Abstract
Introduction:
Structural alterations may correlate with symptom severity in psychotic disorders, but the existing literature on this issue is heterogeneous. In addition, it is not known how cortical thickness and cortical surface area correlate with symptom dimensions of psychosis.
Methods:
Subjects included 455 individuals with schizophrenia, schizoaffective, or bipolar I disorders. Data were obtained as part of the Bipolar Schizophrenia Network for Intermediate Phenotypes study. Diagnosis was made through the Structured Clinical Interview for DSM-IV. Positive and negative symptom subscales were assessed using the Positive and Negative Syndrome Scale. Structural brain measurements were extracted from T1-weight structural MRIs using FreeSurfer v5.1 and were correlated with symptom subscales using partial correlations. Exploratory factor analysis was also used to identify factors among those regions correlating with symptom subscales.
Results:
The positive symptom subscale correlated inversely with gray matter volume (GMV) and cortical thickness in frontal and temporal regions, whereas the negative symptom subscale correlated inversely with right frontal cortical surface area. Among regions correlating with the positive subscale, factor analysis identified four factors, including a temporal cortical thickness factor and frontal GMV factor. Among regions correlating with the negative subscale, factor analysis identified a frontal GMV-cortical surface area factor. There was no significant diagnosis by structure interactions with symptom severity.
Conclusions:
Structural measures correlate with positive and negative symptom severity in psychotic disorders. Cortical thickness demonstrated more associations with psychopathology than cortical surface area.
Key words: positive, negative, cortical thickness, surface area, psychopathology, gray matter
Introduction
Structural imaging studies have established the presence of subtle structural brain alterations in psychotic disorders. For schizophrenia, some of the most consistent findings include reductions in gray matter volume (GMV) of frontal, temporal, and limbic regions,1 whereas bipolar disorder has also been associated with GMV reductions in prefrontal, temporal, and limbic regions.2,3
Studies have found correlations between structural alterations and symptom dimensions of psychosis; however, findings have been heterogeneous. In schizophrenia, inverse correlations between positive symptom severity and GMV of temporal lobe regions, most commonly the superior temporal gyrus (STG), have been frequently reported,4–8 but a minority of studies have observed no correlation or a direct correlation between positive symptom severity and GMV of these regions.9–11 Results have been similarly mixed for the negative symptom dimension, with several studies finding inverse correlations with GMV of frontal regions,12–14 and other studies reporting no correlation or a positive correlation with frontal regions.15–17
Multiple reasons may account for the heterogeneity in findings. Positive symptoms of psychosis can wax and wane with time; thus, results may be influenced by illness acuity of subjects at the time of scan. Other reasons for heterogeneity of results may include variations in technical aspects of imaging methodology, variable adjustment for confounding factors, and differences in subject characteristics, such as duration of illness.
In addition to the heterogeneity of findings on clinical correlations with GMV, the literature is limited regarding correlations with nonvolumetric structural measures. Two constituents of volume, cortical thickness (CT), and cortical surface area (CSA) have demonstrated significant alterations in both schizophrenia18–20 and bipolar disorder.21–23 They may correlate differently with psychopathology because of their distinct neurobiological and genetic origins. According to a prevailing theory of cortical development, CSA is determined by the total number of cortical columns that form the cerebral cortex, whereas CT is determined by the number of cells within each column.24–26 A recent longitudinal neuroimaging study of children found evidence for independent developmental trajectories of CT and CSA.27 In addition, neuroimaging studies of twins have found that CSA and CT are both highly heritable but probably genetically distinct.28
Thus far, the literature has not established whether CT and CSA have distinct correlations with positive and negative symptoms. Correlations have been reported between CT and propensity for hallucinations19 and positive symptoms29 among individuals with schizophrenia. However, one cross-diagnostic study of psychosis did not find an association between CT and symptom dimensions of psychosis.22 Analysis of symptom correlations with CT and CSA may reveal their differential contributions to psychopathology, which in turn could help identify more specific neuropathological processes that drive the emergence of psychotic symptoms. In addition, inclusion of multiple diagnostic categories may reveal whether symptom-structure correlations are trans-diagnostic.
In this study, we examined correlations between symptom dimensions of psychosis and regional GMV, CSA, and CT in schizophrenia, schizoaffective disorder, and bipolar I disorder with psychosis. Correlations were examined using partial correlations between individual regions and subscales, and factor analysis was used to summarize overall structure-symptom relationships. Based on the existing literature,30 we hypothesized that temporal alterations would correlate with the positive subscale, whereas frontal alterations would correlate with the negative subscale. We also hypothesized that CT and CSA would show distinct correlations with symptom dimensions. This is one of the largest sample sizes to date in which associations with dimensions of psychosis have been examined.
Methods
Participants
Subjects included individuals with a DSM-IV diagnosis of schizophrenia, schizoaffective disorder, or bipolar I disorder with psychotic features. Subjects were recruited as part of the Bipolar-Schizophrenia Network for Intermediate Phenotypes, using recruitment methods that have been detailed elsewhere.31,32 Inclusion criteria included the following: (1) age 15–65; (2) English proficiency, as determined by ability to follow task instructions; (3) no known history of neurologic disorders including head injury; (4) no history of substance abuse within the last month or substance dependence within the last 6 months; and (5) negative urine toxicology screen on day of testing. Patients were generally clinically stable and receiving consistent psychopharmacological treatment for 4 weeks before testing. Study protocols were approved by institutional review boards at each study site, and subjects signed informed consent forms.
All subjects received the Structured Clinical Interview for DSM-IV (SCID-IV).33 A consensus process was used to establish diagnosis using results from the SCID-IV, chart review, and review of psychiatric and medical histories. The Positive and Negative Syndrome Scale (PANSS)34 was used to assess positive, negative, and general symptoms in patients. Inter-site standardization of symptom ratings was performed by periodic meetings for rater training, using established “gold standard” interviews. At the beginning of the study, there was a face-to-face training session for all raters, with a requirement for reliability of >0.85. Rater training was repeated annually to reestablish reliability.31
A total of 455 patients had complete datasets available for structural MRI and symptom scales for analysis of structure-symptom correlations.31,32 Of these 455 subjects, 181 individuals were categorized as having schizophrenia, 117 individuals as having schizoaffective disorder, and 157 individuals as having bipolar I disorder with psychotic features. Three hundred fifty-two healthy controls were also evaluated in the larger study; they received structural MRIs but did not receive PANSS assessments.
MRI-Structural Imaging
High-resolution isotropic T1-weighted MPRAGE sequences were obtained. Sites used comparable but slightly different MPRAGE acquisition parameters; full details for each site have been described previously.31 The Alzheimer’s Disease Neuroimaging Initiative (ADNI) protocol was used at all sites to standardize imaging analysis (http://www.loni.ucla.edu/ADNI). All images were subjected to a rigorous data quality control process. First, images were opened, converted to nifti format, and checked for scanner artifacts. If the images passed through this check, they were run through auto-recon 1 in FreeSurfer v5.1.35 Images were then checked for remaining nonbrain tissues (dura or sinus). Trained raters, all reliable above 95%, edited images to remove any remaining nonbrain tissue. An independent rater then determined whether images were adequately cleaned for segmentation, and images were then processed through auto-recon 2 and 3. Freesurfer v5.1 software was used to extract regional GMV, CT, and CSA measurements.
Statistical Analysis
All statistical analysis was done using the program R (Vienna, Austria; 2013, http://www.R-project.org, version 2.15.3). Data were examined for bivariate normality using the multivariate Shapiro-Wilk test (R package: mvnormtest), revealing that clinical symptom measures were not normally distributed. Nonparametric tests were used for further analyses. Diagnostic differences in demographic variables and symptom subscales were tested through the Kruskal-Wallis and chi-squared tests. Partial correlations were performed to correlate individual structural measures with symptom subscales. In addition, a factor analysis approach was used to identify structural factors among regions associated with symptom subscales.
Partial Correlations Between Structural Measures and Symptom Subscales
A series of variables were tested for potential inclusion as covariates. These included age, sex, race, study site, intracranial volume (ICV), socioeconomic status, patient educational level, duration of illness, and antipsychotic medication status (a binary variable representing whether or not the patient was currently on an antipsychotic). Duration of illness was computed by subtracting age at illness onset from current age. Socioeconomic status was represented by patient Hollingshead Index score, whereas patient educational level was represented by patient years of education. Study site was treated as a categorical variable, with each site being “dummy coded” as a binary variable for regression analyses. Variables were retained as covariates if they correlated with either structural measures or symptom subscales, using the Kruskal-Wallis test for categorical variables and the Spearman correlation for continuous variables.
Using partial Spearman correlations, symptom subscales were first correlated with GMV, CSA, and mean CT of each lobe and were Hochberg-adjusted for multiple comparisons (32 comparisons per subscale).36 For each lobe that demonstrated statistically significant correlations with a symptom subscale, subregions of that lobe were then correlated with that symptom subscale in a step-down fashion (see supplementary table 1 for lists of the subregions that constituted each lobe). These correlations were again Hochberg-adjusted for multiple comparisons by the total number of subregions tested for correlations with that symptom subscale.
GMV and CSA for each lobe were computed by adding the GMVs or CSAs of component subregions. A mean CT for each lobe was determined by calculating a weighted average of the cortical thicknesses of component subregions (ie, CT of each subregion multiplied by CSA of that subregion, divided by total CSA for that lobe).
Lobes and subregions that showed significant correlations in the whole-group analysis were then tested for correlations with symptom subscales within each diagnostic group and were corrected for total number of correlations tested within that diagnostic group. Symptom subscales and PANSS total scores were also correlated with total GMV. Finally, a supplemental analysis was done using current antipsychotic dose in chlorpromazine equivalents, which was only available for 295 of 455 patients. Analyses were repeated in this subset of patients with and without chlorpromazine dose as a covariate to evaluate the impact of this covariate.
Exploratory Factor Analysis
To construct an enriched sample of brain regions, all regional structural measures (80 GMV measures, 66 CSA measures, and 66 CT measures) were first screened for correlations with the positive and negative symptom subscales. Regional measures that correlated with the positive subscale at a significance level of P < .05, uncorrected, were entered into factor analysis. These regional measures were regressed against age, ICV, sex, and race. The residuals of these regressions were then used to create the correlation matrix for factor analysis, as has been done with structural brain measures previously 37,38; that is, the correlation matrix for factor analysis consisted of partial correlations among these regional measures, controlling for covariates of age, ICV, sex, and race.
Principal factor extraction (also called “principal axes factoring”) was used to extract factors because of deviations from multivariate normality.39 A Scree test was performed to obtain an initial estimate of number of factors.40,41 In addition, several factor analyses were performed using different numbers of factors and were evaluated for overall factor structure. A final number of factors was chosen if it produced factors with no or few item cross-loadings and at least three variable loadings above 0.45.42 Direct oblique rotation was performed to assess whether factors were correlated with each other. Factor scores for subjects were derived using regression to maximize determinacy of scores.43
Each factor score was entered as a predictor into a regression model with a full set of covariates (see previous section) to verify associations with the positive symptom subscale. Next, all factor scores were entered into a single model with covariates to assess their relative correlations with symptom subscale scores. To assess specificity of correlations between factor scores and symptom subscales, factor scores were also tested for correlations with the negative subscale. Finally, to assess diagnosis by structure interactions, models were rerun with the inclusion of diagnosis and diagnosis by structure interaction terms. Assumptions of multiple regression were tested through visualization of QQ plots and residual-versus-fitted plots.
An identical and separate factor analysis process was performed using those regions that correlated with the negative symptom subscale.
Results
Demographics
Subject demographics and study site demographics are presented in table 1 and supplementary table 2, respectively. Mean values for symptom subscales varied across diagnostic groups and study sites (P < .05 using Kruskal-Wallis rank sum test). Post-hoc comparisons of symptom subscales in schizophrenia and bipolar I disorder indicated that all symptom subscales were significantly higher in the schizophrenia group (P < .05 using Wilcoxon rank-sum test).
Table 1.
Subject Demographics and Symptom Scale Characteristics
| All Patients | Schizophrenia | Schizoaffective | Bipolar I | |
|---|---|---|---|---|
| n | 455 | 181 | 117 | 157 |
| Mean age (SD) | 36.2 (12.7) | 35.8 (12.6) | 36.0 (12.1) | 36.7 (13.3) |
| Sex M: Fa | 220:235 | 118:63 | 56:61 | 46:111 |
| (%M:%F) | 48%:52% | 65%:35% | 48%:52% | 29%:71% |
| Race (%)a,b | AA:184 (40%) | AA:94 (52%) | AA:52 (44%) | AA:38 (24%) |
| CA:242 (53%) | CA:73 (40%) | CA:58 (50%) | CA:111 (71%) | |
| OT:29 (6%) | OT:14 (8%) | OT:7 (6%) | OT:8 (5%) | |
| Mean PANSS positive (SD)c | 16.2 (5.5) | 17.2 (5.4) | 18.6 (4.9) | 13.3 (4.8) |
| Mean PANSS negative (SD)c | 14.9 (5.3) | 16.8 (5.8) | 15.4 (4.5) | 12.3 (4.1) |
| Mean PANSS general (SD)c | 32.2 (9.0) | 32.8 (9.1) | 35.0 (8.7) | 29.5 (8.4) |
| Mean PANSS total (SD)c | 63.3 (16.9) | 66.7 (16.9) | 69.1 (15.4) | 55.1 (14.7) |
| Mean intracranial volume (SD)c | 1437 cc (186 cc) | 1467 cc (197 cc) | 1396 cc (177 cc) | 1434 cc (174 cc) |
| Mean duration of illness (SD)c | 18.6 y (12.3 y) | 16.4 y (11.8 y) | 19.9 y (11.7 y) | 20.1 y (12.9 y) |
| Mean years of education (SD)c | 13.4 y (2.4 y) | 12.8 y (2.3 y) | 13.2 y (2.2 y) | 14.3 y (2.4 y) |
| Mean Hollingshead Score (SD)c,d | 48.2 (15.7) | 53.2 (14.6) | 48.7 (14.6) | 42.1 (15.7) |
| Antipsychotic status (%)a,e | Yes: 85%; No: 15% | Yes: 92%; No: 8% | Yes: 86%; No: 14% | Yes: 75%; No: 25% |
Note: F, female; M, male.
aSignificantly different across diagnostic groups by chi-squared test.
bAA, African American; CA, Caucasian American; OT, Other.
cSignificantly different across diagnostic groups by Kruskal-Wallis test.
dHollingshead occupation score multiplied by 7, added to Hollingshead education score multiplied by 4; higher score indicates lower social class.
eCurrently taking antipsychotics (yes or no).
Partial Correlations Between Structural Measures and Symptom Subscales
Covariate Analysis.
All potential covariates demonstrated correlations with at least one symptom subscale and were retained as covariates in further analyses. In addition, age, sex, race, study site, duration of illness, and intracranial volume correlated with structural measures (P adjusted < .05).
Correlations With Structural Measures in Combined Group.
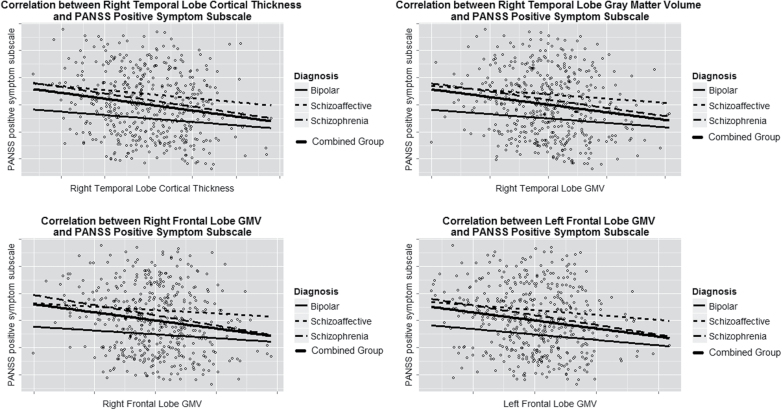
PANSS positive symptom subscale was correlated with frontal and temporal GMV reductions and temporal CT reductions, whereas the PANSS negative subscale was correlated with reductions in right frontal CSA (P adjusted < .05, table 2, figure 1, supplementary figure 1). There were no correlations with the PANSS general subscale.
Table 2.
Structure-Subscale Correlations Within Combined Group and Within Diagnostic Categories
| Lobe | Measurement | Subscale | Combined Group | Schizophrenia | Schizoaffective | Bipolar I | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | P Adjusteda | r | P Valueb | r | P Valueb | r | P Valueb | |||
| Left frontal GMV | GMV | PANSS positive | −.162 | .016 | −.176 | .018 | −.072 | .44 | −.067 | .41 |
| Left pars orbitalis | −.182 | .004 | −.113 | .130 | −.139 | .13 | −.141 | .078 | ||
| Left superior frontal | −.166 | .013 | −.170 | .022 | −.170 | .067 | −.015 | .85 | ||
| Right frontal GMV | GMV | PANSS positive | −.165 | .013 | −.170 | .022 | −.015 | .88 | −.031 | .70 |
| Right superior frontal | −.180 | .005 | −.160 | .032 | −.155 | .095 | .010 | .90 | ||
| Right temporal GMV | GMV | PANSS positive | −.158 | .020 | −.141 | .058 | −.054 | .57 | −.020 | .80 |
| Right superior temporal | −.155 | .031 | −.155 | .036 | −.148 | .11 | −.020 | .80 | ||
| Right fusiform | −.178 | .005 | −.089 | .240 | −.145 | .12 | −.067 | .40 | ||
| Right temporal CT | Cortical thickness | PANSS positive | −.173 | .007 | −.183 | .013 | −.079 | .40 | −.047 | .56 |
| Right middle temporal | −.150 | .045 | −.199 | .0072 | .025 | .79 | −.091 | .26 | ||
| Right superior temporal | −.155 | .032 | −.138 | .064 | −.102 | .27 | −.104 | .19 | ||
| Right insula | −.175 | .006 | −.236 | .0014c | −.075 | .42 | .021 | .80 | ||
| Right frontal area | Surface area | PANSS negative | −.151 | .041 | −.153 | .040 | .081 | .34 | −.023 | .77 |
| Right pars orbitalis | −.138 | .026 | −.145 | .051 | −.150 | .11 | −.093 | .25 | ||
| Right superior frontal | −.138 | .026 | −.101 | .17 | .016 | .86 | −.190 | .018 | ||
| Right precentral | −.159 | .007 | −.150 | .043 | −.064 | .49 | −.162 | .042 | ||
Note: GMV, gray matter volume; CT, cortical thickness.
a P values Hochberg-adjusted as described in the text.
bUnadjusted P value.
cSignificant after Hochberg correction.
Fig. 1.
Significant correlations between lobe measures and positive symptom severity for all patients and diagnostic groups; plotted values are residuals after adjustment for covariates.
Correlations With Structural Measures within Diagnostic Groups.
These structural measures were then evaluated for symptom-subscale correlations within diagnostic groups. Correlations remained significant within the schizophrenia group, but only the correlation with the right insula CT would have survived correction for multiple comparisons. Correlations within bipolar disorder and schizoaffective disorder were largely nonsignificant (table 2).
Correlations With Total GMV.
In the combined group of all patients, total GMV correlated inversely with the PANSS positive subscale (r = −.177, P = .00015) and the PANSS total score (r = −.118, P = .011), but not with the PANSS negative subscale (r = −.061, P = .19) or the PANSS general subscale (r = −.0827, P = .08).
Among subjects with available data on antipsychotic dose in chlorpromazine equivalents, effect sizes of correlations decreased when chlorpromazine equivalent medication dose was included as a covariate, but the overall pattern of correlations was similar.
Exploratory Factor Analysis
Positive Symptom Subscale.
Initial screening revealed that 58 of 212 regions were correlated with the positive subscale (P < .05, uncorrected). Scree test suggested between 4 and 6 factors, and further evaluation supported 4 reliable factors: a temporal CT factor, a frontal GMV factor, a frontoparietal CT factor, and a precuneus GMV-SA factor (table 3, supplementary table 3). There were no cross-loadings of variables. Direct oblique rotation was retained because factors were correlated.
Table 3.
Factor Analysis: Regions Loading Above 0.45 on Factors Derived Through Principal Factors Extraction
| Positive Subscale: Four Factors | Negative Subscale: One Factor | |||
|---|---|---|---|---|
| F1: Temporal CT | F2: Frontal GMV | F3: Frontoparietal CT | F4: Precuneus | F: Frontal GMV-SA |
| L fusiform CT | R lateral orbitofrontal GMV | L superior parietal CT | R precuneus GMV | R superior frontal GMV |
| R middle temporal CT | R superior frontal GMV | R inferior parietal CT | L precuneus GMV | R superior frontal CSA |
| R superior temporal CT | L lateral orbitofrontal GMV | L superior frontal CT | R precuneus CSA | R precentral GMV |
| R fusiform CT | L superior frontal GMV | R lateral occipital CT | R fusiform CSA | R precentral CSA |
| L inferior temporal CT | R superior frontal CSA | R supramarginal CT | R lateral orbitofrontal CSA | |
| R inferior temporal CT | R rostral middle frontal GMV | R superior frontal CT | R pars orbitalis CSA | |
| R insula CT | L superior frontal CSA | L lateral occipital CT | R pars orbitalis GMV | |
| L fusiform GMV | L pars orbitalis GMV | L supramarginal CT | R paracentral CSA | |
| L insula CT | R pars orbitalis GMV | L superior parietal GMV | L superior parietal GMV | |
| R temporal pole CT | L rostral middle frontal GMV | R lingual CT | L posterior cingulate GMV | |
| R banks of superior temporal sulcus CT | ||||
| R fusiform GMV | ||||
| R middle temporal GMV | ||||
Note: GMV, gray matter volume; CT, cortical thickness; L, left; R, right; CSA, cortical surface area.
All four factor scores were significant predictors of the positive subscale in separate regression models with covariates (temporal CT score: B = −1.04, P = .00035; frontal GMV score: B = −1.16, P = .000047; frontoparietal CT score: B = −0.66, P = .024; precuneus GMV-SA score: B = −1.0, P = .00071). When all four factor scores were entered into the same regression model with covariates, the temporal CT score (B = −0.80, P = .028) and frontal GMV score (B = −0.71, P = .032) remained significant predictors of the positive subscale (adjusted R 2 = .176). There was no significant diagnosis by factor interactions. None of the factor scores were significant predictors of the negative subscale.
Negative Symptom Subscale.
Fourteen of 212 regions correlated with the negative subscale upon initial screen (P < .05, uncorrected). Scree test suggested one factor, and extraction of more than one factor led to degenerate factor structure and cross-loading of variables. Further evaluation supported a frontal GMV-SA factor (table 3, supplementary table 4). No rotation was performed because of the single factor structure. This factor score was a significant predictor of the negative subscale (B = −0.99, P = .00032, adjusted R 2 = .139). The diagnosis by factor interaction was not significant. Finally, this factor was a significant predictor of the positive subscale (B = −0.87, P = .0019, adjusted R 2 = .150).
Discussion
This study examined correlations between symptom dimensions and regional GMV, CT, and CSA in a group of individuals with schizophrenia, schizoaffective disorder, or bipolar I disorder with psychotic features. Partial correlations were used to evaluate symptom correlations with individual regions, and factor analysis was used to summarize and compare structure-symptom relationships. The PANSS positive subscale correlated with reductions in both temporal and frontal structural measures. Among regions correlating with the positive subscale, factor analysis identified a temporal CT factor, a frontal GMV factor, a frontoparietal CT factor, and a precuneus GMV-SA factor, of which the temporal CT and frontal GMV factors independently predicted positive symptom severity when all four factors were jointly entered in a regression model.
Temporal regions have been previously implicated in the production of psychotic symptoms. Functional MRI studies have noted activation of temporal regions during real-time auditory hallucinations,44 whereas structural MRI studies have found associations between temporal regions and both hallucinations4,45 and thought disorder.46 Although frontal associations with the positive subscale have been observed less frequently in the literature, several other studies have found correlations between the positive subscale and reductions in overall frontal volume47 or GMV in regions such as the inferior frontal gyrus.48,49 Abnormalities in frontal regions could impact cognitive processes of working memory, attention, and language processing,50 contributing to positive symptoms such as disorganization and delusions.
Comparing diagnostic groups, structure-symptom correlations were larger in the schizophrenia group than in the two other diagnostic categories. However, our factor analysis did not find significant interactions between diagnosis and structural factors on symptom subscales, indicating no major impact of diagnosis on structure-symptom correlations. Overall, findings in the schizophrenia group may reflect their higher level of subtle brain pathology, which may in turn be associated with their more chronic and persistent psychotic symptoms compared with schizoaffective and bipolar I disorders. Differences in exposure to antipsychotic medication are unlikely to completely account for the larger effect sizes of correlations in schizophrenia. Although individuals with schizophrenia typically have greater exposure to antipsychotics than individuals with bipolar disorder, our secondary analyses found that antipsychotic medication dose did not have a major impact on effect sizes of correlations.
The PANSS negative subscale correlated inversely with CSA of right frontal regions, and factor analysis identified a frontal GMV-SA factor among those regions that correlated with the negative subscale. These results corroborate prior studies reporting correlations between frontal reductions and negative symptoms,13,51–53 and inverse correlations between regional cerebral blood flow and negative symptoms on positron emission tomography scans.54 Overall, the negative subscale demonstrated fewer correlations with structural measures than the positive subscale. This observation may indicate the relatively greater contribution of social and nonstructural biological influences in the manifestation of negative symptoms.
As predicted, CT and CSA diverged in their associations with symptom subscales. CT reductions exhibited more correlations with symptom subscales, particularly the positive subscale, than CSA. Interestingly, one recent trans-diagnostic study of schizophrenia and bipolar I found more widespread regional reductions in CT than in CSA.20 This study (and an earlier study with the same subject sample) did not find associations of either type of structural measure with symptom subscales.20,22 Our findings suggest that CT may be more closely associated with symptoms of psychosis than CSA. These findings may reflect the distinct neurobiological processes underlying these two aspects of structure. As mentioned earlier, recent research indicates that CT and CSA may have distinct genetic influences,28 may follow independent developmental trajectories in childhood,27 and may not be highly correlated with each other.20 CT has been shown to fluctuate in response to environmental factors such as cannabis use55,56 and childhood trauma,56 and may represent a “state” marker that tracks more closely with fluctuating positive symptoms than CSA. Given the more widespread correlations of CT with the symptom dimensions, particularly the positive subscale, future investigations may wish to focus on pathophysiological processes of CT as driving the development of psychosis.
Notably, although correlations were significant, their magnitudes were small, indicating that other factors likely make independent contributions to symptom severity. Symptom severity is partly driven by social factors, such as education and socioeconomic status, whose neurobiological effects may not be captured by structural measures. In addition, small effect sizes of structure-symptom correlations may complement the findings of neuropathology studies of schizophrenia, which observe subtle reductions in cortical neuropil and volumes of neuronal cell bodies, rather than loss of neurons.57,58 The effect sizes of our correlations may also reflect the influence of processes that enlarge structural measures. For example, inflammation, which may be important in early stages of schizophrenia,59 could lead to structural enlargement through free water retention, thus reducing the strength of inverse correlations between structure and symptoms. Overall, the small effect sizes of structure-symptom correlations corroborate the concept of psychosis as a disorder of network connectivity,60 involving subtle neurochemical and neurophysiological alterations in the interactions between brain regions. Our observations of frontal and temporal contributions to psychosis are consistent with the possibility that frontotemporal connectivity may be of particular importance in the pathogenesis of positive symptoms.61
This study had several strengths. This is one of the largest sample sizes thus far in which this question has been examined. Inclusion of all three diagnostic categories permitted the examination of associations in psychosis in a trans-diagnostic fashion, and inclusion of CT and CSA permitted exploration of their relative associations with symptom subscales. Many potential confounding factors were included in the analysis. In addition, the subject sample consisted of clinically stable, chronically ill individuals. Structural and physiological abnormalities may change across the course of illness in psychosis,62 and structural alterations may be more relevant in understanding persistent symptoms in a chronic population. Thus, our results may reflect more stable correlations between brain structure and residual, treatment-resistant psychopathology.
There were several limitations. Because data were cross-sectional, it was not possible to draw conclusions about causal relationships. The region-of-interest analysis necessitated a heavy correction for multiple comparisons, which may have obscured some findings. In addition, data on subjects’ lifetime history of antipsychotic use were not collected, although current antipsychotic use was included. Longitudinal antipsychotic use may be associated with gray matter changes63 and may have contributed to structure-symptom correlations in this study. Finally, neuropsychological test results were not analyzed here, although they have been reported elsewhere in this sample.64
In conclusion, among a combined group of individuals with schizophrenia, schizoaffective, and bipolar I disorders, the PANSS positive subscale was inversely correlated with GMV and cortical thickness in frontal and temporal regions, whereas the PANSS negative subscale was inversely correlated with frontal cortical surface area and GMV. Overall, cortical thickness appeared more strongly associated with psychopathology, particularly the positive subscale, than cortical surface area. However, the magnitudes of all correlations were low. These results lend support to associations between structural brain alterations and severity of psychopathology.
Supplementary Material
Supplementary material is available at http://schizophre niabulletin.oxfordjournals.org.
Funding
National Institute of Mental Health (MH078113 to M.S.K, MH077945 to G.D.P., MH077852 to G.T., MH077851 to C.A.T., and MH077862 to J.A.S.
Supplementary Material
Acknowledgments
The authors thank Dr Gunvant Thaker and Dr Will Carpenter for their contributions to the Bipolar-Schizophrenia Network for Intermediate Phenotypes consortium. Dr Keshavan has received research support from Sunovion and GlaxoSmithKline. Dr Padmanabhan has received grant support from the Janssen Academic Research Mentorship program. Dr Pearlson has served on an advisory panel for Bristol-Myers Squibb. Dr Sweeney has been on advisory boards for Bristol-Myers Squibb, Eli Lilly, Pfizer, Roche, and Takeda and has received grant support from Janssen. Dr Tamminga has the following disclosures to make: Intracellular Therapies (ITI, Inc)—Advisory Board, drug development; PureTech Ventures—Ad Hoc Consultant; Eli Lilly Pharmaceuticals—Ad Hoc Consultant; Sunovion—Ad Hoc Consultant; Astellas—Ad Hoc Consultant; Cypress Bioscience—Ad Hoc Consultant; Merck—Ad Hoc Consultant; International Congress on Schizophrenia Research—Organizer, unpaid volunteer; National Alliance on Mental Illness—Council Member, unpaid volunteer; American Psychiatric Association—Deputy Editor. The other authors report no disclosures.
References
- 1. Shepherd AM, Laurens KR, Matheson SL, Carr VJ, Green MJ. Systematic meta-review and quality assessment of the structural brain alterations in schizophrenia. Neurosci Biobehav Rev. 2012;36:1342–1356. [DOI] [PubMed] [Google Scholar]
- 2. Selvaraj S, Arnone D, Job D, et al. Grey matter differences in bipolar disorder: a meta-analysis of voxel-based morphometry studies. Bipolar Disord. 2012;14:135–145. [DOI] [PubMed] [Google Scholar]
- 3. Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E. The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatry. 2008;165:1015–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barta PE, Pearlson GD, Powers RE, Richards SS, Tune LE. Auditory hallucinations and smaller superior temporal gyral volume in schizophrenia. Am J Psychiatry. 1990;147:1457–1462. [DOI] [PubMed] [Google Scholar]
- 5. Flaum M, O’Leary DS, Swayze VW, II, Miller DD, Arndt S, Andreasen NC. Symptom dimensions and brain morphology in schizophrenia and related psychotic disorders. J Psychiatr Res. 1995;29:261–276. [DOI] [PubMed] [Google Scholar]
- 6. Lui S, Deng W, Huang X, et al. Association of cerebral deficits with clinical symptoms in antipsychotic-naive first-episode schizophrenia: an optimized voxel-based morphometry and resting state functional connectivity study. Am J Psychiatry. 2009;166:196–205. [DOI] [PubMed] [Google Scholar]
- 7. Nestor PG, Onitsuka T, Gurrera RJ, et al. Dissociable contributions of MRI volume reductions of superior temporal and fusiform gyri to symptoms and neuropsychology in schizophrenia. Schizophr Res. 2007;91:103–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Onitsuka T, Shenton ME, Salisbury DF, et al. Middle and inferior temporal gyrus gray matter volume abnormalities in chronic schizophrenia: an MRI study. Am J Psychiatry. 2004;161:1603–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Turetsky B, Cowell PE, Gur RC, Grossman RI, Shtasel DL, Gur RE. Frontal and temporal lobe brain volumes in schizophrenia. Relationship to symptoms and clinical subtype. Arch Gen Psychiatry. 1995;52:1061–1070. [DOI] [PubMed] [Google Scholar]
- 10. Fannon D, Chitnis X, Doku V, et al. Features of structural brain abnormality detected in first-episode psychosis. Am J Psychiatry. 2000;157:1829–1834. [DOI] [PubMed] [Google Scholar]
- 11. Molina V, Reig S, Pascau J, et al. Anatomical and functional cerebral variables associated with basal symptoms but not risperidone response in minimally treated schizophrenia. Psychiatry Res. 2003;124:163–175. [DOI] [PubMed] [Google Scholar]
- 12. Benoit A, Bodnar M, Malla AK, Joober R, Lepage M. The structural neural substrates of persistent negative symptoms in first-episode of non-affective psychosis: a voxel-based morphometry study. Front Psychiatry. 2012;3:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baare WF, Hulshoff Pol HE, Hijman R, Mali WP, Viergever MA, Kahn RS. Volumetric analysis of frontal lobe regions in schizophrenia: relation to cognitive function and symptomatology. Biol Psychiatry. 1999;45:1597–1605. [DOI] [PubMed] [Google Scholar]
- 14. Bora E, Fornito A, Radua J, et al. Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr Res. 2011;127:46–57. [DOI] [PubMed] [Google Scholar]
- 15. Lacerda AL, Hardan AY, Yorbik O, Vemulapalli M, Prasad KM, Keshavan MS. Morphology of the orbitofrontal cortex in first-episode schizophrenia: relationship with negative symptomatology. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:510–516. [DOI] [PubMed] [Google Scholar]
- 16. Volpe U, Mucci A, Quarantelli M, Galderisi S, Maj M. Dorsolateral prefrontal cortex volume in patients with deficit or nondeficit schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2012;37:264–269. [DOI] [PubMed] [Google Scholar]
- 17. Nesvag R, Saetre P, Lawyer G, Jonsson EG, Agartz I. The relationship between symptom severity and regional cortical and grey matter volumes in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:482–490. [DOI] [PubMed] [Google Scholar]
- 18. Kuperberg GR, Broome MR, McGuire PK, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60:878–888. [DOI] [PubMed] [Google Scholar]
- 19. Oertel-Knöchel V, Knöchel C, Rotarska-Jagiela A, et al. Association between psychotic symptoms and cortical thickness reduction across the schizophrenia spectrum. Cereb Cortex. 2013;23:61–70. [DOI] [PubMed] [Google Scholar]
- 20. Rimol LM, Nesvåg R, Hagler DJ, et al. Cortical volume, surface area, and thickness in schizophrenia and bipolar disorder. Biol Psychiatry. 2012;71:552–560. [DOI] [PubMed] [Google Scholar]
- 21. Lyoo IK, Sung YH, Dager SR, et al. Regional cerebral cortical thinning in bipolar disorder. Bipolar Disord. 2006;8:65–74. [DOI] [PubMed] [Google Scholar]
- 22. Rimol LM, Hartberg CB, Nesvåg R, et al. Cortical thickness and subcortical volumes in schizophrenia and bipolar disorder. Biol Psychiatry. 2010;68:41–50. [DOI] [PubMed] [Google Scholar]
- 23. Hartberg CB, Sundet K, Rimol LM, et al. Brain cortical thickness and surface area correlates of neurocognitive performance in patients with schizophrenia, bipolar disorder, and healthy adults. J Int Neuropsychol Soc. 2011;17:1080–1093. [DOI] [PubMed] [Google Scholar]
- 24. Mountcastle VB. Modality and topographic properties of single neurons of cat’s somatic sensory cortex. J Neurophysiol. 1957;20:408–434. [DOI] [PubMed] [Google Scholar]
- 25. Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. [DOI] [PubMed] [Google Scholar]
- 26. Rakic P. A small step for the cell, a giant leap for mankind: a hypothesis of neocortical expansion during evolution. Trends Neurosci. 1995;18:383–388. [DOI] [PubMed] [Google Scholar]
- 27. Wierenga LM, Langen M, Oranje B, Durston S. Unique developmental trajectories of cortical thickness and surface area. Neuroimage. 2014;87:120–126. [DOI] [PubMed] [Google Scholar]
- 28. Panizzon MS, Fennema-Notestine C, Eyler LT, et al. Distinct genetic influences on cortical surface area and cortical thickness. Cereb Cortex. 2009;19:2728–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xiao Y, Lui S, Deng W, et al. Altered cortical thickness related to clinical severity but not the untreated disease duration in schizophrenia [published online ahead of print December 18, 2013]. Schizophr Bull. 10.1093/schbul/sbt177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Padmanabhan J, Hooker CI, Keshavan MS. Brain evolution, language and psychopathology in schizophrenia. In: Brambilla P, Marini A, eds. Brain Evolution, Language and Psychopathology in Schizophrenia. New York: Routledge; 2013. [Google Scholar]
- 31. Tamminga CA, Ivleva EI, Keshavan MS, et al. Clinical phenotypes of psychosis in the Bipolar and Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry. 2013;170:1263–1274. [DOI] [PubMed] [Google Scholar]
- 32. Ivleva EI, Bidesi AS, Keshavan MS, et al. Gray matter volume as an intermediate phenotype for psychosis: Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry. 2013;170:1285–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. First M, Spitzer R, Gibbon M, et al. The structured clinical interview for DSM-III-r personality disorders (SCID-II). Part II. Multi-site test-retest reliability study. J Pers Disord. 1995;9:92–104. [Google Scholar]
- 34. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 35. Fischl B. FreeSurfer. Neuroimage. 2012;62:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–802. [Google Scholar]
- 37. Yeh PH, Zhu H, Nicoletti MA, Hatch JP, Brambilla P, Soares JC. Structural equation modeling and principal component analysis of gray matter volumes in major depressive and bipolar disorders: differences in latent volumetric structure. Psychiatry Res. 2010;184:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Colibazzi T, Zhu H, Bansal R, Schultz RT, Wang Z, Peterson BS. Latent volumetric structure of the human brain: exploratory factor analysis and structural equation modeling of gray matter volumes in healthy children and adults. Hum Brain Mapp. 2008;29:1302–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Fabrigar L, Wegener D, MacCallum R, Strahan E. Evaluating the use of exploratory factor analysis in psychological research. Psychol Methods. 1999;4:272–299. [Google Scholar]
- 40. Cattell R. The scree test for number of factors. Multivariate Behav Res. 1966;1:245–276. [DOI] [PubMed] [Google Scholar]
- 41. Stevens J. Applied Multivariate Statistics for the Social Sciences. New York, NY: Routledge, Taylor & Francis Group, LLC; 2009. [Google Scholar]
- 42. Costello A, Osborne J. Best practices in exploratory factor analysis: four recommendations for getting the most out of your analysis. Pract Assess Res Eval. 2005;10:1–9. [Google Scholar]
- 43. Thurstone L. The Vectors of Mind. Chicago: University of Chicago Press; 1935. [Google Scholar]
- 44. Jardri R, Pouchet A, Pins D, Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am J Psychiatry. 2011;168:73–81. [DOI] [PubMed] [Google Scholar]
- 45. Palaniyappan L, Balain V, Radua J, Liddle PF. Structural correlates of auditory hallucinations in schizophrenia: a meta-analysis. Schizophr Res. 2012;137:169–173. [DOI] [PubMed] [Google Scholar]
- 46. Shenton ME, Kikinis R, Jolesz FA, et al. Abnormalities of the left temporal lobe and thought disorder in schizophrenia. A quantitative magnetic resonance imaging study. N Engl J Med. 1992;327:604–612. [DOI] [PubMed] [Google Scholar]
- 47. Andreasen NC, Nopoulos P, Magnotta V, Pierson R, Ziebell S, Ho BC. Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biol Psychiatry. 2011;70:672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Iwashiro N, Suga M, Takano Y, et al. Localized gray matter volume reductions in the pars triangularis of the inferior frontal gyrus in individuals at clinical high-risk for psychosis and first episode for schizophrenia. Schizophr Res. 2012;137:124–131. [DOI] [PubMed] [Google Scholar]
- 49. Suga M, Yamasue H, Abe O, et al. Reduced gray matter volume of Brodmann’s Area 45 is associated with severe psychotic symptoms in patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2010;260:465–473. [DOI] [PubMed] [Google Scholar]
- 50. Antonova E, Sharma T, Morris R, Kumari V. The relationship between brain structure and neurocognition in schizophrenia: a selective review. Schizophr Res. 2004;70:117–145. [DOI] [PubMed] [Google Scholar]
- 51. Wible CG, Anderson J, Shenton ME, et al. Prefrontal cortex, negative symptoms, and schizophrenia: an MRI study. Psychiatry Res. 2001;108:65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Berge D, Carmona S, Rovira M, Bulbena A, Salgado P, Vilarroya O. Gray matter volume deficits and correlation with insight and negative symptoms in first-psychotic-episode subjects. Acta Psychiatr Scand. 2011;123:431–439. [DOI] [PubMed] [Google Scholar]
- 53. Chua SE, Wright IC, Poline JB, et al. Grey matter correlates of syndromes in schizophrenia. A semi-automated analysis of structural magnetic resonance images. Br J Psychiatry. 1997;170:406–410. [DOI] [PubMed] [Google Scholar]
- 54. Lahti AC, Weiler MA, Holcomb HH, Tamminga CA, Carpenter WT, McMahon R. Correlations between rCBF and symptoms in two independent cohorts of drug-free patients with schizophrenia. Neuropsychopharmacology. 2006;31:221–230. [DOI] [PubMed] [Google Scholar]
- 55. Rais M, van Haren NE, Cahn W, et al. Cannabis use and progressive cortical thickness loss in areas rich in CB1 receptors during the first five years of schizophrenia. Eur Neuropsychopharmacol. 2010;20:855–865. [DOI] [PubMed] [Google Scholar]
- 56. Habets P, Marcelis M, Gronenschild E, Drukker M, van Os J, (G.R.O.U.P) GRaOoP. Reduced cortical thickness as an outcome of differential sensitivity to environmental risks in schizophrenia. Biol Psychiatry. 2011;69:487–494. [DOI] [PubMed] [Google Scholar]
- 57. Harrison PJ. The neuropathology of schizophrenia. A critical review of the data and their interpretation. Brain. 1999;122(Pt 4):593–624. [DOI] [PubMed] [Google Scholar]
- 58. Glantz LA, Lewis DA. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch Gen Psychiatry. 2000;57:65–73. [DOI] [PubMed] [Google Scholar]
- 59. Pasternak O, Westin CF, Bouix S, et al. Excessive extracellular volume reveals a neurodegenerative pattern in schizophrenia onset. J Neurosci. 2012;32:17365–17372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lynall ME, Bassett DS, Kerwin R, et al. Functional connectivity and brain networks in schizophrenia. J Neurosci. 2010;30:9477–9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lawrie SM, Buechel C, Whalley HC, Frith CD, Friston KJ, Johnstone EC. Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol Psychiatry. 2002;51:1008–1011. [DOI] [PubMed] [Google Scholar]
- 62. Ren W, Lui S, Deng W, et al. Anatomical and functional brain abnormalities in drug-naive first-episode schizophrenia. Am J Psychiatry. 2013;170:1308–1316. [DOI] [PubMed] [Google Scholar]
- 63. Ho BC, Andreasen NC, Ziebell S, Pierson R, Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry. 2011;68:128–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hill SK, Reilly JL, Keefe RS, et al. Neuropsychological impairments in schizophrenia and psychotic bipolar disorder: findings from the Bipolar and Schizophrenia Network on Intermediate Phenotypes (B-SNIP) study. Am J Psychiatry. 2013;170:1275–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.