Abstract
Objectives:
Although recent studies have demonstrated that patients with schizophrenia and healthy controls did not differ in the speed of age-related decline in cortical thickness and performances on cognitive tests, hemodynamic changes assessed by functional neuroimaging remain unclear. This study investigated age effects on regional brain cortical activity to determine whether there is similar age-related decline in cortical activity as those observed in cortical thickness and cognitive test performance.
Method:
A total of 109 patients with schizophrenia (age range: 16–59 y) and 106 healthy controls (age range: 16–59 y) underwent near-infrared spectroscopy (NIRS) while performing a verbal fluency test (VFT). Group comparison of cortical activity was examined using 2-tailed t tests, adopting the false discovery rate method. The relationship between age and cortical activity was investigated using correlational and multiple regression analyses, adjusting for potential confounding variables. A 2-way ANOVA was conducted to investigate differences in the age effects between diagnostic groups.
Results:
The patient group exhibited significantly decreased cortical activity in several regions of the frontotemporal cortices. However, slopes of age-dependent decreases in cortical activity were similar between patients and healthy individuals at the bilateral frontotemporal regions.
Conclusions:
Our study showed no significant between-group differences in the age-related decline in cortical activity, as measured by NIRS, over the frontotemporal regions during a VFT. The results of our study may indicate a decrease in cortical activity in a relatively limited period around illness onset rather than continuously progressing over the course of the illness.
Key words: schizophrenia, near infrared spectroscopy, age effect, NIRS
Introduction
Schizophrenia historically has been viewed as a chronically deteriorating disease, a concept that has been reinforced by several structural MRI findings of progressive tissue loss in the brain.1,2 However, this historical concept was recently questioned in several studies, including a meta-analysis conducted by Zipursky et al,3 who proposed that the progressive brain changes revealed by MRI in patients with schizophrenia (SZ) might be due to other factors, such as antipsychotic medication, substance use, or lifestyle, rather than the disease course itself.
Recent MRI studies have begun to focus on progressive changes in cortical thickness because cortical thickness is assumed to reflect the underlying neuropathology of schizophrenia.4 It is interesting to note, however, that several cross-sectional studies have found no interaction effects between age and diagnostic group on cortical thickness variation among patients with first-episode psychosis (FEP) or chronic schizophrenia (ChSZ).5–7 Moreover, Nesvag et al8,9 found that the decreases in frontotemporal cortical thickness associated with aging were similar in ChSZ and healthy controls (HC) in both cross-sectional and longitudinal studies. Similarly, Kubota et al10 demonstrated similar age-related decline in cortical thickness between SZ and HC, both globally and regionally, in the prefrontal and temporal cortices. On the other hand, other longitudinal studies11,12 demonstrated accelerated decreases in regional cortical thickness in ChSZ.
With regard to cognitive function, Fucetola et al13 found that, compared with HC, SZ scored lower but demonstrated similar age-related declines in most neuropsychological functions, with the exception of abstraction ability. Moreover, Bora et al14 also found no progressive cognitive decline in patients with ultrahigh risk and first-episode psychosis at follow-ups, indicating cognitive deficits are already established before prodromal phases of psychosis. These findings in neuroimaging and neuropsychological studies may indicate that pathological alterations in schizophrenia may occur at a relatively early stage of illness and are within the normal range in later stages.10,13
However, relatively few functional neuroimaging studies have been conducted comparing age effects for SZ and HC. In a study using positron emission tomography with 18-fluoro-2-deoxyglucose (FDG-PET), Siegel et al,15 demonstrated that, although there was no significant relationship between age and whole-brain glucose metabolic rate, SZ showed a greater decrease in their whole-brain glucose metabolic rate, compared with HC, in the lateral and medial superior frontal cortices during the continuous performance test. Similarly, an FDG-PET study that used a memory task found more pronounced age-related decreases in the lateral, medial-frontal, and anterior-temporal regions in SZ.16 However, the small sample sizes of these PET studies make it difficult to draw definite conclusions.17 Hence, studies with larger numbers of subjects and those using different neuroimaging modalities are warranted to better understand the relationship between aging and cortical activity.
Multichannel near-infrared spectroscopy (NIRS), a functional neuroimaging technology widely used in recent years, can measure the hemodynamics over the surface of the cortices of the bilateral frontotemporal regions.18 This technique enables the detection of spatiotemporal characteristics of brain function by measuring the concentrations of oxy-hemoglobin ([oxy-Hb]) and deoxy-hemoglobin ([deoxy-Hb]), which are assumed to reflect the regional cerebral blood volume as demonstrated by good correlations with functional MRI (fMRI) signals.19 Previous studies have revealed age-related changes in [oxy-Hb] during a letter version of the verbal fluency test (VFT) in HC20,21 or SZ.22 In schizophrenia, we have demonstrated progressive declines in cortical activities as the clinical course progresses (Ultrahigh risk vs FEP vs chronic SZ)23 in our previous work. However, to the best of our knowledge, a comparison of the effects of age on cortical activity between the 2 groups has not been addressed in previous NIRS studies.
Against this background, this study aimed to use NIRS in a large sample of participants to elucidate whether age-related changes in brain cortical activity would differ between SZ and HC during a VFT.
Materials and methods
Participants
One hundred and nine SZ and 106 HCs participated in this study (table 1). There were no significant differences regarding the distributions of age, sex, or premorbid IQ between the 2 groups. All participants were native Japanese speakers. This study complied with the Declaration of Helsinki and was approved by the Research Ethics Committee of the University of Tokyo Hospital (approval No. 630–6). All participants received a complete explanation of the study and gave written informed consent. The SZ group included 28 first-episode patients and 81 chronic patients (age range: 16–59 y), and the HC group included healthy individuals (age range: 16–59 y). Most participants in the SZ group were receiving antipsychotic medication (drug naive [n = 8], typical [n = 24], atypical [n = 50], typical and atypical [n = 26], and 1 person for whom data were missing). Patients were recruited from both outpatient and inpatient services at the University of Tokyo Hospital, the University of Tokyo Health Service Center, and other psychiatry clinics. Patients in this study were diagnosed by 1 of 3 experienced psychiatrists (S.K., R.T., and/or K.K.) according to the criteria defined by the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV).24 For those participants referred from other psychiatry clinics, we would perform structured clinical interview for DSM-IV Axis I Disorders (SCID)25 and/or discuss with their primary psychiatrists to confirm the diagnoses. Healthy participants were recruited from various routes, including public recruitment boards, university recruitment boards, Internet referral, hospital staff and via other participants. All healthy participants were screened using the SCID-Non-Patient edition (SCID-NP) or the Mini-International Neuropsychiatric Interview.26 Exclusion criteria for both groups were neurological illness, traumatic brain injury with any known cognitive consequences or loss of consciousness for more than 5 minutes, a history of electroconvulsive therapy, a history of pervasive developmental disorder, or alcohol/substance abuse or addiction.
Table 1.
Basic Characteristics of Study Participants
| Healthy Controls (N = 106) | Schizophrenia Patients (N = 109) | P value | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Age (y) | 31.9 (7.2) | 33.0 (10.4) | .37 |
| Sex (M/F)a | 53/53 | 55/54 | 1.00 |
| Premorbid IQ | 103.9 (8.6) | 102.4 (11.0) | .26 |
| Edinburghb | 91.5 (17.5) | 88.6 (27.1) | .34 |
| LFT performance | 14.3 (4.1) | 13.2 (4.4) | .07 |
| GAF | 41.7 (12.4) | ||
| DOI (wk) | 428.4 (436.1) | ||
| PANSS | |||
| Positive | 16.2 (5.2) | ||
| Negative | 21.0 (6.7) | ||
| General psychopathology | 37.7 (8.9) | ||
| Total | 74.9 (18.3) | ||
| Medicationc | |||
| Chlorpromazine | 655.8 (623.3) | ||
| Diazepam | 11.7 (15.4) | ||
| Bipiden | 2.5 (2.7) | ||
Note: LFT, Letter Fluency Test; GAF, Global Assessment of Functioning scale; DOI, duration of illness; PANSS, Positive and Negative Symptom Scale.
aChi square test was used for testing group difference. Otherwise, t test was used.
bRight-handedness was defined as >70 points according to Oldfield’s Edinburgh Inventory.
cOne patient’s data of medication was missing.
Clinical Measures
The Global Assessment of Functioning (GAF) scale and the Positive and Negative Syndrome Scale (PANSS)27 were used to assess a patient’s functioning level and symptoms and were administered on the same day as the NIRS measurements. Handedness was evaluated according to the Edinburgh Inventory,28 where right-handedness is defined as a score more than 70 points. Premorbid IQ was estimated with the Japanese version of the National Adult Reading Test.29 If patients were taking antipsychotics, benzodiazepines, or antiparkinsonian agents, we calculated the chlorpromazine, diazepam, or biperiden equivalent doses.30,31
Cognitive Activation Test
Participants were administered the 160-second block-design VFT, which is an effective cognitive test for NIRS measurement in schizophrenia.22,32–35 The 160-second block-design VFT contains 3 different time periods: a 30-second pretask period, a 60-second task period, and a 70-second posttask period. In the 30-second pretask and 70-second posttask periods, patients were instructed to say Japanese vowels (/a/, /i/, /u/, /e/, and /o/) repeatedly to control for and remove task-related motion artifacts during the task periods. During the 60-second task period, patients were instructed to say as many words as possible that started with a phonological syllable presented as an audible instruction by a computer. The task period consisted of 3 continuous 20-second subperiods that were initiated by a single syllable selected from 9 possible syllables (first, /to/, /a/, or /na/; second, /i/, /ki/, or /se/; third, /ta/, /o/,or /ha/). Transitions between the 20-second subperiods were continuous. We recorded the total number of corrected words generated during the task as an index of task performance.
NIRS Instrument
We used a 52-channel NIRS instrument (ETG-4000; Hitachi Medical Co) to measure changes in hemoglobin concentration. The NIRS probe attachments were thermoplastic 3×11 shell sets with 52 channels. The lowest probe line was set along the Fp1-Fp2 line, as defined by the international 10–20 system used in electroencephalography. The distance between pairs of source and detector probes was set to 3.0cm. We defined the measurement area between each probe-set pair as one “channel,” which was sufficient to measure depths between 20mm and 30mm from the scalp. This depth corresponds to the surface of the cerebral cortex.
NIRS measurements were performed while participants sat in a chair with their eyes open with the probe attachment resting on their heads. Participants were instructed to maintain a relaxed state and avoid voluntary movements to minimize motion artifacts. The NIRS instrument measures changes in both oxygenated and deoxygenated hemoglobin concentrations using 2 wavelengths (695nm and 830nm) of near-infrared light (indicated as mM) on the basis of the Beer-Lambert law.36 We could not measure the absolute path length from the scalp to the cerebral cortex; therefore, we recorded hemoglobin concentrations from baseline to activation periods. Relative changes in hemoglobin concentration were indicated by mM·mm.
Acquired NIRS signals were acquired using a time resolution of 0.1 second. We set the moving average window to 10 seconds to remove short-term artifacts such as heartbeats and small movements. We determined changes in the task-related signal by calculating a linear fit to 2 baseline periods that included the last 10 second of the pretask period and the last 10 second of the posttask period. Obvious waveform artifacts were removed using a computer program adopted in our previous studies.22,37 This program excluded whole signal changes for each rejected channel; thus, the number of available channels varied among individuals (all patients: 31–52 [mean, 48.0; SD, 4.3]; SZ group: 31–52 [mean, 48.3; SD, 4.3]; HC group: 34–52 [mean, 47.6; SD, 4.3]; ns).
The spatial information of each channel was estimated by using data from the Functional Brain Science Laboratory at Jichi Medical University in Japan.38,39 According to the LONI Probabilistic Brain Atlas (LPBA40),40 NIRS channels can record functional hemodynamics within the frontal, temporal, and parietal cortices, bilaterally. Similar to our previous study,34,35 we anatomically labeled NIRS channels only after the LPBA region of highest probability was determined (see supplementary material I for LPBA40 anatomical labels). We used the mean changes in [oxy-Hb] measured during the VFT as an index of cortical activity because [oxy-Hb] better reflects brain cortical activity and demonstrates stronger correlations with blood oxygenation level–dependent signals measured by fMRI.41,42
Statistical Analysis
Group Comparison of Cortical Activity During the VFT.
Two independent sample t tests were conducted to examine group differences in demographic variables and mean [oxy-Hb] changes during the VFT in 52 channels, adopting the false discovery rate (FDR) method.43 Sex differences were examined using a chi square test.
Correlational Analyses Between Age and Cortical Activity in HC and SZ Groups.
First, Pearson correlations were calculated between age and mean [oxy-Hb] changes in 52 channels for both groups, using FDR to correct for multiple comparisons. We set the value specifying the maximum FDR to 0.05 so that there were no more than 5% false positives on average.43 Second, to elucidate the independent contributions of age to mean [oxy-Hb] changes in those channels that showed significant correlations, we performed stepwise multiple regression analyses for both groups. In these analyses, mean [oxy-Hb] change was the dependent variable, and we controlled for other potential confounding variables such as gender (dummy parameterized, male = 1, female = 0), premorbid IQ, and VFT performance in both groups and added factors such as GAF score, PANSS score, and antipsychotic dose in the analyses of the SZ group, with a probability of F for conservative entry and removal criteria of 0.05 and 0.1, respectively. For significant findings, effect sizes were indicated using the standardized regression coefficient (β). Because multiple regressions focused on NIRS channels where brain activity was shown to be significantly correlated with age after FDR-method, no further correction was applied and predictors were considered significant at P < .05.34 In addition, we also investigated the correlation between age and VFT performance.
Comparison of Age Effects Between Groups.
First, we conducted a 2-way ANOVA to evaluate the interaction between age (104 younger and 111 older subjects, divided by the median age, 32.0 y; the younger group: mean age 25.4, SD 4.2 y; the older group: mean age 39.2, SD 7.0 y) and diagnostic groups (HC = 1, SZ = 0). Second, we compared age regression slopes of the HC and SZ groups. Two-tailed t tests were applied to compare z-transformed r coefficients of age and cortical activity between the 2 groups. A FDR-corrected P < .05 was considered significant. All data were analyzed using SPSS 18.0 (IBM Inc).
Results
Cortical Activity During the VFT in Both Groups
Compared with HC group, the SZ group demonstrated significantly lower cortical activity in 31 channels during the VFT (ch10, 11, 17, 19, 21, 23–32, 34, 36, 38–42, and 44–52; FDR-corrected P < .0298). These channels were approximately located at the bilateral middle frontal gyrus (MFG), bilateral inferior frontal gyrus (IFG), bilateral precentral gyrus, bilateral middle temporal gyrus (MTG), bilateral superior temporal gyrus (STG), left postcentral gyrus, and left superior frontal gyrus (SFG).
Correlational Analyses of Cortical Activity and Age in Both Groups
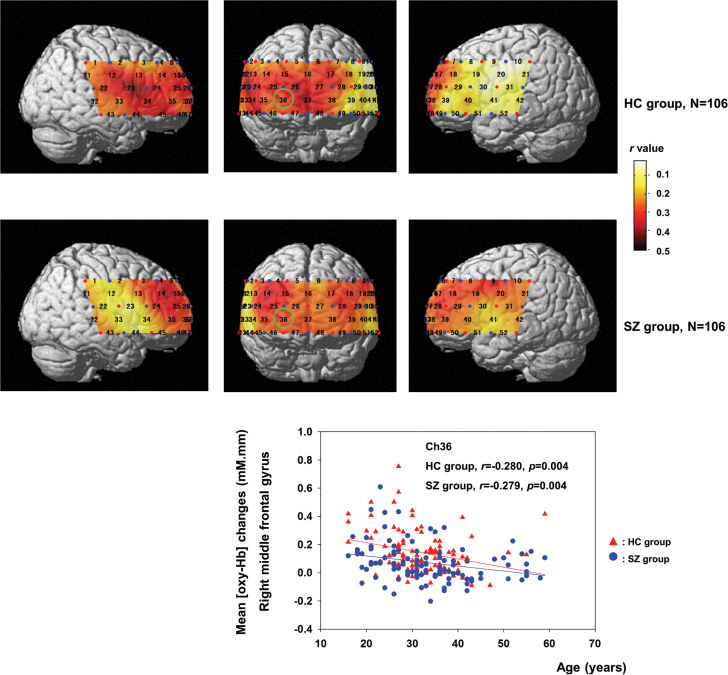
In the SZ group, 39 channels demonstrated significant correlations between age and cortical activity (ch3–5, 7–11, 13–21, 24–29, 31–32, 35–40, 42–43, and 45–50; FDR-corrected P < .0375; r = −.214 to −.378). In the HC group, there were significant correlations between age and cortical activity in 30 channels (ch1, 4, 6, 11–18, 22–28, 32–38, and 44–48; FDR-corrected P < .0289; r = −.218 to −.456), as shown in figure 1.
Fig. 1.
This figure illustrates the correlation coefficients (r) for 52 channels in patients with schizophrenia and healthy individuals. The Arabic numerals represent channel locations. The scatter plot demonstrates the age regression slope for channel 36 (as denoted by a green circle), approximately located at the right middle frontal gyrus.
Among those 39 channels demonstrating significant correlation with age in the SZ group, multiple regression analyses revealed significant contributions of age in 16 channels (β = −.193 to −.332; uncorrected P < .05) among other confounding factors (table 2). In addition, the total PANSS score did not show significant contributions to brain cortical activity. On the other hand, significant contributions were found for male in 28 channels (ch8–10, 13–14, 18–21, 24–29, 31–32, 35–36, 38–40, 42–43, 46–47, and 49–50), GAF in 3 channels (ch19, 21, and 32), VFT performance in 7 channels (ch16–18, 26–27, 37, and 47), premorbid IQ in 9 channels (ch16–18, 26–27, 36–37, and 47–48), and antipsychotic dosage in 17 channels (ch8, 14, 19, 21, 24–25, 28–29, 32, 35, 38–40, 42, 46 and 49–50), as shown in table 2. Among those 30 channels demonstrating significant correlation with age in the HC group, significant contributions were found for age in 28 channels (ch6, 11–18, 22–28, 32–38, and 44–48), sex in 4 channels (ch4, 14–15, and 26), premorbid IQ in 1 channel (ch33), and VFT performance in 10 channels (ch1, 4, 14, 18, 25, 28, 35–36, 38, and 46), as shown in table 3.
Table 2.
Summary of Stepwise Multiple Regression Analysis in Channels Showing Significantly Correlated With Age in Schizophrenia Patients
| No. of Channelsa,b | R 2 | Adjusted R 2 | Independent Variables | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | Gender | CPZ | Other Factors | ||||||
| β | P | β | P | β | P | ||||
| Right middle frontal gyrus | |||||||||
| Ch3 | .075 | .065 | −.273 | .005 | |||||
| Ch4 | .110 | .102 | −.332 | <.001 | |||||
| Ch14 | .218 | .195 | −.290 | .002 | .218 | .023 | −.228 | .012 | |
| Ch15 | .108 | .099 | −.329 | <.001 | |||||
| Ch25 | .159 | .142 | .362 | <.001 | −.245 | <.001 | |||
| Ch26 | .197 | .173 | .238 | <.001 | VFT: β = −.225, P = .016; IQ: β = .324, P < .001 | ||||
| Ch36 | .138 | .121 | .277 | .003 | IQ: β = .212, P = .025 | ||||
| Ch47 | .214 | .190 | .240 | .010 | VFT: β = −.222, P = .021; IQ: β = .342, P < .001 | ||||
| Left middle frontal gyrus | |||||||||
| Ch7 | .064 | .055 | −.254 | .010 | |||||
| Ch8 | .136 | .119 | .317 | .001 | −.246 | .010 | |||
| Ch17 | .173 | .149 | −.277 | .003 | VFT: β = −.193, P = .049; IQ: β = .305, P = .002 | ||||
| Ch18 | .247 | .217 | −.226 | .014 | .197 | .033 | VFT: β = −.225, P = .013; IQ: β = .310, P < .001 | ||
| Ch19 | .195 | .171 | .302 | .001 | −.297 | .002 | GAF: β = −.232, P = .014 | ||
| Ch28 | .183 | .167 | .394 | <.001 | −.233 | .011 | |||
| Ch38 | .099 | .080 | .280 | .005 | −.201 | .042 | |||
| Ch49 | .122 | .104 | .299 | .002 | −.233 | .017 | |||
| Right inferior frontal gyrus | |||||||||
| Ch24 | .212 | .189 | −.222 | .019 | .297 | .002 | −.213 | .019 | |
| Ch35 | .176 | .151 | −.193 | .048 | .276 | .006 | −.200 | .033 | |
| Ch45 | .046 | .036 | −.216 | .034 | |||||
| Ch46 | .154 | .137 | .356 | <.001 | −.245 | .012 | |||
| Left inferior frontal gyrus | |||||||||
| Ch29 | .129 | .111 | .310 | .001 | −.231 | .016 | |||
| Ch39 | .154 | .138 | .332 | .010 | −.270 | .004 | |||
| Ch40 | .122 | .104 | .292 | .003 | −.242 | .014 | |||
| Ch50 | .126 | .107 | .245 | .013 | −.292 | .003 | |||
| Right superior frontal gyrus | |||||||||
| Ch16 | .182 | .157 | −.226 | .016 | VFT: β = −.302, P = .002; IQ: β = .278, P = .005 | ||||
| Ch37 | .240 | .216 | −.225 | .014 | VFT: β = −.242, P = .011; IQ: β = .419, P < .001 | ||||
| Left superior frontal gyrus | |||||||||
| Ch27 | .207 | .183 | .259 | .005 | VFT: β = −.262, P = .002; IQ: β = .302, P = .002 | ||||
| Ch48 | .170 | .153 | −.255 | .008 | IQ: β = .311, P = .001 | ||||
| Right supramarginal gyrus | |||||||||
| Ch11 | .046 | .037 | −.215 | .029 | |||||
| Left supramarginal gyrus | |||||||||
| Ch10 | .078 | .068 | .279 | .005 | |||||
| Ch21 | .161 | .135 | .267 | .006 | −.256 | .010 | GAF: β = −.241, P = .014 | ||
| Right precentral gyrus | |||||||||
| Ch13 | .105 | .088 | −.198 | .047 | .203 | .041 | |||
| Left precentral gyrus | |||||||||
| Ch9 | .165 | .147 | −.286 | .005 | .213 | .036 | |||
| Left postcentral gyrus | |||||||||
| Ch20 | .131 | .122 | .362 | <.001 | |||||
| Ch31 | .106 | .096 | .326 | .002 | |||||
| Right middle temporal gyrus | |||||||||
| Ch32 | .154 | .128 | .252 | .009 | −.242 | .013 | GAF: β = −.232, P = .018 | ||
| Ch43 | .062 | .053 | .250 | .012 | |||||
| Left middle temporal gyrus | |||||||||
| Ch42 | .177 | .159 | .379 | <.001 | −.242 | .012 | |||
Note: No., number; Ch, channel; CPZ, chlorpromazine; VFT, Vetter Fluency Test performance; IQ, intelligence quotient; GAF, Global Assessment of Functioning scale.
aAge, gender, premorbid IQ, VFT performance, GAF, Positive and Negative Syndrome Scale (PANSS) score, and antipsychotic dose were included in the multiple linear regression analysis.
bAge, gender, premorbid IQ, VFT performance, GAF, PANSS score and antipsychotic dose did not show significant contributions to cortical activity in Ch5.
Table 3.
Summary of Stepwise Multiple Regression Analysis of Channels Showing Significantly Correlated With Age in Healthy Individuals
| No. of Channelsa | R 2 | Adjusted R 2 | Independent Variables | ||||
|---|---|---|---|---|---|---|---|
| Age | Gender | Other Factors | |||||
| β | P | β | P | ||||
| Right middle frontal gyrus | |||||||
| Ch4 | .131 | .113 | .254 | .009 | VFT: β = −.225, P = .020 | ||
| Ch14 | .182 | .157 | −.237 | .012 | .215 | .022 | VFT: β = −.190, P = .043 |
| Ch15 | .206 | .190 | −.258 | <.006 | .334 | <.001 | |
| Ch25 | .162 | .145 | −.311 | .001 | VFT: β = −.207, P = .030 | ||
| Ch26 | .180 | .163 | −.351 | <.001 | .189 | .044 | |
| Ch36 | .137 | .119 | −.236 | .014 | VFT: β = −.246, P = .011 | ||
| Ch47 | .090 | .080 | −.300 | .003 | |||
| Left middle frontal gyrus | |||||||
| Ch17 | .112 | .103 | −.355 | .001 | |||
| Ch18 | .131 | .114 | −.223 | .019 | VFT: β = −.250, P = .009 | ||
| Ch28 | .135 | .118 | −.257 | .007 | VFT: β = −.223, P = .018 | ||
| Ch38 | .100 | .082 | −.211 | .032 | VFT: β = −.199, P = .043 | ||
| Right inferior frontal gyrus | |||||||
| Ch24 | .110 | .101 | −.332 | .001 | |||
| Ch34 | .117 | .108 | −.342 | .001 | |||
| Ch35 | .139 | .122 | −.211 | .026 | VFT: β = −.275, P = .004 | ||
| Ch45 | .149 | .140 | −.386 | <.001 | |||
| Ch46 | .138 | .121 | −.189 | .047 | VFT: β = −.292, P = .003 | ||
| Right superior frontal gyrus | |||||||
| Ch16 | .072 | .064 | −.269 | .005 | |||
| Ch37 | .142 | .133 | −.377 | <.001 | |||
| Left superior frontal gyrus | |||||||
| Ch6 | .082 | .073 | −.287 | .003 | |||
| Ch27 | .096 | .087 | −.310 | .001 | |||
| Ch48 | .125 | .115 | −.353 | <.001 | |||
| Right supramarginal gyrus | |||||||
| Ch1 | .059 | .050 | VFT: β = −.244, P = .015 | ||||
| Ch11 | .048 | .038 | −.218 | .027 | |||
| Right precentral gyrus | |||||||
| Ch13 | .089 | .080 | −.299 | .002 | |||
| Ch23 | .208 | .198 | −.456 | <.001 | |||
| Right postcentral gyrus | |||||||
| Ch12 | .134 | .125 | −.366 | <.001 | |||
| Right superior temporal gyrus | |||||||
| Ch22 | .094 | .083 | −.306 | .004 | |||
| Ch33 | .137 | .117 | −.298 | .004 | IQ: β = −.278, P = .007 | ||
| Ch44 | .103 | .093 | −.231 | .002 | |||
| Right middle temporal gyrus | |||||||
| Ch32 | .062 | .052 | −.249 | .017 | |||
Note: No., number; Ch, channel; VFT, Vetter Fluency Test performance; IQ, intelligence quotient.
aAge, gender, premorbid IQ, and VFT performance were included in the multiple linear regression analysis.
Comparison of Age Effects on Cortical Activity Between Groups
The 2-way ANOVA did not reveal significant interactions between age and diagnostic group for any of the 52 channels. In addition, the age regression slopes did not significantly differ between the 2 groups in all 52 channels (see supplementary material II for details).
Discussion
To our knowledge, this is the first study to investigate age effects on brain cortical activity during a VFT in a large sample of healthy individuals and SZ using multichannel NIRS. The main findings showed similar age effects on cortical activity at the bilateral frontotemporal regions of participants in both groups.
Age Effects on Brain Activity During the VFT
In this study, we found age-dependent decreases in cortical activity over the frontotemporal regions during a VFT using NIRS instrument in both HC and SZ groups. This is consistent with previous research that has found aging effects on morphological or functional changes of the brain in HC and SZ. In healthy individuals, structural MRI studies have reported prominent loss of volume44–48 and/or cortical thickness49–51 over the frontotemporal regions in the normal aging process. Several PET studies also have demonstrated age-dependent decreases in cerebral blood flow, cerebral blood volume, or cerebral glucose metabolism rate during the resting state or during a cognitive task in healthy individuals.52–54 Age effects on functional MRI blood oxygenation level–dependent signals also can be observed during resting state,55 language tasks,56 or auditory stimuli.57
On the other hand, in SZ, previous studies have found effects of aging on the structure and function of the brain. Several longitudinal and cross-sectional volumetric MRI studies have demonstrated progressive gray matter loss globally or regionally.1,2,58–62 Moreover, recent studies have demonstrated age effects on cortical thickness in SZ.7,9,10,63 Though rare, functional neuroimaging studies have demonstrated age-dependent decreases in cerebral blood flow, cerebral blood volume, or cerebral glucose metabolism in schizophrenia by using simple-photon emission computed tomography64 or PET studies.16,18,65
In this study, the age-related decline in cortical activity of HC during the cognitive task was consistent with findings of previous NIRS studies that used a calculation task,66,67 a working memory task,68 a color-word-Stroop task,69 and a VFT.21,70 In contrast, Heinzel et al20 demonstrated a positive correlation between cortical activity and age over the bilateral MFG and bilateral supramarginal gyri regions during the VFT, suggesting possible compensation for an age-related decrease in the bilateral brain function. However, Heinzel et al recruited healthy subjects aged 51–82 years, which is different from our sample (individuals aged 16–59 y in both HC and SZ groups).
In the SZ group, we demonstrated age-related decline in cortical activity similar to those reported by Takizawa et al22 but not those by Shinba et al.71 Shinba et al did not find significant correlations between age and cortical activity in the prefrontal area during a random number generation test in 13 schizophrenia patients as measured using 2-channel NIRS. However, in this study, we used a multichannel NIRS instrument and recruited a large number of participants, which enabled us to examine the age effects on brain functions in greater detail.
There are several possible explanations for the age-related declines in cortical activity demonstrated in our study. First, reduced activation of frontotemporal regions during a VFT in older subjects might be due to altered functional brain organization.20,66 During a VFT, the effortful retrieval of words involves behavioral strategies, such as clustering of words from 1 category within a phonological or semantic task condition and switching between categories, (ie, the effective search process of finding new categories).72 As elderly subjects have been shown to exhibit relatively decreased switching compared with young subjects,73 different strategies might contribute to age-related neural differences. Second, the mechanisms of coupling between brain cell activity and blood flow are altered during the aging process. Such alterations may attenuate the temporary physiological mismatch between oxygen delivery and consumption during brain activation.74 Third, the age-dependent decrease in cerebral perfusion and metabolism in the resting state is hypothesized to result from the combined effects of direct neuronal loss, cellular biological impairment, and functional deafferentation,52 and such mechanisms also could explain the aging effects on brain cortical activity.21 Fourth, one may argue that due to brain atrophy during aging, the brain volume under investigation is smaller in older adults. Thus, NIRS sensitivity may be affected by the scalp-cortex distance (SCD),75 which may have affected our results. Therefore, future studies investigating the relationship between cortical activity measured by NIRS and underlying changes in brain structures are warranted. Fifth, aging is accompanied by the degeneration of the vascular system.76 Therefore, regional cerebral flow may decrease with age as vessel stiffness is enhanced, causing a reduction in the cerebral metabolic rate of oxygen.77 If the age-related declines in cortical activity were due to age-related alterations in vasculature, both mean [oxy-Hb] and [deoxy-Hb] changes should show similar patterns of correlation with age. Because we did not find significant associations between age and mean [deoxy-Hb] changes in this study, we consider this explanation less plausible (please see supplementary material III for details).
Similar Age-Related Decline in Cortical Activity Over Frontotemporal Areas Between the SZ and HC Groups
In this study, we found similar age effects on cortical activities over the bilateral frontotemporal regions during a VFT in both groups. This is inconsistent with the findings of a PET study that showed a more negative slope of age in cerebral glucose metabolic rate in SZ in the lateral and medial-frontal and anterior-temporal regions.17 The differences between the study done by Shihabuddin et al and ours could be attributed to different sample sizes. Study done by Shihabuddin et al included 42 study subjects (18 patients with schizophrenia or schizoaffective disorder and 24 control subjects), while ours recruited a larger number of participants (n = 215) with a wide age range (16–59 y), which enabled investigations of age effects on brain functions with increased statistical power. Therefore, it is difficult to directly compare the results of these 2 studies.
Although no other NIRS-related studies have examined age effects on brain function between HC and SZ groups, our previous cross-sectional study revealed that brain cortical activity in SZ decreases as the clinical course progresses (Ultrahigh risk vs FEP vs chronic SZ) compared with HC.23 Meanwhile, more recent studies have suggested that substantial progressive brain morphological alterations are most prominent in a relatively early stage of the illness62,78,79 but not at later stages.8,62,78–82 Therefore, Kubota et al10 concluded that pathological alterations in cortical thickness might occur at a relatively early phase of schizophrenia and changes might be within the normal range in later phases. Our study demonstrated cortical areas similar to those identified by studies that focused on the effect of age on cortical thickness, such as the bilateral SFG,10 bilateral MFG,9,10 right IFG,7 and right MTG.10 Because NIRS measures cortical brain activity, the hypothesis proposed by Kubota et al10 regarding pathological alterations in cortical thickness may be a reasonable explanation of our findings.
Limitations
Several limitations of this study should be considered. First, considering the cross-sectional design, the effects of age on brain activity should be interpreted with caution. Although our results provided some interesting findings, a cross-sectional design does not allow decisive conclusions to be drawn regarding disease-related decreased brain activity and the progression of reduced cortical activations over the course of illness. Second, we did not measure the SCD in all participants because SCD may affect NIRS sensitivity. Cui et al83 investigated this topic using simultaneous NIRS and fMRI and suggested that the measured NIRS signals would contain high-frequency noises when SCD is longer than 22mm. In our study, the SCDs of the prefrontal and temporal regions in participants were mostly within 14mm. Moreover, there have been several NIRS studies focusing on dementia with reliable results.84,85 Therefore, we believe brain atrophy has negligible impact on the results. Future studies should simultaneously measure MRI and NIRS in order to investigate the effect of SCD. Third, patients’ antipsychotic medication could be a confounding variable because the effects of medication on cortical activity may be influenced by dosage, medication type (typical vs atypical), and patients’ adherence to treatment. In our previous studies, we did not find significant correlations between antipsychotic dosage and cortical activities.22,34,86 However, in this study, we found significant effects of antipsychotic dosage on cortical activities in SZ. Further, we did not evaluate the effects of different types of antipsychotic medication due to limited sample sizes. Because it was proposed that the types and dosages of antipsychotics might be associated with brain structural changes,3 these factors should be taken into consideration. However, because this study was naturalistic and did not control for medication usage, we could not further analyze the effects of medication on cortical activities. Finally, the small number of participants at older age in this study limited our conclusions on late-life trajectory. Future studies on these topics are warranted.
Conclusion
This study revealed that schizophrenia was associated with reduced cortical activity during a VFT and that similar age-related declines in cortical activity were found over the bilateral frontotemporal regions in SZ and HCs. Consistent with previous MRI and neuropsychological studies that suggest reductions of cortical thickness or cognitive impairments in schizophrenia occur relatively early in illness, results of our study may indicate a decrease in cortical activity that occurs in a relatively limited period around illness onset rather than progressing over the course of the illness.
Supplementary Material
Supplementary material is available at http://schizophre- niabulletin.oxfordjournals.org.
Funding
The Ministry of Health, Labour, and Welfare (Health and Labour Science Research Grants for Comprehensive Research on Disability Health and Welfare, H23-seishin-ippan-002 to R.T. and Y.N.); the Japan Society for the Promotion of Science/Ministry of Education, Culture, Sports, Science and Technology (21249064); Grant-in-Aid for Scientific Research on Innovative Areas (Comprehensive Brain Science Network and Adolescent Mind and Self-Regulation 23118001, 23118004 to K.K. and 23791309 to R.T.); Intramural Research Grant for Neurological and Psychiatric Disorders of NCNP (23-10 to R.T. and Y.N.); the Japan Research Foundation for Clinical Pharmacology (to R.T.); Takeda Science Foundation (to Y.N.).
Supplementary Material
Acknowledgments
We thank Mika Yamagishi, Hanako Sakurada, Yuki Kawakubo, Kohei Marumo, Shingo Kawasaki, and Masaru Kinou for their substantial support in the NIRS measurements for this study. Dr Chou wants to thank Dr Chin-Hong Chan and the Taiwan Astellas Foundation for arranging fellowship program in the University of Tokyo. This work was partly the result of the “Development of biomarker candidates for social behavior” project carried out under the Strategic Research Program for Brain Sciences by the MEXT. K.K. has potential conflicts of interest. The University of Tokyo and the Research and Developmental Center, Hitachi Medical Corporation have had official contracts for a collaborative study on the clinical application of near-infrared spectroscopy in psychiatric disorders. For this study, the Hitachi Group provided project grants (K.K.: JPY 300000 per year since July 31, 2003). The other authors have no relevant conflicts of interest.
Reference
- 1. Olabi B, Ellison-Wright I, McIntosh AM, Wood SJ, Bullmore E, Lawrie SM. Are there progressive brain changes in schizophrenia? A meta-analysis of structural magnetic resonance imaging studies. Biol Psychiatry. 2011;70:88–96. [DOI] [PubMed] [Google Scholar]
- 2. Vita A, De Peri L, Deste G, Sacchetti E. Progressive loss of cortical gray matter in schizophrenia: a meta-analysis and meta-regression of longitudinal MRI studies. Transl Psychiatry. 2012;2:e190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zipursky RB, Reilly TJ, Murray RM. The myth of schizophrenia as a progressive brain disease. Schizophr Bull. 2013;39:1363–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Garey L. When cortical development goes wrong: schizophrenia as a neurodevelopmental disease of microcircuits. J Anat. 2010;217:324–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Narr KL, Bilder RM, Toga AW, et al. Mapping cortical thickness and gray matter concentration in first episode schizophrenia. Cereb Cortex. 2005;15:708–719. [DOI] [PubMed] [Google Scholar]
- 6. Narr KL, Toga AW, Szeszko P, et al. Cortical thinning in cingulate and occipital cortices in first episode schizophrenia. Biol Psychiatry. 2005;58:32–40. [DOI] [PubMed] [Google Scholar]
- 7. Kuperberg GR, Broome MR, McGuire PK, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Arch Gen Psychiatry. 2003;60:878–888. [DOI] [PubMed] [Google Scholar]
- 8. Nesvåg R, Bergmann Ø, Rimol LM, et al. A 5-year follow-up study of brain cortical and subcortical abnormalities in a schizophrenia cohort. Schizophr Res. 2012;142:209–216. [DOI] [PubMed] [Google Scholar]
- 9. Nesvåg R, Lawyer G, Varnäs K, et al. Regional thinning of the cerebral cortex in schizophrenia: effects of diagnosis, age and antipsychotic medication. Schizophr Res. 2008;98:16–28. [DOI] [PubMed] [Google Scholar]
- 10. Kubota M, Miyata J, Yoshida H, et al. Age-related cortical thinning in schizophrenia. Schizophr Res. 2011;125:21–29. [DOI] [PubMed] [Google Scholar]
- 11. Cobia DJ, Smith MJ, Wang L, Csernansky JG. Longitudinal progression of frontal and temporal lobe changes in schizophrenia. Schizophr Res. 2012;139:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Haren NE, Schnack HG, Cahn W, et al. Changes in cortical thickness during the course of illness in schizophrenia. Arch Gen Psychiatry. 2011;68:871–880. [DOI] [PubMed] [Google Scholar]
- 13. Fucetola R, Seidman LJ, Kremen WS, Faraone SV, Goldstein JM, Tsuang MT. Age and neuropsychologic function in schizophrenia: a decline in executive abilities beyond that observed in healthy volunteers. Biol Psychiatry. 2000;48:137–146. [DOI] [PubMed] [Google Scholar]
- 14. Bora E, Murray RM. Meta-analysis of cognitive deficits in ultra-high risk to psychosis and first-episode psychosis: do the cognitive deficits progress over, or after, the onset of psychosis? [published online ahead of print June 14, 2013]. Schiz Bull. :10.1093/schbul/sbt085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Siegel BV, Reynolds C, Lohr JB, et al. Changes in regional cerebral metabolic rate with age in schizophrenics and normal adults. Dev Brain Dysfunct. 1994;7:132–146. [Google Scholar]
- 16. Shihabuddin L, Buchsbaum MS, Hazlett EA, et al. Dorsal striatal size, shape, and metabolic rate in never-medicated and previously medicated schizophrenics performing a verbal learning task. Arch Gen Psychiatry. 1998;55:235–243. [DOI] [PubMed] [Google Scholar]
- 17. Buchsbaum MS, Hazlett EA. Functional brain imaging and aging in schizophrenia. Schizophr Res. 1997;27:129–141. [DOI] [PubMed] [Google Scholar]
- 18. Strangman G, Boas DA, Sutton JP. Non-invasive neuroimaging using near-infrared light. Biol Psychiatry. 2002;52:679–693. [DOI] [PubMed] [Google Scholar]
- 19. Sato H, Yahata N, Funane T, et al. A NIRS-fMRI investigation of prefrontal cortex activity during a working memory task. Neuroimage. 2013;83:158–173. [DOI] [PubMed] [Google Scholar]
- 20. Heinzel S, Metzger FG, Ehlis AC, et al. ; TREND Study Consortium. Aging-related cortical reorganization of verbal fluency processing: a functional near-infrared spectroscopy study. Neurobiol Aging. 2013;34:439–450. [DOI] [PubMed] [Google Scholar]
- 21. Kameyama M, Fukuda M, Uehara T, Mikuni M. Sex and age dependencies of cerebral blood volume changes during cognitive activation: a multichannel near-infrared spectroscopy study. Neuroimage. 2004;22:1715–1721. [DOI] [PubMed] [Google Scholar]
- 22. Takizawa R, Kasai K, Kawakubo Y, et al. Reduced frontopolar activation during verbal fluency task in schizophrenia: a multi-channel near-infrared spectroscopy study. Schizophr Res. 2008;99:250–262. [DOI] [PubMed] [Google Scholar]
- 23. Koike S, Takizawa R, Nishimura Y, et al. Different hemodynamic response patterns in the prefrontal cortical sub-regions according to the clinical stages of psychosis. Schizophr Res. 2011;132:54–61. [DOI] [PubMed] [Google Scholar]
- 24. American Psychiatric Association. Diagnostic and Statistical Manual of MentalDisorders. 4th ed. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- 25. First M.B., Spitzer R.L., Gibbon M., et al. Structured Clinical Interview for DSM-IV Axis I Disorders. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1997. (Japanese version: Kitamura, T.,Okano, T., Tokyo, Japan: Nihon Hyoron-sha publishers; 2003). [Google Scholar]
- 26. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of clinical psychiatry 1998;59(suppl 20):22–33;quiz 34–57. [PubMed] [Google Scholar]
- 27. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 28. Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. [DOI] [PubMed] [Google Scholar]
- 29. Matsuoka K, Uno M, Kasai K, Koyama K, Kim Y. Estimation of premorbid IQ in individuals with Alzheimer’s disease using Japanese ideographic script (Kanji) compound words: Japanese version of National Adult Reading Test. Psychiatry Clin Neurosci. 2006;60:332–339. [DOI] [PubMed] [Google Scholar]
- 30. American Psychiatric Association. Practice guideline for the treatment of patients with schizophrenia. Am J Psychiatry. 1997;154(suppl 4):1–63. [DOI] [PubMed] [Google Scholar]
- 31. Inagaki A, Inada T. Dose equivalence of psychotropic drugs. Part 18: dose equivalence of psychotropic drugs: 2006-version. Japanese J Clin Psychopharmacol. 2006;9:1443–1447. [Google Scholar]
- 32. Nishimura Y, Takizawa R, Koike S, et al. Association of decreased prefrontal hemodynamic response during a verbal fluency task with EGR3 gene polymorphism in patients with schizophrenia and in healthy individuals. Neuroimage. 2014;85(pt 1):527–534. [DOI] [PubMed] [Google Scholar]
- 33. Sakakibara E, Takizawa R, Nishimura Y, et al. Genetic influences on prefrontal activation during a verbal fluency task in adults: a twin study based on multichannel near-infrared spectroscopy. Neuroimage. 2014;85(pt 1):508–517. [DOI] [PubMed] [Google Scholar]
- 34. Chou PH, Koike S, Nishimura Y, et al. Distinct effects of duration of untreated psychosis on brain cortical activities in different treatment phases of schizophrenia: a multi-channel near-infrared spectroscopy study. Prog Neuropsychopharmacol Biol Psychiatry. 2014;49:63–69. [DOI] [PubMed] [Google Scholar]
- 35. Takizawa R, Fukuda M, Kawasaki S, et al. ; Joint Project for Psychiatric Application of Near-Infrared Spectroscopy (JPSY-NIRS) Group. Neuroimaging-aided differential diagnosis of the depressive state. Neuroimage. 2014;85(pt 1):498–507. [DOI] [PubMed] [Google Scholar]
- 36. Jöbsis FF. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977;198:1264–1267. [DOI] [PubMed] [Google Scholar]
- 37. Marumo K, Takizawa R, Kinou M, et al. Functional abnormalities in the left ventrolateral prefrontal cortex during a semantic fluency task, and their association with thought disorder in patients with schizophrenia. Neuroimage. 2014;85(pt 1):518–526. [DOI] [PubMed] [Google Scholar]
- 38. Singh AK, Okamoto M, Dan H, Jurcak V, Dan I. Spatial registration of multichannel multi-subject fNIRS data to MNI space without MRI. Neuroimage. 2005;27:842–851. [DOI] [PubMed] [Google Scholar]
- 39. Tsuzuki D, Jurcak V, Singh AK, Okamoto M, Watanabe E, Dan I. Virtual spatial registration of stand-alone fNIRS data to MNI space. Neuroimage. 2007;34:1506–1518. [DOI] [PubMed] [Google Scholar]
- 40. Shattuck DW, Mirza M, Adisetiyo V, et al. Construction of a 3D probabilistic atlas of human cortical structures. Neuroimage. 2008;39:1064–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hoshi Y, Tamura M. Detection of dynamic changes in cerebral oxygenation coupled to neuronal function during mental work in man. Neurosci Lett. 1993;150:5–8. [DOI] [PubMed] [Google Scholar]
- 42. Strangman G, Culver JP, Thompson JH, Boas DA. A quantitative comparison of simultaneous BOLD fMRI and NIRS recordings during functional brain activation. Neuroimage. 2002;17:719–731. [PubMed] [Google Scholar]
- 43. Singh AK, Dan I. Exploring the false discovery rate in multichannel NIRS. Neuroimage. 2006;33:542–549. [DOI] [PubMed] [Google Scholar]
- 44. Tisserand DJ, Pruessner JC, Sanz Arigita EJ, et al. Regional frontal cortical volumes decrease differentially in aging: an MRI study to compare volumetric approaches and voxel-based morphometry. Neuroimage. 2002;17:657–669. [PubMed] [Google Scholar]
- 45. Raz N, Gunning-Dixon F, Head D, Rodrigue KM, Williamson A, Acker JD. Aging, sexual dimorphism, and hemispheric asymmetry of the cerebral cortex: replicability of regional differences in volume. Neurobiol Aging. 2004;25:377–396. [DOI] [PubMed] [Google Scholar]
- 46. Taki Y, Goto R, Evans A, et al. Voxel-based morphometry of human brain with age and cerebrovascular risk factors. Neurobiol Aging. 2004;25:455–463. [DOI] [PubMed] [Google Scholar]
- 47. Raz N, Lindenberger U, Rodrigue KM, et al. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. [DOI] [PubMed] [Google Scholar]
- 48. Smith CD, Chebrolu H, Wekstein DR, Schmitt FA, Markesbery WR. Age and gender effects on human brain anatomy: a voxel-based morphometric study in healthy elderly. Neurobiol Aging. 2007;28:1075–1087. [DOI] [PubMed] [Google Scholar]
- 49. Fjell AM, Westlye LT, Amlien I, et al. High consistency of regional cortical thinning in aging across multiple samples. Cereb Cortex. 2009;19:2001–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Salat DH, Buckner RL, Snyder AZ, et al. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–730. [DOI] [PubMed] [Google Scholar]
- 51. McGinnis SM, Brickhouse M, Pascual B, Dickerson BC. Age-related changes in the thickness of cortical zones in humans. Brain Topogr. 2011;24:279–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Marchal G, Rioux P, Petit-Taboué MC, et al. Regional cerebral oxygen consumption, blood flow, and blood volume in healthy human aging. Arch Neurol. 1992;49:1013–1020. [DOI] [PubMed] [Google Scholar]
- 53. Meltzer CC, Cantwell MN, Greer PJ, et al. Does cerebral blood flow decline in healthy aging? A PET study with partial-volume correction. J Nucl Med. 2000;41:1842–1848. [PubMed] [Google Scholar]
- 54. Murphy DG, DeCarli C, McIntosh AR, et al. Sex differences in human brain morphometry and metabolism: an in vivo quantitative magnetic resonance imaging and positron emission tomography study on the effect of aging. Arch Gen Psychiatry. 1996;53:585–594. [DOI] [PubMed] [Google Scholar]
- 55. Asllani I, Habeck C, Borogovac A, Brown TR, Brickman AM, Stern Y. Separating function from structure in perfusion imaging of the aging brain. Hum Brain Mapp. 2009;30:2927–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rotte M. Age-related differences in the areas of Broca and Wernicke using functional magnetic resonance imaging. Age Ageing. 2005;34:609–613. [DOI] [PubMed] [Google Scholar]
- 57. Juckel G, Karch S, Kawohl W, et al. Age effects on the P300 potential and the corresponding fMRI BOLD-signal. Neuroimage. 2012;60:2027–2034. [DOI] [PubMed] [Google Scholar]
- 58. Hulshoff Pol HE, Schnack HG, Bertens MG, et al. Volume changes in gray matter in patients with schizophrenia. Am J Psychiatry. 2002;159:244–250. [DOI] [PubMed] [Google Scholar]
- 59. Mathalon DH, Sullivan EV, Lim KO, Pfefferbaum A. Progressive brain volume changes and the clinical course of schizophrenia in men: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:148–157. [DOI] [PubMed] [Google Scholar]
- 60. Kasai K, Shenton ME, Salisbury DF, et al. Progressive decrease of left superior temporal gyrus gray matter volume in patients with first-episode schizophrenia. Am J Psychiatry. 2003;160:156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kasai K, Shenton ME, Salisbury DF, et al. Progressive decrease of left Heschl gyrus and planum temporale gray matter volume in first-episode schizophrenia: a longitudinal magnetic resonance imaging study. Arch Gen Psychiatry. 2003;60:766–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Whitford TJ, Grieve SM, Farrow TF, et al. Progressive grey matter atrophy over the first 2-3 years of illness in first-episode schizophrenia: a tensor-based morphometry study. Neuroimage. 2006;32:511–519. [DOI] [PubMed] [Google Scholar]
- 63. Wiegand LC, Warfield SK, Levitt JJ, et al. Prefrontal cortical thickness in first-episode psychosis: a magnetic resonance imaging study. Biol Psychiatry. 2004;55:131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Goldstein PC, Brown GG, Marcus A, Ewing JR. Effects of age, neuropsychological impairment, and medication on regional cerebral blood flow in schizophrenia and major affective disorder. Henry Ford Hosp Med J. 1990;38:202–206. [PubMed] [Google Scholar]
- 65. Schultz SK, O’Leary DS, Boles Ponto LL, et al. Age and regional cerebral blood flow in schizophrenia: age effects in anterior cingulate, frontal, and parietal cortex. J Neuropsychiatry Clin Neurosci. 2002;14:19–24. [DOI] [PubMed] [Google Scholar]
- 66. Hock C, Müller-Spahn F, Schuh-Hofer S, Hofmann M, Dirnagl U, Villringer A. Age dependency of changes in cerebral hemoglobin oxygenation during brain activation: a near-infrared spectroscopy study. J Cereb Blood Flow Metab. 1995;15:1103–1108. [DOI] [PubMed] [Google Scholar]
- 67. Takizawa R, Nishimura Y, Yamasue H, Kasai K. Anxiety and performance: the disparate roles of prefrontal subregions under maintained psychological stress [published online ahead of print February 20, 2013]. Cereb Cortex. :10.1093/cercor/bht036. [DOI] [PubMed] [Google Scholar]
- 68. Kwee IL, Nakada T. Dorsolateral prefrontal lobe activation declines significantly with age–functional NIRS study. J Neurol. 2003;250:525–529. [DOI] [PubMed] [Google Scholar]
- 69. Schroeter ML, Zysset S, Kruggel F, von Cramon DY. Age dependency of the hemodynamic response as measured by functional near-infrared spectroscopy. Neuroimage. 2003;19:555–564. [DOI] [PubMed] [Google Scholar]
- 70. Herrmann MJ, Walter A, Ehlis AC, Fallgatter AJ. Cerebral oxygenation changes in the prefrontal cortex: effects of age and gender. Neurobiol Aging. 2006;27:888–894. [DOI] [PubMed] [Google Scholar]
- 71. Shinba T, Nagano M, Kariya N, et al. Near-infrared spectroscopy analysis of frontal lobe dysfunction in schizophrenia. Biol Psychiatry. 2004;55:154–164. [DOI] [PubMed] [Google Scholar]
- 72. Troyer AK, Moscovitch M, Winocur G. Clustering and switching as two components of verbal fluency: evidence from younger and older healthy adults. Neuropsychology. 1997;11:138–146. [DOI] [PubMed] [Google Scholar]
- 73. Lanting S, Haugrud N, Crossley M. The effect of age and sex on clustering and switching during speeded verbal fluency tasks. J Int Neuropsychol Soc. 2009;15:196–204. [DOI] [PubMed] [Google Scholar]
- 74. Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci U S A. 1986;83:1140–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Haeussinger FB, Heinzel S, Hahn T, Schecklmann M, Ehlis AC, Fallgatter AJ. Simulation of near-infrared light absorption considering individual head and prefrontal cortex anatomy: implications for optical neuroimaging. PLoS One. 2011;6:e26377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Vermeij A, van Beek AH, Olde Rikkert MG, Claassen JA, Kessels RP. Effects of aging on cerebral oxygenation during working-memory performance: a functional near-infrared spectroscopy study. PLoS One. 2012;7:e46210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Marín J. Age-related changes in vascular responses: a review. Mech Ageing Dev. 1995;79:71–114. [DOI] [PubMed] [Google Scholar]
- 78. Sun D, Phillips L, Velakoulis D, et al. Progressive brain structural changes mapped as psychosis develops in ‘at risk’ individuals. Schizophr Res. 2009;108:85–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Andreasen NC, Nopoulos P, Magnotta V, Pierson R, Ziebell S, Ho BC. Progressive brain change in schizophrenia: a prospective longitudinal study of first-episode schizophrenia. Biol Psychiatry. 2011;70:672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bose SK, Mackinnon T, Mehta MA, et al. The effect of ageing on grey and white matter reductions in schizophrenia. Schizophr Res. 2009;112:7–13. [DOI] [PubMed] [Google Scholar]
- 81. Takahashi T, Wood SJ, Kawasaki Y, Suzuki M, Velakoulis D, Pantelis C. Lack of progressive gray matter reduction of the superior temporal subregions in chronic schizophrenia. Schizophr Res. 2010;117:101–102. [DOI] [PubMed] [Google Scholar]
- 82. Yoshida T, McCarley RW, Nakamura M, et al. A prospective longitudinal volumetric MRI study of superior temporal gyrus gray matter and amygdala-hippocampal complex in chronic schizophrenia. Schizophr Res. 2009;113:84–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Cui X, Bray S, Bryant DM, Glover GH, Reiss AL. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. Neuroimage. 2011;54:2808–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Araki T, Wake R, Miyaoka T, et al. The effects of combine treatment of memantine and donepezil on Alzheimer’s Disease patients and its relationship with cerebral blood flow in the prefrontal area [published online ahead of print January 17, 2014]. Int J Geriatr Psychiatry. :10.1002/gps.4074. [DOI] [PubMed] [Google Scholar]
- 85. Tomioka H, Yamagata B, Takahashi T, et al. Detection of hypofrontality in drivers with Alzheimer’s disease by near-infrared spectroscopy. Neurosci Lett. 2009;451:252–256. [DOI] [PubMed] [Google Scholar]
- 86. Kinou M, Takizawa R, Marumo K, et al. Differential spatiotemporal characteristics of the prefrontal hemodynamic response and their association with functional impairment in schizophrenia and major depression. Schizophr Res. 2013;150:459–467. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.