ||||||
|---|---|---|---|---|---|---|
| Control Subjects (n = 24) | Schizophrenia Subjects (n = 30) | F Statistic | P Value | |||
| Mean | SD | Mean | SD | |||
| Empathy measures | ||||||
| Interpersonal reactivity index | ||||||
| Fantasy | 14.54 | 6.65 | 14.46 | 6.24 | <0.01 | .97 |
| Perspective taking | 20.58 | 4.65 | 16.67 | 4.72 | 9.29 | .004 |
| Empathic concern | 20.33 | 4.66 | 18.83 | 5.09 | 1.25 | .27 |
| Personal distress | 8.33 | 4.20 | 13.43 | 4.69 | 17.30 | <.001 |
| Total score | 63.79 | 11.99 | 63.40 | 13.96 | <0.01 | .91 |
| EPT trial accuracy | 0.92 | 0.09 | 0.84 | 0.13 | 6.52 | .01 |
| EPT reaction time, correct trials (s) | 1.34 | 0.36 | 1.52 | 0.36 | 3.47 | .07 |
| Control trial accuracy | 0.91 | 0.10 | 0.85 | 0.16 | 2.20 | .14 |
| Control trial reaction time, correct trials (s) | 1.04 | 0.36 | 1.26 | 0.55 | 2.76 | .10 |
| Social functioning | ||||||
| Social competencea | 4.36 | 0.61 | 3.23 | 0.84 | 24.29 | <.001 |
| Social attainment | 141.79 | 13.17 | 127.20 | 12.62 | 17.15 | <.001 |
Note: EPT, emotional perspective-taking.
aCompleted by n = 18 control subjects and n = 25 schizophrenia subjects.
Between-Group Differential Activation
Between-group analyses determined that schizophrenia subjects hypoactivated a network of brain regions that may be involved with cognitive empathy as compared with control subjects. Clusters of peak activation difference included bilateral IFG, right SMA (extending to the aMCC), the right TPJ (including the superior temporal sulcus), and the right precuneal sulcus. We did not find between-group differences in activation of the anterior insula, while schizophrenia subjects deactivated the left mPFC and right visual posterior region of the precuneus to a greater extent than controls.
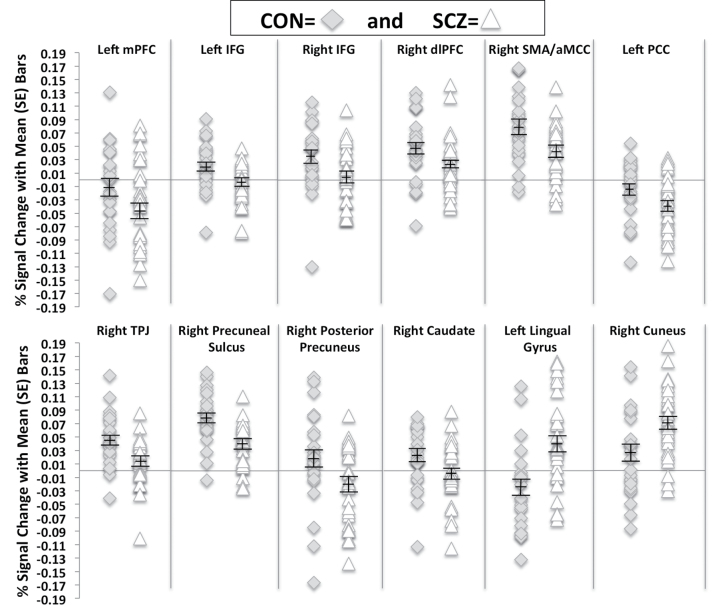
In addition to the hypothesized regions involved in cognitive empathy, we found that schizophrenia subjects hypoactivated the dorsolateral PFC (dlPFC) and caudate nucleus as well as hyperactivated the lingual gyrus and cuneus as compared with control subjects. Lastly, we found that schizophrenia subjects deactivated the left posterior cingulate cortex (PCC) during accurate EPT trial performance to a greater extent than control subjects. The percent signal change for all clusters (by group) are presented in figure 1, while Montreal Neurological Institute coordinates for all peak activations can be found in table 3.
Fig. 1.
Percent signal change during accurate emotional perspective-taking (EPT) trials within schizophrenia subjects and control subjects. Plots include mean and SE bars. Medial prefrontal cortex (mPFC); inferior frontal gyrus (IFG); dorsolateral PFC (dlPFC); supplementary motor area (SMA); anterior midcingulate cortex (aMCC); posterior cingulate cortex (PCC); temporoparietal junction (TPJ).
Table 3.
Peak % Change in BOLD Activation in Study Participants
| Region With Peak Activation | Hem | “BA” | Peak Coordinates | Peak T Value | Cluster Size | ||
|---|---|---|---|---|---|---|---|
| L/R | A/P | S/I | |||||
| Control subjects > schizophrenia subjects | |||||||
| Frontal regions | |||||||
| mPFC | L | 9 | −0 | 54 | 32 | 4.82 | 96 |
| IFG | L | 11,47 | −35 | 35 | −8 | 6.17 | 176 |
| R | 47 | 52 | 32 | −2 | 7.35 | 165 | |
| dlPFC | R | 9 | 38 | 24 | 30 | 6.84 | 88 |
| SMA (including aMCC) | R | 6,32 | 2 | 6 | 52 | 7.56 | 253 |
| PCC | L | −0 | −36 | 48 | 5.88 | 104 | |
| Temporoparietal regions | |||||||
| TPJ (including STS) | R | 22,40 | 68 | −34 | 28 | 8.53 | 531 |
| Parietooccipital regions | |||||||
| Precuneal sulcus | R | 7 | 18 | −64 | 32 | 6.56 | 300 |
| Posterior precuneus | R | 2 | −64 | 30 | 7.63 | 90 | |
| Subcortical region | |||||||
| Caudate mucleus | R | 8 | 10 | 2 | 5.37 | 82 | |
| Schizophrenia subjects > control subjects | |||||||
| Occipital regions | |||||||
| Lingual gyrus | L | −0 | −72 | −2 | −6.36 | 133 | |
| Cuneus | R | 17 | 10 | −96 | 4 | −7.54 | 128 |
Note: Blood oxygenation level dependent (BOLD), hemisphere (Hem), left (L), right (R), Brodmann’s area (BA), medial prefrontal cortex (mPFC), inferior frontal gyrus (IFG), dorsolateral PFC (dlPFC), supplementary motor area (SMA), anterior midcingulate cortex (aMCC), posterior cingulate cortex (PCC), temporoparietal junction (TPJ), superior temporal sulcus (STS).
Brain-Behavior Correlations
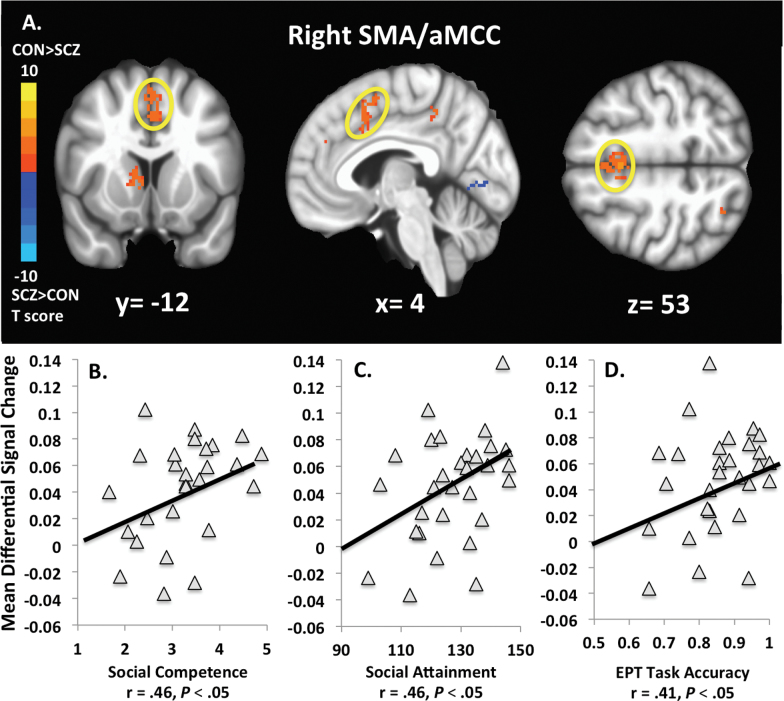
In schizophrenia subjects, the cluster with peak activation in the right SMA (extending to the aMCC) was correlated with social competence (r = .46, P < .05), social attainment (r = .46, P < .05), and accuracy during EPT performance (r = .41, P < .05) (figure 2). The right visual posterior region of the precuneus negatively correlated with self-reported cognitive empathy (r = −.45, P < .05), and the right precuneal sulcus correlated with social attainment (r = .35, P = .07) and IRI fantasy (r = .36, P = .06) at the trend level. In control subjects, we found that the right visual posterior region of the precuneus was negatively correlated with IRI personal distress (r = −.49, P < .05). The remaining pairwise correlations between the regions hypothesized to be involved with cognitive empathy (ie, IFG, mPFC, SMA/aMCC, right TPJ, and precuneus) and all study measures completed by schizophrenia and control subjects did not attain significance.
Fig. 2.
Activation maps displaying the blood-oxygen-level-dependent (BOLD) change within the right SMA/aMCC cluster during accurate emotional perspective-taking (EPT) trials between schizophrenia (SCZ) subjects and control (CON) subjects (A). Right SMA/aMCC activation was greater in CON as compared with SCZ (T = 2.58, df = 29, P < .05). CON > SCZ activation is reflected by warmer colors (T > 0), while SCZ > CON is reflected by cooler colors (T < 0). Activation in this region in SCZ was correlated with higher levels of social competence (B), social attainment (C), and EPT task accuracy (D).
Exploratory Correlations
The following exploratory correlations were found to be significant for schizophrenia subjects: right cuneus with social competence (r = −.40, P < .05), left lingual gyrus with the chlorpromazine equivalent at the trend level (r = .37, P = .054). No other correlations between brain activation and the remaining study measures were significant for either group (including clinical symptoms and duration of illness for schizophrenia subjects). Among the empathy and social functioning measures completed by the schizophrenia subjects, we found that EPT trial accuracy correlated with self-reported cognitive empathy (r = .40, P < .05), social competence (r = .47, P < .05), and social attainment (r = .35, P = .07) at the trend level, while self-reported cognitive empathy correlated with social attainment (r = .59, P < .001) and social competence at the trend level (r = .33, P = .09). Social competence and social attainment were not correlated (r = .26, P = .18).
Discussion
We examined whether brain activation associated with accurate performance during a cognitive empathy task differed between schizophrenia subjects and control subjects and whether empathy-related brain activation was correlated with measures of empathy and social functioning. Our results suggest that schizophrenia subjects, as compared with controls, were characterized by hypoactivation in a network of brain regions known to support empathic processing as well as hyperactivation in occipital regions during accurate performance on a cognitive empathy task. Among schizophrenia subjects, task activation in the right SMA extending to the aMCC was correlated with measures of social competence and social attainment. These findings extend our previous work demonstrating that schizophrenia subjects have deficits in self-reported and performance-based cognitive empathy that were associated with social functioning.28,29
Empathy and Social Functioning
Schizophrenia subjects demonstrated reduced accuracy on the cognitive empathy task performed in the scanner, which is consistent with prior studies using the fMRI version and full versions of this task.21,25,29 The schizophrenia subjects also demonstrated lower social competence and social attainment than control subjects, which is expected given the current participants were a subsample from a prior studying reporting on measures of social functioning.29
Neural Alterations in Cognitive Empathy
Schizophrenia subjects hypoactivated several regions (ie, bilateral IFG, right TPJ, and right precuneal sulcus) involved in cognitive empathy during accurate task performance. Specifically, we found bilateral hypoactivation in Brodmann’s area (BA) 47 within the IFG of schizophrenia subjects and a recent meta-analysis supports the involvement of BA 47 in empathy.49 IFG activation has also been observed during affective mentalizing and the processing of emotions,49–51 while the right TPJ has been implicated in distinguishing one’s own emotional state from the feelings and perspectives of others.52 Research suggests the precuneus is associated with self-reflection, perspective-taking, and the default mode network (DMN).53 Overall, the observed hypoactivation in this region was consistent with recent studies on the neural correlates of cognitive empathy in schizophrenia.21,22,31
Our results showed some divergence from prior studies of cognitive empathy. First, schizophrenia subjects in the present study were characterized by hyperactivation of the lingual gyrus and cuneus, while prior research did not observe empathy-related hyperactivation in any region.21 Although these findings were unexpected, a recent study of adolescents with traumatic brain injuries to the IFG and other prefrontal regions found that participants hyperactivated both the lingual gyrus and cuneus during perspective-taking task performance.54 Thus, our observation of hyperactivation in these regions could suggest a potential compensatory mechanism for accurate task completion. This interpretation would be consistent with prior studies implicating activity in the cuneus as a compensatory region associated with emotional processing.55 However, cuneus hyperactivation in schizophrenia subjects was associated with poorer social competence, which suggests this additional activation could be interfering with social functioning. The observed hyperactivation in this study compared with prior research could be explained by greater statistical power given the much larger sample size in this study compared with prior studies.21–23
We found that schizophrenia subjects deactivated the mPFC, PCC, and right visual posterior regions of the precuneus (extending into PCC) during task performance. Due to the role of these regions in the DMN,56–58 this pattern of activation could potentially be explained as the schizophrenia subjects needing to “turn off” the DMN to a greater extent in order to engage in task performance for which they still had poorer accuracy than controls.59,60 Also, we observed that schizophrenia subjects were characterized by hypoactivation in the dlPFC and caudate nucleus, which are regions involved in working memory61 and typically hypoactive during working memory performance in this population.62,63 Prior research suggests that working memory performance is correlated with accuracy on a lab-based version of the EPT task.29 Thus, the observed hypoactivation in these regions suggests that schizophrenia subjects had difficulty engaging this working memory circuitry to complete the task (eg, maintain contents of stimulus, retrieve and maintain prior memories to help interpret the stimulus).
Correlations With Neural Alterations
In addition to being characterized by hypoactivation in schizophrenia subjects, the cluster with peak activation in the SMA extending to the aMCC was significantly correlated with social competence, social attainment, and task accuracy in this group. A recent meta-analysis suggests that the SMA and aMCC are core regions of an empathy neural network.5 Furthermore, the cytoarchitecture and primate literature suggest the aMCC extends to the SMA and that the SMA may be vulnerable to impairments in aMCC function.64,65 Specifically, the aMCC has been implicated in emotion and pain discrimination, empathy for pain, and the preparation of behavioral responses to stressful events.7,66–68 Similarly, the SMA has been implicated in empathic responding, the perception and mental imagery of action, and mobilizing behavioral responses to aversive stimuli.5,7,69–73 These processes might be involved when someone cognitively empathizes with emotional scenes referenced with action-based content and images of full-body social interactions as presented by the stimuli in this study.
The observed correlations involving the SMA/aMCC cluster are consistent with prior studies of schizophrenia suggesting that higher-level social cognitive brain activation correlates with measures of empathy and social functioning.13,33 Also, our results contrast a recent finding that brain activation during performance on a theory-of-mind task was negatively associated with social functioning in schizophrenia subjects.32 The emphasis on nonemotional mental states in the theory-of-mind task as opposed to EPT could possibly explain this divergence in the findings.
We found a significant negative correlation between self-reported cognitive empathy and activation in the right visual posterior region of the precuneus. Given the potential involvement of this region in the DMN,58 this relationship could suggest that schizophrenia subjects who have difficulty deactivating the DMN may have greater impairments in cognitive empathy. However, future research using resting-state data would need to examine this relationship more carefully. Also, we did not observe any significant correlations between empathy-related brain activation and self-reported cognitive empathy. Given that the correlation between self-reported cognitive empathy and the SMA/aMCC cluster trended toward significance (r = .31, P = .11), it is possible that we did not have the statistical power to observe this particular effect. We did not observe any significant correlations between brain activation and social functioning measures in control subjects. These non-findings could be explained by a lack of variability in the control subjects’ social functioning measures, which were designed to capture deficits related to schizophrenia and were typically at ceiling in the controls.
Study Implications
Our results suggest that cognitive empathy could be a critical treatment target for improving social functioning in schizophrenia subjects, which is an important consideration for the field as interventions targeting social cognition continue to be developed.74–76 Based on the observed brain-behavior correlations in the present study, clinical interventions that increase SMA/aMCC activation or increase accurate cognitive empathy may help facilitate improved social functioning for schizophrenia subjects living in the community. Future research could examine the association between treatments targeting empathic behavior and empathy-related brain activation. For instance, recent work by Davis et al30 suggests that treatment with oxytocin is related to improved performance on the same cognitive empathy task used in the current study. Thus, additional research is needed to understand the neural mechanisms contributing to this change.
Our findings also suggest that SMA/aMCC activation could potentially be used as biomarkers to monitor the success of treatment. For example, the observed SMA/aMCC activation during an empathic task could be used as neurofeedback to improve empathic abilities or to monitor the effectiveness of cognitive behavioral therapy. There is evidence that treatment with repetitive transcranial magnetic stimulation can increase SMA and aMCC activity.77 Alternatively, interventions aimed at improving social cognition could also be examined in this context, as some of the resultant benefits could generalize to empathic behavior.78–80
Limitations
Although our findings provide insight into the relationship between cognitive empathy brain activation and social functioning, they must be interpreted in the context of some limitations. First, the results may not generalize to individuals during the prodromal or first-episode stages of schizophrenia given an illness duration of more than 10 years in our sample. Second, recent studies suggest that lower-level auditory processing deficits (ie, cue and pitch detection) may have an important role in higher-level social cognitive tasks assessing prosody and sarcasm.81–83 Similarly, mentalizing processes rely on intact lower-level visual processing.84,85 The current study did not account for potential lower-level visual processing contributions to cognitive empathy, and future research might consider evaluating this relationship in the context of social functioning. Third, there was limited specificity regarding the relationship between empathy and social functioning given that we did not examine the neural correlates of affective empathy and their association with social competence and social attainment. Future research examining the neural correlates of these processes might further inform our understanding of how empathy is associated with social functioning.
Conclusions
Schizophrenia subjects were characterized by hypoactivation in neural regions known to support empathic behavior, which were associated with poor social functioning. Thus, cognitive empathy is a potential treatment target that may be contributing to everyday functioning. As such, measures of SMA/aMCC activation could be used as biomarkers to help guide future research to improve treatment and outcome. Altogether, these findings suggest that the poor everyday functioning observed for decades in individuals with schizophrenia could be influenced by alterations in the neurobiological substrates for empathy.
Supplementary Material
Supplementary material is available at http://schizophre niabulletin.oxfordjournals.org.
Funding
This study was primarily funded by the Department of Psychiatry and Behavioral Sciences at Northwestern University Feinberg School of Medicine. Support for this work was also provided by grants to Dr J.G.C. (R01 MH056584) and grants to Dr H.C.B. (DA014118, DA026002, DA026104, DA027804) from the National Institute on Drug Abuse, and grants (DABK39-03-0098, DABK39-03-C-0098) from the Office of National Drug Control Policy, Washington, DC; Warren Wright Adolescent Center at Northwestern Memorial Hospital’s Stone Institute of Psychiatry.
Supplementary Material
Acknowledgments
The authors would like to acknowledge the research staff at the Northwestern University Schizophrenia Research Group for study coordination and data collection, and all participants for volunteering their time to be in this study. We would also like to acknowledge Dr Philip D. Harvey, PhD, from the Miller School of Medicine at the University of Miami for recommending the social functioning measures and Dr Eva-Maria Seidel, PhD, from the University of Vienna, Austria, for her assistance with task preparation. Lastly, we would like to thank University of Michigan’s Training Course in fMRI (PI Jonides: R25 MH071279).
This work was presented during a poster symposium at the 2013 International Congress on Schizophrenia Research, Orlando, FL, April 20th–24th. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Batson CD. These things called empathy: eight related but distinct phenomena. In: Decety J, Ickes W, eds. The Social Neuroscience of Empathy. Cambridge, MA: MIT Press; 2009:4–15. [Google Scholar]
- 2. Zaki J, Ochsner K. Reintegrating the study of accuracy into social cognition research. Psychol Inq. 2011;22:159–182. [Google Scholar]
- 3. Shamay-Tsoory SG. The neural bases for empathy. Neuroscientist. 2011;17:18–24. [DOI] [PubMed] [Google Scholar]
- 4. Decety J. Dissecting the neural mechanisms mediating empathy. Emotion Review. 2011;3:92–108. [Google Scholar]
- 5. Fan Y, Duncan NW, de Greck M, Northoff G. Is there a core neural network in empathy? An fMRI based quantitative meta-analysis. Neurosci Biobehav Rev. 2011;35:903–911. [DOI] [PubMed] [Google Scholar]
- 6. Gonzalez-Liencres C, Shamay-Tsoory SG, Brüne M. Towards a neuroscience of empathy: ontogeny, phylogeny, brain mechanisms, context and psychopathology. Neurosci Biobehav Rev. 2013;37:1537–1548. [DOI] [PubMed] [Google Scholar]
- 7. Lamm C, Batson CD, Decety J. The neural substrate of human empathy: effects of perspective-taking and cognitive appraisal. J Cogn Neurosci. 2007;19:42–58. [DOI] [PubMed] [Google Scholar]
- 8. Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 2009;13:334–340. [DOI] [PubMed] [Google Scholar]
- 9. Meyer ML, Masten CL, Ma Y, et al. Empathy for the social suffering of friends and strangers recruits distinct patterns of brain activation. Soc Cogn Affect Neurosci. 2013;8:446–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rameson LT, Morelli SA, Lieberman MD. The neural correlates of empathy: experience, automaticity, and prosocial behavior. J Cogn Neurosci. 2012;24:235–245. [DOI] [PubMed] [Google Scholar]
- 11. Schnell K, Bluschke S, Konradt B, Walter H. Functional relations of empathy and mentalizing: an fMRI study on the neural basis of cognitive empathy. Neuroimage. 2011;54:1743–1754. [DOI] [PubMed] [Google Scholar]
- 12. Völlm BA, Taylor AN, Richardson P, et al. Neuronal correlates of theory of mind and empathy: a functional magnetic resonance imaging study in a nonverbal task. Neuroimage. 2006;29:90–98. [DOI] [PubMed] [Google Scholar]
- 13. Hooker CI, Verosky SC, Germine LT, Knight RT, D’Esposito M. Mentalizing about emotion and its relationship to empathy. Soc Cogn Affect Neurosci. 2008;3:204–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schulte-Rüther M, Markowitsch HJ, Fink GR, Piefke M. Mirror neuron and theory of mind mechanisms involved in face-to-face interactions: a functional magnetic resonance imaging approach to empathy. J Cogn Neurosci. 2007;19:1354–1372. [DOI] [PubMed] [Google Scholar]
- 15. Farrow TF, Zheng Y, Wilkinson ID, et al. Investigating the functional anatomy of empathy and forgiveness. Neuroreport. 2001;12:2433–2438. [DOI] [PubMed] [Google Scholar]
- 16. Nummenmaa L, Hirvonen J, Parkkola R, Hietanen JK. Is emotional contagion special? An fMRI study on neural systems for affective and cognitive empathy. Neuroimage. 2008;43:571–580. [DOI] [PubMed] [Google Scholar]
- 17. Frith CD, Frith U. Interacting minds–a biological basis. Science. 1999;286:1692–1695. [DOI] [PubMed] [Google Scholar]
- 18. Saxe R. Uniquely human social cognition. Curr Opin Neurobiol. 2006;16:235–239. [DOI] [PubMed] [Google Scholar]
- 19. Saxe R, Kanwisher N. People thinking about thinking people. The role of the temporo-parietal junction in “theory of mind”. Neuroimage. 2003;19:1835–1842. [DOI] [PubMed] [Google Scholar]
- 20. Ruby P, Decety J. How would you feel versus how do you think she would feel? A neuroimaging study of perspective-taking with social emotions. J Cogn Neurosci. 2004;16:988–999. [DOI] [PubMed] [Google Scholar]
- 21. Derntl B, Finkelmeyer A, Voss B, et al. Neural correlates of the core facets of empathy in schizophrenia. Schizophr Res. 2012;136:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee SJ, Kang do H, Kim CW, et al. Multi-level comparison of empathy in schizophrenia: an fMRI study of a cartoon task. Psychiatry Res. 2010;181:121–129. [DOI] [PubMed] [Google Scholar]
- 23. Harvey PO, Zaki J, Lee J, Ochsner K, Green MF. Neural substrates of empathic accuracy in people with schizophrenia. Schizophr Bull. 2013;39:617–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lee J, Zaki J, Harvey PO, Ochsner K, Green MF. Schizophrenia patients are impaired in empathic accuracy. Psychol Med. 2011:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Derntl B, Finkelmeyer A, Toygar TK, et al. Generalized deficit in all core components of empathy in schizophrenia. Schizophr Res. 2009;108:197–206. [DOI] [PubMed] [Google Scholar]
- 26. Achim AM, Ouellet R, Roy MA, Jackson PL. Assessment of empathy in first-episode psychosis and meta-analytic comparison with previous studies in schizophrenia. Psychiatry Res. 2011;190:3–8. [DOI] [PubMed] [Google Scholar]
- 27. Langdon R, Coltheart M, Ward PB. Empathetic perspective-taking is impaired in schizophrenia: evidence from a study of emotion attribution and theory of mind. Cogn Neuropsychiatry. 2006;11:133–155. [DOI] [PubMed] [Google Scholar]
- 28. Smith MJ, Horan WP, Karpouzian TM, Abram SV, Cobia DJ, Csernansky JG. Self-reported empathy deficits are uniquely associated with poor functioning in schizophrenia. Schizophr Res. 2012;137:196–202. [DOI] [PubMed] [Google Scholar]
- 29. Smith MJ, Horan WP, Cobia DJ, et al. Performance-based empathy mediates the influence of working memory on social competence in schizophrenia. Schizophr Bull. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Davis MC, Lee J, Horan WP, et al. Effects of single dose intranasal oxytocin on social cognition in schizophrenia. Schizophr Res. 2013;147:393–397. [DOI] [PubMed] [Google Scholar]
- 31. Benedetti F, Bernasconi A, Bosia M, et al. Functional and structural brain correlates of theory of mind and empathy deficits in schizophrenia. Schizophr Res. 2009;114:154–160. [DOI] [PubMed] [Google Scholar]
- 32. Das P, Lagopoulos J, Coulston CM, Henderson AF, Malhi GS. Mentalizing impairment in schizophrenia: a functional MRI study. Schizophr Res. 2012;134:158–164. [DOI] [PubMed] [Google Scholar]
- 33. Pinkham AE, Hopfinger JB, Ruparel K, Penn DL. An investigation of the relationship between activation of a social cognitive neural network and social functioning. Schizophr Bull. 2008;34:688–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barratt W. The Barratt Simplified Measure of Social Status (BSMSS): measuring SES. Indiana State University, Terre Haute, IN. 2005.
- 35. First MB, Spitzer RL, Miriam G, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient Edition. New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 36. Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Andreasen NC. The Scale for the Assessment of Positive Symptoms. Iowa City, IA: The University of Iowa; 1983. [Google Scholar]
- 38. Andreasen NC. The Scale for the Assessment of Negative Symptoms. Iowa City, IA: The University of Iowa; 1983. [Google Scholar]
- 39. Davis MH. Measuring individual differences in empathy: evidence for a multidimensional approach. J Pers Soc Psychol. 1983;44:113–126. [Google Scholar]
- 40. Harvey PD, Raykov T, Twamley EW, Vella L, Heaton RK, Patterson TL. Validating the measurement of real-world functional outcomes: phase I results of the VALERO study. Am J Psychiatry. 2011;168:1195–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Harvey PD, Velligan DI, Bellack AS. Performance-based measures of functional skills: usefulness in clinical treatment studies. Schizophr Bull. 2007;33:1138–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Patterson TL, Moscona S, McKibbin CL, Davidson K, Jeste DV. Social skills performance assessment among older patients with schizophrenia. Schizophr Res. 2001;48:351–360. [DOI] [PubMed] [Google Scholar]
- 43. Schneider LC, Struening EL. SLOF: a behavioral rating scale for assessing the mentally ill. Soc Work Res Abstr. 1983;19:9–21. [DOI] [PubMed] [Google Scholar]
- 44. Derntl B, Finkelmeyer A, Eickhoff S, et al. Multidimensional assessment of empathic abilities: neural correlates and gender differences. Psychoneuroendocrinology. 2010;35:67–82. [DOI] [PubMed] [Google Scholar]
- 45. Gur RC, Sara R, Hagendoorn M, et al. A method for obtaining 3-dimensional facial expressions and its standardization for use in neurocognitive studies. J Neurosci Methods. 2002;115:137–143. [DOI] [PubMed] [Google Scholar]
- 46. Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–173. [DOI] [PubMed] [Google Scholar]
- 47. Chen G, Saad ZS, Nath AR, Beauchamp MS, Cox RW. FMRI group analysis combining effect estimates and their variances. Neuroimage. 2012;60:747–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Benjamini Y, Drai D, Elmer G, Kafkafi N, Golani I. Controlling the false discovery rate in behavior genetics research. Behav Brain Res. 2001;125:279–284. [DOI] [PubMed] [Google Scholar]
- 49. Liakakis G, Nickel J, Seitz RJ. Diversity of the inferior frontal gyrus–a meta-analysis of neuroimaging studies. Behav Brain Res. 2011;225:341–347. [DOI] [PubMed] [Google Scholar]
- 50. Rizzolatti G, Fabbri-Destro M, Cattaneo L. Mirror neurons and their clinical relevance. Nat Clin Pract Neurol. 2009;5:24–34. [DOI] [PubMed] [Google Scholar]
- 51. Jabbi M, Keysers C. Inferior frontal gyrus activity triggers anterior insula response to emotional facial expressions. Emotion. 2008;8:775–780. [DOI] [PubMed] [Google Scholar]
- 52. Decety J, Lamm C. The role of the right temporoparietal junction in social interaction: how low-level computational processes contribute to meta-cognition. Neuroscientist. 2007;13:580–593. [DOI] [PubMed] [Google Scholar]
- 53. Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. [DOI] [PubMed] [Google Scholar]
- 54. Newsome MR, Scheibel RS, Hanten G, et al. Brain activation while thinking about the self from another person’s perspective after traumatic brain injury in adolescents. Neuropsychology. 2010;24:139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Delvecchio G, Sugranyes G, Frangou S. Evidence of diagnostic specificity in the neural correlates of facial affect processing in bipolar disorder and schizophrenia: a meta-analysis of functional imaging studies. Psychol Med. 2012:1–17. [DOI] [PubMed] [Google Scholar]
- 56. Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Buckner RL, Andrews-Hanna JR, Schacter DL. The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci. 2008;1124:1–38. [DOI] [PubMed] [Google Scholar]
- 58. Fransson P, Marrelec G. The precuneus/posterior cingulate cortex plays a pivotal role in the default mode network: evidence from a partial correlation network analysis. Neuroimage. 2008;42:1178–1184. [DOI] [PubMed] [Google Scholar]
- 59. Mayer JS, Roebroeck A, Maurer K, Linden DE. Specialization in the default mode: task-induced brain deactivations dissociate between visual working memory and attention. Hum Brain Mapp. 2010;31:126–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Singh KD, Fawcett IP. Transient and linearly graded deactivation of the human default-mode network by a visual detection task. Neuroimage. 2008;41:100–112. [DOI] [PubMed] [Google Scholar]
- 61. Schroll H, Vitay J, Hamker FH. Working memory and response selection: a computational account of interactions among cortico-basalganglio-thalamic loops. Neural Netw. 2012;26:59–74. [DOI] [PubMed] [Google Scholar]
- 62. Anticevic A, Repovs G, Barch DM. Working memory encoding and maintenance deficits in schizophrenia: neural evidence for activation and deactivation abnormalities. Schizophr Bull. 2013;39:168–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Deserno L, Sterzer P, Wüstenberg T, Heinz A, Schlagenhauf F. Reduced prefrontal-parietal effective connectivity and working memory deficits in schizophrenia. J Neurosci. 2012;32:12–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Vogt BA, Vogt L. Cytology of human dorsal midcingulate and supplementary motor cortices. J Chem Neuroanat. 2003;26:301–309. [DOI] [PubMed] [Google Scholar]
- 65. Morecraft RJ, Van Hoesen GW. Cingulate input to the primary and supplementary motor cortices in the rhesus monkey: evidence for somatotopy in areas 24c and 23c. J Comp Neurol. 1992;322:471–489. [DOI] [PubMed] [Google Scholar]
- 66. Torta DM, Cauda F. Different functions in the cingulate cortex, a meta-analytic connectivity modeling study. Neuroimage. 2011;56:2157–2172. [DOI] [PubMed] [Google Scholar]
- 67. Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005;6:533–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bruneau EG, Pluta A, Saxe R. Distinct roles of the ‘shared pain’ and ‘theory of mind’ networks in processing others’ emotional suffering. Neuropsychologia. 2012;50:219–231. [DOI] [PubMed] [Google Scholar]
- 69. Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mukamel R, Ekstrom AD, Kaplan J, Iacoboni M, Fried I. Single-neuron responses in humans during execution and observation of actions. Curr Biol. 2010;20:750–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Keysers C, Gazzola V. Expanding the mirror: vicarious activity for actions, emotions, and sensations. Curr Opin Neurobiol. 2009;19:666–671. [DOI] [PubMed] [Google Scholar]
- 72. Jabbi M, Bastiaansen J, Keysers C. A common anterior insula representation of disgust observation, experience and imagination shows divergent functional connectivity pathways. PLoS One. 2008;3:e2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shamay-Tsoory SG, Lester H, Chisin R, et al. The neural correlates of understanding the other’s distress: a positron emission tomography investigation of accurate empathy. Neuroimage. 2005;27:468–472. [DOI] [PubMed] [Google Scholar]
- 74. Pinkham AE, Harvey PD. Future directions for social cognitive interventions in schizophrenia. Schizophr Bull. 2013;39:499–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fiszdon JM, Reddy LF. Review of social cognitive treatments for psychosis. Clin Psychol Rev. 2012;32:724–740. [DOI] [PubMed] [Google Scholar]
- 76. Kurtz MM, Richardson CL. Social cognitive training for schizophrenia: a meta-analytic investigation of controlled research. Schizophr Bull. 2012;38:1092–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. van den Wildenberg WP, Burle B, Vidal F, van der Molen MW, Ridderinkhof KR, Hasbroucq T. Mechanisms and dynamics of cortical motor inhibition in the stop-signal paradigm: a TMS study. J Cogn Neurosci. 2010;22:225–239. [DOI] [PubMed] [Google Scholar]
- 78. Horan WP, Kern RS, Tripp C, et al. Efficacy and specificity of social cognitive skills training for outpatients with psychotic disorders. J Psychiatr Res. 2011;45:1113–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wölwer W, Frommann N. Social-cognitive remediation in schizophrenia: generalization of effects of the Training of Affect Recognition (TAR). Schizophr Bull. 2011;37(suppl 2):S63–S70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Roberts DL, Velligan DI. Can social functioning in schizophrenia be improved through targeted social cognitive intervention? Rehabil Res Pract. 2012;2012:742106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Leitman DI, Laukka P, Juslin PN, Saccente E, Butler P, Javitt DC. Getting the cue: sensory contributions to auditory emotion recognition impairments in schizophrenia. Schizophr Bull. 2010;36:545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gold R, Butler P, Revheim N, et al. Auditory emotion recognition impairments in schizophrenia: relationship to acoustic features and cognition. Am J Psychiatry. 2012;169:424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Kantrowitz JT, Hoptman MJ, Leitman DI, Silipo G, Javitt DC. The 5% difference: early sensory processing predicts sarcasm perception in schizophrenia and schizo-affective disorder. Psychol Med. 2013:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Brunet E, Sarfati Y, Hardy-Baylé MC, Decety J. Abnormalities of brain function during a nonverbal theory of mind task in schizophrenia. Neuropsychologia. 2003;41:1574–1582. [DOI] [PubMed] [Google Scholar]
- 85. Pelphrey KA, Morris JP, McCarthy G. Grasping the intentions of others: the perceived intentionality of an action influences activity in the superior temporal sulcus during social perception. J Cogn Neurosci. 2004;16:1706–1716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.