Abstract
Bipolar illness is a debilitating neuropsychiatric disorder associated with alterations in the ventral anterior cingulate cortex (vACC), a brain region thought to regulate emotional behavior. Although recent data-driven functional connectivity studies provide evidence consistent with this possibility, the role of vACC in bipolar illness and its pattern of whole brain connectivity remain unknown. Furthermore, no study has established whether vACC exhibits differential whole brain connectivity in bipolar patients with and without co-occurring psychosis and whether this pattern resembles that found in schizophrenia. We conducted a human resting-state functional connectivity investigation focused on the vACC seed in 73 remitted bipolar I disorder patients (33 with psychosis history), 56 demographically matched healthy comparison subjects, and 73 demographically matched patients with chronic schizophrenia. Psychosis history within the bipolar disorder group corresponded with significant between-group connectivity alterations along the dorsal medial prefrontal surface when using the vACC seed. Patients with psychosis history showed reduced connectivity (Cohen’s d = −0.69), whereas those without psychosis history showed increased vACC coupling (Cohen’s d = 0.8) relative to controls. The vACC connectivity observed in chronic schizophrenia patients was not significantly different from that seen in bipolar patients with psychosis history but was significantly reduced compared with that in bipolar patients without psychosis history. These robust findings reveal complex vACC connectivity alterations in bipolar illness, which suggest differences depending on co-occurrence of lifetime psychosis. The similarities in vACC connectivity patterns in schizophrenia and psychotic bipolar disorder patients may suggest the existence of common mechanisms underlying psychotic symptoms in the two disorders.
Key words: bipolar illness, schizophrenia, connectivity, resting-state, medial prefrontal cortex
Introduction
Bipolar disorder (BD) is a severe neuropsychiatric illness that profoundly affects mood regulation1 and is a leading cause of disability worldwide.2 Complete understanding of its neurobiology can inform better treatments, aid in predicting prognosis, and promote early detection. Functional disturbances in prefrontal cortex (PFC) regions involved in affect and mood regulation3 are repeatedly implicated in BD. Specifically, the ventral anterior cingulate cortex (vACC) is an important locus of dysfunction in BD.4–6 This region, described as sub/perigenual cingulate cortex, is linked to emotional regulation in humans4,7 and in nonhuman primates.8–10 Initially, Drevets and colleagues found evidence for reduced gray matter volume and metabolism in depressed bipolar and unipolar individuals in this region. Subsequently, Mayberg and colleagues observed decreased sub/perigenual cingulate blood flow in pathological depression and normal sadness, which predicted treatment response through pharmacological11 and cognitive behavioral therapy.12 Direct stimulation of this region reduced depressive symptomatology in treatment-resistant individuals with mood disorders.13 While the exact mechanism for how vACC dysfunction leads to mood disorders is still unclear, Ongur and colleagues14 found a reduction in glia among individuals with familial BD in this region, suggesting that the observed neuroimaging anomalies may be sensitive to both clinical state and predisposition to affective dysregulation. While the vACC operates as a component of a broader system involved in mood regulation,3 network-level alterations of this region in bipolar illness remain largely uncharacterized.15 Here we explicitly focused on characterizing connectivity of this critical node involved in mood regulation in BD.
Recently, using resting-state neuroimaging, Chai and colleagues15 found increased connectivity in symptomatic individuals with BD between the vACC, insula, and ventrolateral PFC. The relatively small sample size, however, likely restricted the power to fully map whole brain vACC connectivity deficits in BD. Furthermore, no study examined the heterogeneity of this complex illness with respect to vACC connectivity. For instance, many BD patients experience frank psychosis.16 Therefore, some studies divide BD patients based on the presence or absence of psychotic symptoms.17,18 Recent results suggest that occurrence of psychosis in bipolar illness may indeed be associated with a distinct pattern of PFC dysconnectivity,19 in line with observations in schizophrenia.20 Specifically, prior studies in BD found reductions in global connectivity between vACC and the rest of PFC in a fully data-driven manner19; this effect was driven by more severe disturbances in BD patients with history of psychosis. Furthermore, an independent amygdala connectivity analysis converged on a similar vACC node that exhibited increased connectivity with the amygdala in psychotic BD.19 It remains unknown, however, if BD patients with psychosis show a unique pattern of vACC connectivity alterations relative to patients without psychosis.
Collectively, we tested the hypothesis that bipolar patients with psychosis show a distinct pattern of whole brain vACC connectivity from those without psychosis history, which may be associated with alterations in PFC circuits implicated in emotional regulation. In turn, we studied a separate large group of schizophrenia patients (N = 73), matched to BD groups. The schizophrenia group served as a vital clinical control to test the secondary hypothesis that dysconnectivity for bipolar patients with psychosis history resembles alterations in schizophrenia but is distinct from bipolar patients without psychosis history.
Materials and methods
Participants
We recruited 73 patients diagnosed with bipolar I disorder (20 males and 53 females) and a demographically matched group of 56 healthy control subjects (HCS; 24 males and 32 females). Patients were remitted at the time of the study and were divided into 2 demographically matched groups based on history of psychosis, using standardized procedures17: 33 patients reported a history of psychosis in the course of bipolar illness (BPP); 40 patients reported no lifetime history of psychosis (BPW; table 1). All patients were recruited in the Hartford, Connecticut area through outpatient clinics and community mental health services. HCS were recruited through community advertising and flyers. Signed informed consent approved by the Hartford Hospital and Yale University Institutional Review Board was obtained from all participants.
Table 1.
Demographics
| Bipolar I, Psychosis Hx | Bipolar I, No Psychosis Hx | Healthy Comparison | Schizophrenia | Significance (HC, BPP, BPW) | Significance (BPW vs BPP) | Significance (BPP vs SCZ) | Significance (BPW vs SCZ) | Significance (HC, BPP, BPW, SCZ) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M (SD) | M (SD) | M (SD) | M (SD) | F Value/ Chi Square | P Value | t Value/ Chi Square | P Value, 2-Tail | t Value/ Chi Square | P Value, 2-Tail | t Value/ Chi Square | P Value, 2-Tail | F Value/ Chi Square | P Value | |
| Sample size | 33 | 40 | 56 | 73 | — | — | — | — | — | — | — | — | — | — |
| # Female (%) | 21 (63%) | 32 (80%) | 32 (57%) | 24 (33%) | 5.52 | .06 | 2.43 | .12 | 8.80 | .00 | 22.95 | .00 | 25.45 | .00 |
| Age (M ± SD) | 34.18 (10.9) | 30.20 (11.5) | 31.25 (10.3) | 32.99 (10.9) | 1.29 | .28 | 1.51 | .14 | 0.52 | .60 | 1.27 | .21 | 1.08 | .36 |
| Education | 13.94 (1.6) | 14.45 (2.1) | 15.11 (2.1) | 13.79 (1.8) | 3.75 | .03 | 1.14 | .26 | 0.39 | .70 | 1.73 | .09 | 5.37 | .00 |
| Mother’s education | 13.67 (3.0) | 14.26 (2.3) | 13.63 (2.6) | 13.79 (3.0) | 0.77 | .47 | 0.96 | .34 | 0.20 | .84 | 0.84 | .41 | 0.45 | .72 |
| Father’s education | 14.64 (3.4) | 15.00 (3.8) | 12.98 (3.9) | 14.05 (3.6) | 3.97 | .02 | 0.43 | .67 | 0.78 | .44 | 1.30 | .20 | 2.71 | .05 |
| Mean parental education | 14.15 (2.9) | 14.63 (2.7) | 13.30 (2.9) | 13.92 (3.1) | 2.45 | .09 | 0.67 | .50 | 0.33 | .75 | 1.09 | .28 | 1.54 | .21 |
| Clinical course | ||||||||||||||
| Age at diagnosis | 18.27 (6.1) | 18.73 (6.9) | N/A | (not available) | — | — | 0.29 | .77 | — | — | — | — | — | — |
| Duration of illness | 15.91 (10.9) | 11.48 (9.1) | N/A | (not available) | — | — | 1.89 | .06 | — | — | — | — | — | — |
| Current symptomatology | ||||||||||||||
| Depression (HAMD) | 3.12 (3.1) | 4.33 (4.0) | 0.33 (0.7) | (not available) | 25.80 | .0000 | 1.41 | .16 | — | — | — | — | — | — |
| Mania (YMRS) | 2.45 (3.3) | 2.88 (3.7) | 0.15 (0.4) | (not available) | 14.55 | .0000 | 0.51 | .61 | — | — | — | — | — | — |
| Psychosis (BPRS) | 28.79 (4.4) | 27.90 (3.6) | 24.56 (1.0) | (not available) | 23.56 | .0000 | 0.95 | .35 | — | — | — | — | — | — |
| PANSS | (not available) | (not available) | (not available) | 60.22 (14.6) | — | — | — | — | — | — | — | — | — | — |
| Medications, n (%) | ||||||||||||||
| Mood stabilizer(s) | 18 (54%) | 19 (47%) | N/A | 20 (27%) | — | — | 0.36 | .55 | 7.28 | .01 | 4.62 | .03 | — | — |
| Antidepressant(s) | 11 (33%) | 20 (50%) | N/A | 25 (34%) | — | — | 2.06 | .15 | 0.01 | .93 | 2.68 | .10 | — | — |
| Atypical antipsychotic(s) | 15 (45%) | 10 (25%) | N/A | 60 (82%) | — | — | 3.36 | .07 | 14.82 | .00 | 35.86 | .00 | — | — |
| Anxiolytic/ benzodiazepine(s) | 12 (36%) | 14 (35%) | N/A | 13 (18%) | — | — | 0.01 | .90 | 4.34 | .04 | 4.20 | .04 | — | — |
| Lithium | 8 (24%) | 5 (12%) | N/A | 6 (8%) | — | — | 1.70 | .19 | 5.09 | .02 | 0.54 | .46 | — | — |
| Unmedicated | 6 (18%) | 6 (15%) | N/A | 7 (9%) | — | — | 0.13 | .72 | 1.56 | .21 | 0.74 | .39 | — | — |
| Typical antipsychotic(s) | 0 (0%) | 1 (2%) | N/A | 12 (16%) | — | — | 0.84 | .36 | 6.12 | .01 | 4.93 | .03 | — | — |
| CPZ equivalents | (not available) | (not available) | (not available) | 232.39 (204.2) | — | — | — | — | — | — | — | — | — | — |
| Data quality considerations | ||||||||||||||
| Signal-to-noise | 217.71 (51.1) | 216.04 (54.0) | 215.45 (58.9) | 212.12 (62.3) | 55.19 | .98 | 0.14 | .89 | 0.45 | .65 | 0.33 | .74 | 0.09 | .97 |
| % Frames Scrubbed | 11.74 (9.5) | 10.82 (10.1) | 9.74 (10.4) | 18.51 (16.7) | 0.42 | .66 | 0.43 | .67 | 2.65 | .01 | 3.07 | .00 | 6.06 | .00 |
Note: PANSS, Positive and Negative Syndrome Scale; BPP, bipolar patients with psychosis history; BPW, bipolar patients without psychosis history; SCZ, schizophrenia patients, BPRS, Brief Psychiatric Rating Scale; HAMD, Hamilton Depression Rating Scale; Hx, history; YMRS, Young Mania Rating Scale; CPZ, chlorpromazine (calculated as per Andreasen et al 52 ); PANSS, Positive and Negative Syndrome Scale; HC, healthy controls. Age, education levels, parental education, age at diagnosis and duration of illness are expressed in years. Of note, no pair-wise BPP-BPW comparisons reached significance. In cases where there were significant differences between clinical groups (eg, gender proportion and % frames scrubbed), we used these variables as covariates. No result was changed when covarying for variables with significant between-group effects.
All eligible patients met either bipolar I disorder or schizophrenia diagnostic criteria as determined by experienced MA-/PhD-level clinician using Structured Clinical Interview (SCID) for the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV).21 Comorbid Axis I diagnosis of anxiety disorders and/or substance abuse (remitted for at least 6 months prior to this study) were allowed in order to increase representativeness of the samples. Eligible HCS had no current or lifetime mood or psychotic Axis I disorder as assessed by SCID-NP and no history of psychotic or mood disorders in first-degree relatives. No participant had history of major medical or neurological condition and had intelligence quotient > 80 as assessed by Wechsler Abbreviated Intelligence Scale.22 HCS had higher educational attainment (P = .03). However, educational differences are influenced by the illness course23 and were thus not controlled. HCS also showed lower attained level of father’s education, which was included as a covariate. Also, including covariates for prior alcohol/drug use, anxiety, age, illness duration, or gender did not alter findings. Lastly, 73 demographically matched schizophrenia patients were selected from a sample characterized in our previous study,19 collected on the same scanner with identical parameters (49 males and 24 females). While generally well matched (table 1), schizophrenia patients differed somewhat from bipolar groups in gender proportion. When used as a covariate, however, gender did not alter reported findings.
Current Symptoms and Medication
Current symptomatology for bipolar patients was assessed using Young Mania Rating Scale (YMRS),24 21-item Hamilton Depression Scale,25 and the expanded version of the Brief Psychiatric Rating Scale.26 All participants were remitted for at least 2 weeks prior to the participation in the study, determined by standardized cutoff values on YMRS and HAM-D scales (≥7). Eighteen percent of BPW and 15% of BPP groups were unmedicated. Bipolar groups were well matched for medications and current symptomatology (see table 1). Symptom severity for schizophrenia patients was determined using the Positive and Negative Syndrome Scale.27 Nine percent of schizophrenia patients were unmedicated. Schizophrenia patients showed statistically significant differences in medications profile for mood stabilizers, atypical antipsychotics, anxiolytics, and typical antipsychotics, compared with bipolar patients, given difference in medication regiments. However, reported effects were not altered when covaried for presence/absence of a given medication class. Also, medication presence/absence across medication classes was not associated with reported findings for bipolar patients (although see “Limitations”).
Data Acquisition
All scanning was preformed at the Olin Neuropsychiatry Research Center using a Siemens-Allegra 3T scanner. Functional images sensitive to blood oxygenation level-dependent (BOLD) signal were collected with axial slices parallel to the anterior-posterior commissure (AC-PC) using a T2*-weighted gradient-echo, echo-planar sequence (repetition time [TR]/echo time [TE] = 1500/27 ms, flip angle = 60°, field of view = 24 × 24cm, acquisition matrix = 64 × 64, voxel size = 3.43 × 3.43×4 mm), ensuring whole brain coverage. Functional data collection lasted 5.25 minutes, resulting in 210 volumes (29 slices/volume, interslice gap = 1 mm). Subjects were instructed to remain awake in the scanner and keep their eyes open. A video camera was used to monitor all subjects, ensuring they stayed awake. Subjects were omitted from analyses if they fell asleep or if their head movement exceeded 1 mm along any axis. Structural images were acquired using a T1-weighted, 3D magnetization-prepared rapid gradient-echo sequence (TR/TE/TI = 2200/4.13/766 ms, flip angle = 13°, voxel size [isotropic] = 0.8 mm, image size = 240×320×208 voxels), with axial slices parallel to the AC-PC line.
Data Preprocessing and Analysis
All preprocessing followed our prior work and standard approaches in the connectivity literature.20,28 Briefly, we performed (1) slice-time correction, (2) removal of first 5 images from each run, (3) rigid body motion correction, (4) 12-parameter affine transform of the structural image to the Talairach coordinate system, and (5) coregistration of volumes to the structural image with 3 × 3 × 3 mm resampling. Furthermore, we employed the following rigorous quality assurance criteria for each participant, to ensure comparable BOLD quality: (1) signal-to-noise ratios (SNR) >100. SNR was computed by obtaining the mean signal and SD for a given slice across the BOLD run, while excluding all nonbrain voxels across all frames28; (2) no BOLD run with a single frame movement greater than 1 functional voxel as noted above; (3) all BOLDs were movement-scrubbed29,30 and subjects with more than 50% frames flagged as potentially affected by movement artifacts were completely excluded from analyses. First, frames in which sum of the displacement across all 6 rigid body movement correction parameters exceeded 0.5mm (assuming 50 mm cortical sphere radius) were identified. Second, root mean square (RMS) of differences in intensity between the current and preceding frame was computed across all voxels and divided by mean intensity. Frames in which normalized RMS exceeded the value of 3 were identified. We marked frames for exclusion that were flagged by either criterion. In addition, the 1 proceeding and 2 frames following the flagged frame were removed. Subject with more than 50% frames flagged were completely excluded from analyses. After these criteria were implemented, there were no between-group differences in SNR or proportion of removed scrubbed frames for the bipolar samples and controls (table 1). There was, however, a higher proportion of frames scrubbed for the schizophrenia sample relative to the other samples (18.51% of frames removed), suggesting that schizophrenia patients did move more on average. Because of this difference, we ensured that the proportion of the removed frames did not significantly affect any of the reported effects involving the schizophrenia sample. We specifically used the proportion of scrubbed frames as a covariate across reported analyses. Even when proportion of flagged frames was used as a covariate variable, main effects remained unaltered.
We also removed additional spurious signal in resting-state data, as is standard practice.31 Briefly, all images underwent high-pass (0.009 Hz) and low-pass (0.08 Hz) temporal filtering and subsequent removal of nuisance signal from ventricles, deep white matter, and global mean signal, 6 rigid body motion correction parameters, and their first derivatives using in-house Matlab tools.32 Critically, all nuisance regressors underwent identical filtering prior to their removal.
Seed-Based Functional Connectivity Analysis
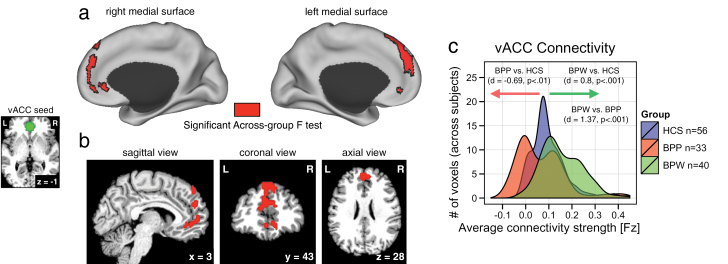
It is important to consider the heterogeneity of the vACC region and its selection, given possible large differences in final effects reflecting subtle variability in seed placement. The vACC resting-state functional connectivity MRI (rs-fcMRI) analyses replicated our prior approaches.19 The vACC seed was guided by prior studies in the following way: as noted, previously studies found 2 proximal vACC clusters showing reduced connectivity with the PFC (x = 3, y = 32, z = 1) but amygdala overconnectivity in bipolar illness (x = 1; y = 41; z = −3).19 To remain sensitive to this general locus of dysconnectivity, we selected the Euclidian midpoint across the 2 clusters and placed a 9 mm-radius sphere in this location (x = 2, y = 37, z = −1; figure 1, left panel). To obtain further specificity and avoid partial volume effects, for each subject, we defined a subset of vACC voxels via FreeSurfer segmentation33 that explicitly overlapped with the a priori defined seed.
Fig. 1.
Alterations in vACC connectivity in bipolar illness with and without psychosis history. Red foci mark regions that showed a significant 1-way between-group ANOVA displayed on the surface (a) and in a volume view (b) to allow complete visualization of the cluster extent. As evident from the map, a large portion of superior medial cortex showed a significant between-group effect in vACC functional connectivity. The small panel on the left illustrates the vACC seed location (x = 2, y = 37, z = −1), which was selected based on our prior data-driven findings19 (see Materials and Methods). (c) The distributions of average connection strengths for each voxel showing a significant between-group effect are plotted for each individual group. The X-axis refers to the strength of connectivity effect in the identified region (using Fisher’s Fz values). The Y-axis quantifies the voxel count at a given strength of connectivity across groups (as opposed to individual subjects). Effect sizes (Cohen’s d) were calculated across subjects using the average signal across the entire cluster in panel (a), verifying marked alterations in vACC seed connectivity across groups (for complete pair-wise P and t values see table 2). The effect was largely driven by a reduction in vACC connectivity for bipolar patients with psychosis history (BPP) group (red distribution), whereas the bipolar patients without psychosis history (BPW) group showed increased vACC connectivity (green distribution) relative to healthy control subjects (HCS; blue distribution). Note: The frequency histograms are shown to illustrate the underlying nature (ie, directionality) of the group effects, not to quantify the difference (which is obligated by the voxel selection).
Before any rs-fcMRI calculations, we spatially smoothed the BOLD signal with a 6 mm full-width-at-half-maximum Gaussian kernel. Subject-specific whole brain vACC maps were computed by extracting the average time series across all vACC voxels and computing a correlation with all other voxels. We computed a Fisher r-to-Z transform, yielding a Fisher-Z connectivity map for each participant where each voxel’s value represents its connectivity with the vACC. To test hypothesized between-group differences, maps were entered into a second-level 1-way ANOVA with 3 between-group levels (HCS, BPP, BPW), which was computed within FSL’s Randomise tool with 10 000 permutations.34 Whole brain type I error correction was accomplished via threshold-free-cluster-enhancement implemented in Randomise.35
Schizophrenia analyses were then computed explicitly within the regions that showed between-group F-test differences for the bipolar sample. The key rationale for this analysis was to examine whether the primary alterations found between BPP and BPW patients resembled those in schizophrenia (as opposed to a whole brain search). We took this stepwise approach (1) to remain powered within a defined search space showing maximal effects for the bipolar sample and (2) to explicitly inform our understanding of whether the BPP effects (vs BPW) are more in line with schizophrenia observations.
All formal effect size calculations were computed using standard approaches across subjects via Cohen’s d 36 by extracting the Fisher-Z value for all subjects across all voxels showing the main effect in figure 1. This was done to characterize the magnitude of between-group effects across voxels surviving the whole brain correction, as done previously.37 Findings were visualized using Caret 5.5 (http://brainvis.wustl.edu/wiki/index.php/Caret:Download) and NeuroLens (http://www.neurolens.org) software. All connectivity distribution plots and effect sizes were computed within the R statistical computing environment (http://www.r-project.org).
Results
vACC Connectivity in Bipolar Illness
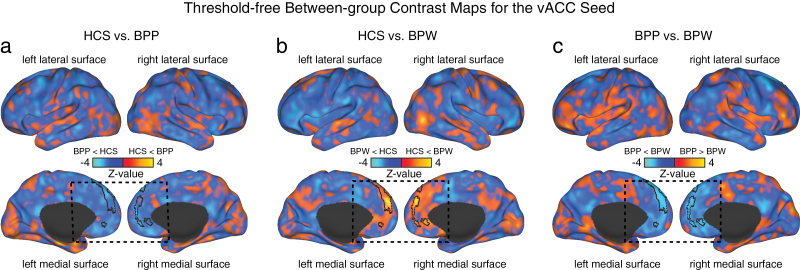
We first tested for whole brain group differences using an a priori defined vACC seed (figure 1, left), between BPW, BPP, and HCS groups. The 1-way ANOVA analysis showed a significant effect in a large cluster along the medial PFC surface, extending superiorly (figure 1a and b). The result was driven by a difference between BPP and BPW groups, whereby the BPP group showed reduced connectivity to the vACC seed relative to HCS (Cohen’s d = −0.69, P < .0027, 2-tailed, see table 2 for all pair-wise comparisons). In contrast, the BPW group showed increased connectivity between the identified cluster and the vACC seed relative to the HCS (Cohen’s d = 0.8, P < .0002, 2-tailed) and also relative to the BPP group (Cohen’s d = 1.37, P < 4.9 × 10− 7, 2-tailed). This finding suggests that areas along the medial PFC surface show dissociations in connectivity with vACC in bipolar illness with and without psychosis history. To further characterize the patterns of between-group differences, we computed follow-up pair-wise t-tests without a threshold applied. This analysis highlights the qualitative pattern of functional alteration in vACC connectivity between the bipolar groups (figure 2a–c, borders mark the significant between-group effect for ease of inspection).
Table 2.
Between-Group Connectivity Results
| BPW vs HCS | BPP vs HCS | BPP vs BPW | SCZ vs HCS | SCZ vs BPW | SCZ vs BPP | |
|---|---|---|---|---|---|---|
| Mean P value | < .001*** | < .01** | < .001*** | .07 | < .001*** | .18 |
| Mean t value | 3.93 | 3.09 | 5.54 | 1.85 | 5.42 | 1.36 |
| Mean effect size (Cohen’s d) | 0.80 | −0.69 | 1.37 | 0.33 | 1.03 | −0.30 |
Note: BPP, Bipolar patients with psychosis history; BPW, Bipolar patients without psychosis history; SCZ, schizophrenia patients; HCS, healthy control subjects. All statistics were calculated across all voxels for the identified cluster that survived the 1-way ANOVA F-test presented in figure 1. All Cohen’s d effect sizes were calculated across subjects, using standard procedures. 36 Cohen’s d was obtained by extracting the average Fisher’s r-to-Z connectivity value for each subject across the entire identified cluster as in figure 1. This was done to characterize the magnitude of between-group effects across voxels surviving the whole brain correction and to provide a guide regarding sample sizes needed for future replications. Significant between-group effect at P < .05*, P < .01**, P < .001***.
Fig. 2.
Threshold-free between-group contrast maps for the vACC seed. To further qualitatively characterize the nature of between-group effects, we highlight each pair-wise group comparison for the vACC seed. (a) Direct threshold-free contrast between HCS and BPP groups. Yellow-orange foci mark areas where BPP showed increased vACC coupling relative to HCS, whereas blue foci mark areas where BPP showed decreased coupling relative to HCS. The border on the medial surface highlights regions that survived the 1-way ANOVA (as shown in figure 1). Consistent with the effect size calculations, there was generally decreased medial prefrontal cortex (PFC) connectivity with the vACC seed for the BPP group (dashed box). (b) In contrast, BPW group was associated with increased medial PFC connectivity with the vACC seed relative to HCS, as evident from the effect size estimates (table 2). (c) Direct comparison of BPP vs BPW groups revealed reductions for the vACC seed along the medial PFC surface for the BPP group relative to the BPW group. Collectively, these results support the hypothesis that vACC connectivity differs between bipolar groups with and without psychosis history.
Differences Between Bipolar and Schizophrenia Patients
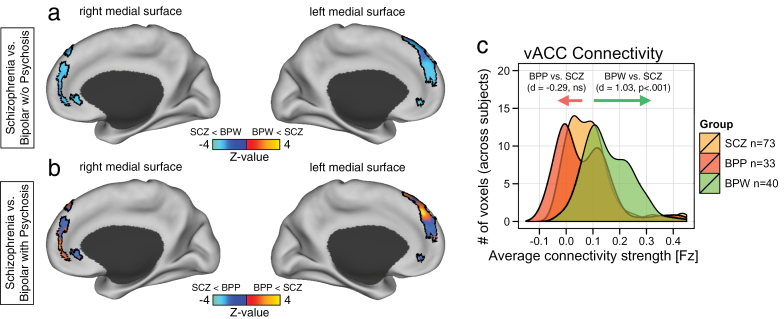
We examined a large and demographically matched schizophrenia sample (table 1) to determine if either of the bipolar groups resembled effects in schizophrenia. We found that the BPW group showed marked increases in vACC-medial PFC coupling relative to the schizophrenia group (figure 3a; Cohen’s d = 1.03, P < 3.5 × 10− 7, 2-tailed). This was evident from the direct pair-wise contrast maps of the schizophrenia vs BPW group, where the schizophrenia sample showed notable reductions relative to the BPW group along the identified medial PFC cluster (blue foci in figure3a). Conversely, the BPP group showed similar, albeit mildly reduced, effects relative to the schizophrenia sample (Cohen’s d = −0.29, P = .18, not significant; figure 3b). This was evident from the rather mixed pattern of signal along the identified medial PFC cluster (see figure 3b maps for qualitative inspection). These findings support the hypothesis that psychotic BD is associated with vACC dysconnectivity similar to schizophrenia. In contrast, the BPW group showed clear differences from both BPP and schizophrenia samples. When contrasting the schizophrenia and HCS groups, we found only modest alterations in vACC connectivity (Cohen’s d = .33, P = .07, 2-tailed), highlighting the utility of the schizophrenia sample as a control clinical group in the present analysis.
Fig. 3.
Differences in medial PFC connectivity in bipolar illness and schizophrenia. Pair-wise group comparison for the vACC seed was performed with an independently collected schizophrenia sample (SCZ), demographically matched to the entire bipolar sample (see table 1). Left panels show direct threshold-free contrast mapped within the medial PFC borders marking the cluster that survived the 1-way ANOVA for the bipolar analysis (as shown in figure 1). (a) Direct contrast of BPW and SCZ groups shows a reduced pattern of vACC seed coupling for SCZ group relative to BPW group, corresponding to the finding identified for the BPP relative to BPP group (see figure 2c). (b) In contrast, comparison of BPP vs SCZ samples revealed a more mixed pattern of increased and reduced connectivity for the outlined regions, indicating no specific directional effect. (c) Effect size estimates (Cohen’s d) confirmed robust increase in connectivity for BPW relative to SCZ group. Conversely, there was a substantially smaller difference for BPP relative to SCZ group (evident by virtually complete overlap for the SCZ/BPP distributions in panel (c). For complete pair-wise statistics see table 2.
Discussion
We examined whole brain connectivity vACC alterations in BD with and without psychosis history. To provide cross-diagnostic relevance of identified vACC connectivity alterations in bipolar illness, we compared bipolar findings with a large sample of patients with chronic schizophrenia. Our results highlight 2 observations: (1) the vACC whole brain connectivity pattern differed between BPP and BPW groups and (2) unlike the BPW group, the BPP group displayed vACC dysconnectivity similar to schizophrenia. To our knowledge, these are the first findings showing distinct patterns of whole brain vACC disturbances in bipolar illness with and without co-occurring psychosis. Our results underscore that neural circuit dysfunction may map onto similarities in lifetime symptoms rather than predefined diagnostic categories. These cross-diagnostic analyses provide a step toward understanding possible overlapping disturbances in neural circuits that may be shared between psychiatric categories with similar symptom presentation (in this case psychosis).38
Ventral Anterior Cingulate in BD
We found evidence for vACC dysconnectivity in remitted bipolar patients. Most prior work examining vACC metabolism4 or connectivity15,39 in BD examined symptomatic individuals. Indeed, attenuation of imaging signal in this region has been associated with improvement of depressive symptoms.40 Our observation of altered connectivity in clinically stable remitted patients suggest that, while vACC connectivity may be influenced by clinical state, at least some aspects of vACC dysconnectivity could be a BD endophenotype. Findings of reduced glia among individuals with BD in the vACC suggest that bipolar illness may be associated with neurophysiological changes in this region that are not entirely driven by clinical presentation.14 If so, it is possible that connectivity measures may be sensitive to this neurophysiological signal. This assertion is speculative; therefore, longitudinal studies across illness phases are required to address the hypothesis that clinical state or current symptom presentation is independent of vACC dysconnectivity in BD.
The vACC is thought to regulate emotional behavior through afferent and efferent connections to orbitofrontal cortex, insula, amygdala, and hypothalamus, among other regions.41 This region is thought to integrate between limbic and cortical aspects of a distributed brain networks, which are disrupted in depression11 and in nondepressed individuals experiencing sadness.7 Our data suggest that connectivity of this region to other areas of medial PFC is disrupted in BD. Consistent with this possibility, Etkin and colleagues42 articulated a key role of the entire medial PFC surface in emotional behavior. Research in basic cognitive neuroscience points to a dichotomy along ventral/dorsal medial prefrontal areas for distinct emotional computations.42 Phillips and colleagues hypothesized a dysregulation in bipolar illness across the dorsal medial PFC system for voluntary emotional regulation and the ventral system for automatic emotional regulation.43 Here we observed a vACC connectivity alteration extending across ventral and dorsal medial PFC. This cortical territory has been implicated in emotional behavior such as arousal monitoring, top-down emotional regulation, emotional conflict monitoring, or representational value of emotional stimuli, to name a few.42 The elevated pattern of connectivity between these cortical regions and vACC found in BPW may reflect increased synaptic plasticity, caused by an ongoing demand to engage in affective regulation. It is interesting, however, that connectivity along this area also dissociates between BD with and without psychosis history. One possibility is that the pathophysiology of BD with co-occurring psychosis follows a more severe course and is, perhaps, more qualitatively similar to the clinical trajectory observed in schizophrenia. Identified patterns of dysconnectivity are also proximal to areas reported in relation to individual differences in anxiety in healthy adults.44 Although we did not observe a significant effect in relation to presence or absence of anxiety, this may possibly reflect differences in the specific circuits studied here, the fact that we measured presence of clinical anxiety rather than individual variation present in the normal population, as well as the baseline anxiety status of the present clinical sample.
The vACC dysconnectivity observed here was restricted to medial PFC regions and did not include other distributed neocortical or subcortical regions possibly implicated in BD. Interestingly, we also failed to observe significant connectivity alterations between the selected vACC region and the amygdala. This could reflect 2 alternative possibilities: (1) There really are no meaningful alterations elsewhere, not even weak ones; (2) there are distributed effects, but they are more modest and therefore not detected with present sample sizes and the level of voxel-wise protection. Thus, the principal reason for such null effects may reflect that certain functional alterations are occurring just below the statistical significance threshold (as can be seen on the unthresholded maps in figure 2). In that sense, the absence of significant amygdala effects (or effects elsewhere) does not rule out the possibility that other relevant circuitry may in fact be perturbed in BD. Indeed, at lower thresholds (figure 2), effects extended to other nodes that have been associated with internal thought processes and “rumination,” suggesting that the disturbances in vACC connectivity may not be exclusively localized to PFC circuits. Future studies with even larger samples will be needed to explore such effects that may be more subtle but nevertheless functionally important.
Related to this point, by seeding a similar region, Chai and colleagues15 found evidence for aberrant connectivity with the dorsolateral and ventrolateral PFC. Their study, however, computed a direct comparison between BD and schizophrenia patients at the whole brain level, requiring a stringent type I error correction. The observed discrepancies may reflect differences in size, composition, and clinical presentation of the samples beings studied and some differences in neuroimaging methods. Specifically, our bipolar sample included individuals who were euthymic; our measure of psychosis related to prior history (as opposed to current symptoms). Therefore, symptomatic individuals could exhibit connectivity disruptions of the vACC with other areas, as reported by Chai and colleagues.15 Also, it is worth noting that the seed location used in this study was based on prior data-driven effects, and it deviates somewhat from the seed location reported by Chai and colleagues. For this reason, we also verified that presently reported results were highly similar when using either seed (see supplementary figure 1). This suggests that the present result is robust across a larger portion of the ventral anterior cingulate cortical territory and may not necessarily be sensitive to the particular seed selection. Importantly, as done by Chai and colleagues, we restricted our study to the vACC (as opposed to other circuits that may be implicated in BD). This focused search was primarily motivated by prior work implicating this area in mood regulation.3 That said, future studies will need to systematically examine network-level questions involving broader circuits involved in emotion processing,45 as well as lateral PFC circuits that may be involved in emotional regulatory processes disrupted in mood disorders.46 Collectively, these findings extend earlier observations by suggesting that vACC connectivity is different in bipolar patients that have experienced co-occurring psychotic symptoms, which may reflect neural alterations that also co-occur in schizophrenia.
Bipolar Illness With Psychosis History Resembles Schizophrenia
Our study revealed that BPP individuals exhibit vACC connectivity alterations more similar to the alterations exhibited by the chronic schizophrenia group than to those exhibited by the BPW group. This supports the emerging motivation to study neural circuit alterations across neuropsychiatric diagnoses, rather than studying narrowly predefined diagnostic categories. The medial PFC and nearby nodes of the default mode network (DMN) are known to exhibit alterations in BD.47 Schizophrenia is also associated with alterations of the DMN47 but is thought to, perhaps, involve a more disturbed alteration of the DMN,48 rather than the anterior PFC circuits specifically,3 which have been implicated in emotional processing (see above). Another resting-state study using independent-component analysis compared BD patients with psychosis to patients with schizophrenia and found that psychotic BD was also associated with abnormalities in the posterior DMN and precuneus,49 though the same study did not observe shared disturbances in the anterior medial PFC reported here, possibly due to differences in connectivity approaches.
It is important to consider that we did not directly compare schizophrenia and bipolar illness at the whole brain level, as done previously.15,37 Instead, the key objective was to characterize alterations in BD as a function of psychosis history at the whole brain level. In turn, we show that the effects found for psychotic BD patients resemble connectivity alterations identified in chronic schizophrenia in this same medial PFC area. Therefore, the schizophrenia sample served as a well-powered clinical control group, highlighting that bipolar individuals indeed show a unique pattern of vACC dysconnectivity as a function of psychosis. These effects indicate that there may exist at least some shared circuit alterations that jointly contribute to symptoms across psychiatric diagnoses. Further delineation of such system-level similarities and differences across psychiatric diagnoses will be vital to inform the Research Domain Criteria initiative (RDoC) to develop better biomarkers for complex psychiatric symptoms.50 Relatedly, although beyond the scope of the present focused investigation, additional insights will be derived from direct follow-up whole brain comparison between BD and schizophrenia groups that are adequately powered to detect subtle system-level alterations. That is, while psychotic BD patients resemble schizophrenia patients in their vACC connectivity pattern to this particular cluster, it is likely that there are dissociations elsewhere.
Limitations
At the time of the scan, bipolar patients were remitted, whereas schizophrenia patients were symptomatic and in their chronic illness phase. Consequently, it could be argued that, unlike the schizophrenia sample, we were not capturing a “neural signature” of current psychotic symptoms in bipolar patients but a more general endophenotype of the illness itself. However, the observation that the BPP group closely resembled schizophrenia patients suggests this finding may be relevant for understanding psychosis broadly. Carefully matched future studies will need to confirm the presence of the same connectivity patterns in currently symptomatic psychotic bipolar patients. This will help discern whether observed effects remain stable within a diagnostic (sub)group or scale as a function of specific symptom severity and/or illness phase. Similarly, it will be important to establish whether the chronicity/progression of either BD or schizophrenia alter reported effects. Given the importance of mood disturbances in bipolar illness, the influence of mood on the observed effects will need to be addressed with a more careful characterization of mania/depression symptoms in schizophrenia. Also, while we made best efforts to control for head motion, using the state-of-the-art methods,51 movement issues warrant ongoing careful consideration in the clinical connectivity literature. Specifically, studies finding disruptions in local vs long-distance connections (as seen here) will require prospective replication, given the possible differential impact of motion depending on distance.51
All patients were medicated at the time of their participation. Although medications did not change the observed effects when we covaried for medication class, it is still important to consider the possible effect of medications, especially given the difference in treatment practice for bipolar illness and schizophrenia. Because patients across both bipolar groups were carefully matched on medication classes, this argues against medications confounds. However, it will be important to replicate present findings in an unmedicated sample or to establish if any medications reverse the observed effect. Another limitation relates to prior history of substance use, which is a major concern for severe mental illness. While none of the participants met current substance abuse/dependence criteria at the time of the scan, prior substance/alcohol history was difficult to fully control for and should be considered in future studies. Also, given the correlational nature of the measures and possible dynamical circuit alterations over time, we cannot make any conclusions about causal relationship between observed effects and the illness phases.
Conclusions
This study provides two novel insights into our understanding of bipolar illness: (1) vACC, a node implicated in emotional regulation and pathophysiology of bipolar illness, shows differential connectivity as a function of psychosis history in bipolar illness and (2) schizophrenia findings qualitatively resembled observations in bipolar patients with psychosis but not those without psychosis history. Such differences in vACC network function indicate that distinct mechanisms may be at play in psychotic BD, possibly consistent with pathophysiology observed in schizophrenia. Present findings represent a building block toward the RDoC initiative to develop biomarker-driven diagnostic systems that can map onto specific behavioral symptoms.50
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
National Institutes of Health grant (MH080912 to PI: D.C.G.); National Institutes of Health grant (DP5OD012109-01 to PI: A.A.), National Institute on Alcohol Abuse and Alcoholism grant (2P50AA012870-11 to PI: J.H.K.), National Institutes of Health grant (MH096801 to PI: M.W.C.); National Institutes of Health grants (MH43775, MH077945, MH074797 to PI: G.D.P.); Fulbright Foundation (to A.S.); Brain and Behavior Research Foundation Young Investigator Award (to PI: A.A.).
Supplementary Material
Acknowledgments
We thank Anderson Winkler and Margaret Brumbaugh for help with initial organization of the database. J.H.K. consults for several pharmaceutical and biotechnology companies with compensation less than $10,000 per year. All other authors declare that they have no conflict of interest.
References
- 1. Keener MT, Phillips ML. Neuroimaging in bipolar disorder: a critical review of current findings. Curr Psychiatry Rep. 2007;9:512–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murray CJL, Lopez AD. The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability From Diseases, Injuries and Risk Factors in 1990 and Projected to 2020. Vol 1 Cambridge, MA: Harvard University Press; 1996. [Google Scholar]
- 3. Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2010;35:192–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Drevets WC, Price JL, Simpson JR, Jr, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. [DOI] [PubMed] [Google Scholar]
- 5. Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectr. 2008;13:663–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang F, Kalmar JH, He Y, et al. Functional and structural connectivity between the perigenual anterior cingulate and amygdala in bipolar disorder. Biol Psychiatry. 2009;66:516–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry. 1999;156:675–682. [DOI] [PubMed] [Google Scholar]
- 8. Hamani C, Mayberg H, Stone S, Laxton A, Haber S, Lozano AM. The subcallosal cingulate gyrus in the context of major depression. Biol Psychiatry. 2011;69:301–308. [DOI] [PubMed] [Google Scholar]
- 9. Ongür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000;10:206–219. [DOI] [PubMed] [Google Scholar]
- 10. Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2:417–424. [DOI] [PubMed] [Google Scholar]
- 11. Mayberg HS, Brannan SK, Mahurin RK, et al. Cingulate function in depression: a potential predictor of treatment response. Neuroreport. 1997;8:1057–1061. [DOI] [PubMed] [Google Scholar]
- 12. Goldapple K, Segal Z, Garson C, et al. Modulation of cortical-limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry. 2004;61:34–41. [DOI] [PubMed] [Google Scholar]
- 13. Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. [DOI] [PubMed] [Google Scholar]
- 14. Ongür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95:13290–13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chai XJ, Whitfield-Gabrieli S, Shinn AK, et al. Abnormal medial prefrontal cortex resting-state connectivity in bipolar disorder and schizophrenia. Neuropsychopharmacology. 2011;36:2009–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goodwin FK, Jamison KR. Manic Depressive Illness. New York, NY: Oxford University Press; 1990. [Google Scholar]
- 17. Glahn DC, Bearden CE, Barguil M, et al. The neurocognitive signature of psychotic bipolar disorder. Biol Psychiatry. 2007;62:910–916. [DOI] [PubMed] [Google Scholar]
- 18. Pearlson GD, Wong DF, Tune LE, et al. In vivo D2 dopamine receptor density in psychotic and nonpsychotic patients with bipolar disorder. Arch Gen Psychiatry. 1995;52:471–477. [DOI] [PubMed] [Google Scholar]
- 19. Anticevic A, Brumbaugh MS, Winkler AM, et al. Global prefrontal and fronto-amygdala dysconnectivity in bipolar I disorder with psychosis history. Biol Psychiatry. 2013;73:565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cole MW, Anticevic A, Repovs G, Barch D. Variable global dysconnectivity and individual differences in schizophrenia. Biol Psychiatry. 2011;70:43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for the DSM-IV-TR Axis I Disorders. Washington, DC: American Psychiatric Press; 2001. [Google Scholar]
- 22. Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- 23. Glahn DC, Bearden CE, Bowden CL, Soares JC. Reduced educational attainment in bipolar disorder. J Affect Disord. 2006;92:309–312. [DOI] [PubMed] [Google Scholar]
- 24. Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. [DOI] [PubMed] [Google Scholar]
- 25. Hamilton M. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol. 1967;6:278–296. [DOI] [PubMed] [Google Scholar]
- 26. Ventura J, Green MF, Shaner A, Liberman RP. Training and quality assurance with the Brief Psychiatric Rating Scale: “The drift busters.” Int J Meth Psychiatr Res. 1993;3:221–244. [Google Scholar]
- 27. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 28. Anticevic A, Repovs G, Barch DM. Emotion effects on attention, amygdala activation, and functional connectivity in schizophrenia. Schizophr Bull. 2012;38:967–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Steps toward optimizing motion artifact removal in functional connectivity MRI; a reply to Carp. Neuroimage. 2013;76:439–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Biswal BB, Mennes M, Zuo XN, et al. Toward discovery science of human brain function. Proc Natl Acad Sci U S A. 2010;107:4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Repovs G, Csernansky JG, Barch DM. Brain network connectivity in individuals with schizophrenia and their siblings. Biol Psychiatry. 2011;69:967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fischl B, Salat DH, Busa E, et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. [DOI] [PubMed] [Google Scholar]
- 34. Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. [DOI] [PubMed] [Google Scholar]
- 36. Cohen J. A power primer. Psychol Bull. 1992;112:155–159. [DOI] [PubMed] [Google Scholar]
- 37. Anticevic A, Cole MW, Repovs G, et al. Connectivity, pharmacology, and computation: toward a mechanistic understanding of neural system dysfunction in schizophrenia. Front Psychiatry. 2013;4:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Keshavan MS, Morris DW, Sweeney JA, et al. A dimensional approach to the psychosis spectrum between bipolar disorder and schizophrenia: the Schizo-Bipolar Scale. Schizophr Res. 2011;133:250–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Anand A, Li Y, Wang Y, Lowe MJ, Dzemidzic M. Resting state corticolimbic connectivity abnormalities in unmedicated bipolar disorder and unipolar depression. Psychiatry Res. 2009;171:189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hamani C, Machado DC, Hipólide DC, et al. Deep brain stimulation reverses anhedonic-like behavior in a chronic model of depression: role of serotonin and brain derived neurotrophic factor. Biol Psychiatry. 2012;71:30–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Barbas H. Flow of information for emotions through temporal and orbitofrontal pathways. J Anat. 2007;211:237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011;15:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Phillips ML, Ladouceur CD, Drevets WC. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol Psychiatry. 2008;13:829, 833–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim MJ, Gee DG, Loucks RA, Davis FC, Whalen PJ. Anxiety dissociates dorsal and ventral medial prefrontal cortex functional connectivity with the amygdala at rest. Cereb Cortex. 2011;21:1667–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kober H, Barrett LF, Joseph J, Bliss-Moreau E, Lindquist K, Wager TD. Functional grouping and cortical-subcortical interactions in emotion: a meta-analysis of neuroimaging studies. Neuroimage. 2008;15:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mamah D, Barch DM, Repovš G. Resting state functional connectivity of five neural networks in bipolar disorder and schizophrenia. J Affect Disord. 2013;150:601–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Calhoun VD, Sui J, Kiehl K, Turner J, Allen E, Pearlson G. Exploring the psychosis functional connectome: aberrant intrinsic networks in schizophrenia and bipolar disorder. Front Psychiatry. 2011;2:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, Krystal JH. The role of default network deactivation in cognition and disease. Trends Cogn Sci. 2012;16:584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Khadka S, Meda SA, Stevens MC, et al. Is aberrant functional connectivity a psychosis endophenotype? A resting state functional magnetic resonance imaging study. Biol Psychiatry. 2013;74:458–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cuthbert BN, Insel TR. Toward new approaches to psychotic disorders: the NIMH Research Domain Criteria project. Schizophr Bull. 2010;36:1061–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Power JD, Mitra A, Laumann TO, Snyder AZ, Schlaggar BL, Petersen SE. Methods to detect, characterize, and remove motion artifact in resting state fMRI. Neuroimage. 2014;84:320–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho BC. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.