Abstract
Both auditory hallucinations (AH) and visual hallucinations may occur in schizophrenia. One of the main hypotheses underlying their occurrence involves the increased activity of the mesolimbic pathway, which links the ventral tegmental area (VTA) and the nucleus accumbens (NAcc). However, the precise contribution of the mesolimbic pathway in hallucinations across various sensory modalities has not yet been explored. We compared the resting-state functional connectivity (rs-FC) of the NAcc among 16 schizophrenia patients with pure AH, 15 with both visuoauditory hallucinations (VAH), and 14 without hallucinations (NoH). A between-group comparison was performed using random-effects ANCOVA (rs-FC of the bilateral NAcc as the dependent variable, groups as the between-subjects factor, age and Positive and Negative Syndrome Scale scores as covariates; q(false discovery rate [FDR]) < .05). Compared to the NoH group, the AH group exhibited significantly enhanced NAcc rs-FC with the left temporal superior gyrus, the cingulate gyri, and the VTA, whereas the VAH group, compared to the AH group, exhibited significantly enhanced NAcc rs-FC with the bilateral insula, putamen, parahippocampal gyri, and VTA. The strength in rs-FC between the NAcc and the VTA appeared to be positively associated with the presence of hallucinations, but the NAcc FC patterns changed with the complexity of these experiences (ie, 0, 1, or 2 sensory modalities), rather than with severity. This might support the aberrant salience hypothesis of schizophrenia. Moreover, these findings suggest that future clinical and neurobiological studies of hallucinations should evaluate not only the global severity of symptoms but also their sensorial features.
Key words: hallucinations, visual, schizophrenia, salience, ventral tegmental area, nucleus accumbens, striatum, MRI
Introduction
Hallucinations are erroneous percepts without corresponding environmental objects. Hallucinations may occur in schizophrenia, classified within “positive symptoms.”1 Auditory hallucinations (AH) are common in schizophrenia and mainly involve hearing voices.2 However, hallucinations may involve other sensory modalities. Visual hallucinations (VH) have been estimated to affect 27% of schizophrenic patients during their lifetime.3 Functional magnetic resonance imaging (fMRI) studies indicate that AH and VH elicit specific patterns of cerebral activity. Previous studies have associated AH with networks involved in speech and salience.4 VH data are more cursory, although aberrant activity of the visual association areas and hippocampal complex may be involved.5,6
At the molecular level, hallucinations during schizophrenia might result from increased dopaminergic (DAergic) transmission.7 Since the 1970s, the mesolimbic pathway has been a proposed target of DAergic hyperactivity in schizophrenia. This pathway projects from the ventral tegmental area (VTA) to the ventral striatum (vStr).8,9 Furthermore, the nucleus accumbens (NAcc; the most ventral portion of the striatum) has been proposed as the central therapeutic site of action for antipsychotic medications.10,11 This hypothesis has been supported by the results of imaging studies, which have found increased metabolism of the vStr during positive symptoms12 and have correlated NAcc activation to the vividness of hallucinations.13 Moreover, several authors have proposed that DAergic transmission may indirectly contribute to hallucinations by inducing aberrant salience processes, impairing sensory integration.14
Nevertheless, the extent to which the mesolimbic pathway is involved in the pathophysiology of hallucinations and depends on the elicited sensory modalities remains unclear. VH in schizophrenia have been linked to neurotransmitters other than dopamine,15,16 as well as VH of other pathologies that induce hallucinations.17,18 These findings may therefore contradict the generalization that all hallucinations have purely DAergic origins.
Here, we surmised that the mesolimbic pathway was differentially involved in the sensory modality of hallucinations. We hypothesized that the level of mesolimbic pathway activity underlies the intensity of the resting-state functional connectivity (rs-FC) among the various brain structures involved in this pathway. Therefore, we compared the rs-FC of the NAcc among 3 groups of patients with schizophrenia, where the first group exhibited pure AH, the second group exhibited both visual and auditory hallucinations (VAH), and a control group consisted of schizophrenia patients without current hallucinations (NoH). Based on this design, we expected that the presence of VH may impact the NAcc rs-FC in the VAH subjects, by comparison with the AH subjects. We also wished to observe whether both AH and VAH groups exhibited more sustained rs-FC with structures of the mesolimbic pathways, especially the VTA, when compared with the NoH group.
Materials and methods
Participants
Forty-five subjects meeting the DSM-IV-TR criteria for schizophrenia were enrolled in the study. Each patient was clinically evaluated using the Positive and Negative Syndrome Scale (PANSS)19 and items #1 and #6 of the Scale for the Assessment of Positive Symptoms (SAPS)20 to specify their symptoms and quantify their severity. Three groups were thus defined: (a) the AH group (n = 16), with treatment-resistant AH and with no previous experience of other types of hallucinations, including VH (ie, SAPS-it #1 ≥ 4 and SAPS-it #3 to SAPS-it #6 = 0); (b) the VAH group (n = 15), consisting of patients with treatment-resistant AH and VH, which could occur simultaneously or separately, but with no other types of hallucinations (ie, SAPS-it #1 ≥ 4 and SAPS-it #6 ≥ 4 and SAPS-it #3 to SAPS-it #5 = 0); and (c) the NoH group (n = 14), consisting of subjects without hallucinations in the previous 3 months (ie, SAPS-it #1 to SAPS-it #7 = 0).
Subjects with a history of alcohol or drug dependence, head trauma, seizure, or neurological disease were excluded. Participants were also excluded if they reported substance use (except tobacco) during the week preceding the fMRI scan. Groups were matched for age, sex, handedness, symptom severity, and antipsychotic medication dosage based on olanzapine equivalent daily doses21 (table 1). The selected sample partially overlapped (22 subjects out of 44) a sample used in a previously published study.6 The present study was approved by the local ethics committee (CPP Nord-Ouest IV, France). All subjects provided written informed consent prior to their participation.
Table 1.
Patient Characteristics and Clinical Measures
| AH | VAH | NoH | P Value | |
|---|---|---|---|---|
| Sample size (n) | 16 | 15 | 14 | |
| Sex (male/female) | 12/4 | 10/5 | 10/4 | .87 |
| Handedness ratio (right/left-handed) |
15/1 | 12/3 | 11/3 | .44 |
| Age (mean ± SD) | 29.83±6.5 | 33.14±12 | 44.7±11.7 | .0029 |
| Olanzapine equivalent dose (mean ± SD) |
23.9±7.9 | 20.7±7.9 | 20.3±7.2 | .5 |
| PANSS scores | ||||
| Total (mean ± SD) | 79.71±21.8 | 75.64±18.5 | 68.44±14.4 | .2 |
| Positive (mean ± SD) | 20.11±4.5 | 26.12±9.9 | 9.64±2.9 | <10E-4 |
| Negative (mean ± SD) | 22.67±8.3 | 18.8±5.9 | 29.93±6.2 | .002 |
| General (mean ± SD) | 36.22±6.2 | 36.63±8.9 | 27.57±11.5 | .035 |
Note:
No significant differences between were found in sex and handedness ratios, and in average antipsychotic dose. Age and Total PANSS score were included in the ANCOVA as covariates. AH, auditory hallucinations; VAH, visuoauditory hallucinations; NoH, no hallucinations.
Clinical Data Analyses
Statistical analyses of the demographic and clinical data were performed using XLSTAT2013 software (Addinsoft, http://www.xlstat.com). Depending on the number of groups, continuous variables were compared using Mann-Whitney or Kruskal-Wallis tests, while categorical variables were compared using chi-square tests. The threshold for statistical significance was defined as P <.05. Findings are summarized in table 1.
Procedure, MRI Acquisition, and Preprocessing
All participants underwent a 10-minute anatomical T1-weighted MRI sequence (3D multishot turbo-field-echo scan; 150 transverse slices, field of view = 256mm2, and voxel size = 1mm3; 1.5 Tesla Intera Achieva scanner, Philips), then 300 volumes of T2*-weighted Blood Oxygen-Level Dependent (BOLD) signal fMRI sequences were acquired (single-shot sensitivity-encoded echo-planar imaging sequence, 30 transverse slices, field of view = 240mm2, voxel size = 4mm3, repetition time = 3000ms, echo time = 70ms). The first 2 volumes of each functional scan were discarded for scan equilibration. During acquisition, the participants remained in an eyes-closed, awake resting state. Headphones and earplugs were used to attenuate the noise of the scanner.
Both the anatomical and functional data were preprocessed and analyzed using the BrainVoyager software (BVQX v2.4, http://www.brainvoyager.com). Anatomical data were corrected using an intensity inhomogeneity correction algorithm and normalized in Talairach space using piece-wise linear transformation.22 Functional data were preprocessed using a slice scan time correction, 3D head motion correction, and a smoothing kernel with a 3D Gaussian filter of 5.0mm spatial full width at half maximum value. Signal oscillations of frequencies greater than 0.1 Hz were removed via high-pass filtering using pairs of discrete sine and cosine waves (this transform also included linear trend removal). The slice-based native space functional data were coregistered to the high-resolution 3D structural scan using a Gaussian/Laplacian-scale pyramid algorithm per patient.
Because it has been shown that changes in rs-FC may depend on the hallucination state,23 we ensured that none of the participants experienced hallucinations during the scanning session using a post-fMRI interview.
Seed-Based Functional Connectivity
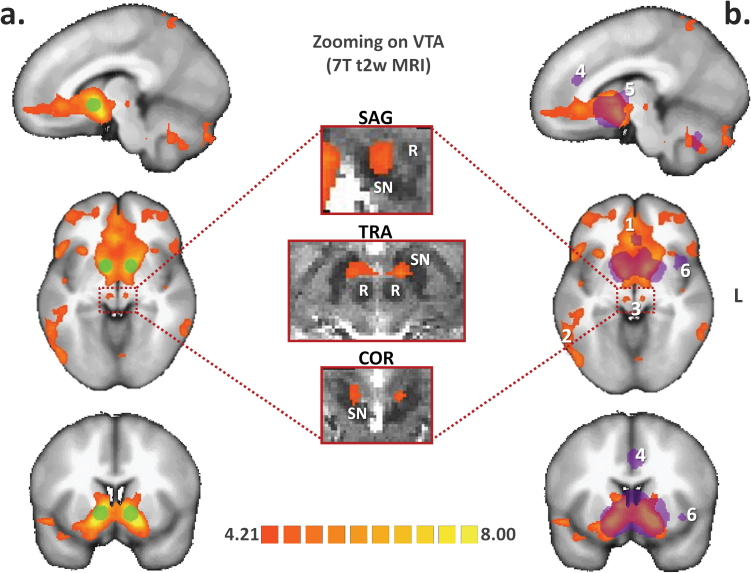
Seed regions corresponding to the bilateral NAcc were defined as 6-mm-diameter spherical regions of interest centered on the x-y-z Talairach peak coordinates from the meta-analysis by Cauda et al24 (figure 1).
Fig. 1.
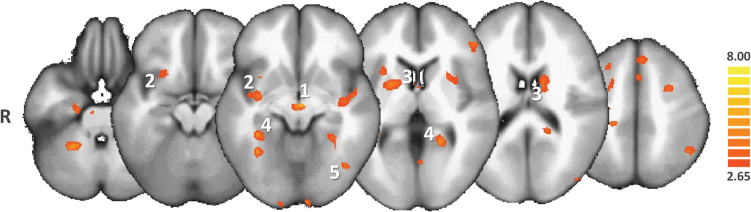
Resting-state connectivity of the nucleus accumbens (NAcc) in schizophrenia. (a) Functional connectivity analysis was seeded on the bilateral NAcc (green spherical region of interest) in a group of 45 patients with schizophrenia. The resulting statistical map was projected onto the icbm452 brain atlas (t(43) > 4.21; q(false discovery rate [FDR]) < .05). (b) The NAcc projection system evidenced that schizophrenia overlaps and extends beyond findings from a recent coordinate-based meta-analysis of positron emission tomography and functional magnetic resonance imaging (MRI) studies conducted in healthy subjects (purple volume of interest, from Cauda et al24), notably at the (1) level of frontal and (2) occipitotemporal regions as well as (3) the ventral tegmental area (VTA). Specific involvement of the VTA was confirmed by projecting the seed-based functional connectivity map onto an enlarged view of the brainstem from a 7-Tesla normalized T2-weighted Gradient Recalled Echo MRI sequence (middle column, 7T data were obtained with the courtesy of the Neurospin Research Center, Saclay, France). Conversely, reduced connectivity strength was evidenced by the NAcc seeds in schizophrenia patients at the level of the (4) dorsal anterior cingulate cortex, (5) associative striatum, and (6) left anterior insula. L: left side of the brain; R: red nucleus; SN: substantia nigra.
We referred to the general linear model (GLM) using z-normalized predictors to obtain individual rs-FC maps. Nuisance covariates, such as cardiac and respiratory cycles, were included in the analyses to reduce the effects of physiological processes. Seven covariates of no-interest were entered into the GLM: cerebrospinal fluid signal and head motion parameters (x/y/z corrections applied to translation and rotation). After fitting the GLM and accounting for the effects of temporal serial correlations via AR2 modeling, a random-effects GLM was generated for all participants.
First- and Second-Level Group Analyses
A first-level analysis was performed that consisted of a multisubject RFX-GLM of the entire sample. Brain areas exhibiting significant functional connectivity with the NAcc using the RFX-GLM were then compared to previously published findings from Cauda et al, which grouped 57 fMRI-positron emission tomography studies of healthy individuals and introduced them into a meta-analytic connectivity model24 (figure 1).
The second-level analysis consisted of a direct between-groups comparison of the sample using a random-effects analysis of covariance (RFX-ANCOVA). Estimated beta-values for each subject obtained in the first-level analysis were entered as dependent variables into the second-level analysis, and group was used as a between-subjects factor. In addition to the estimated subject-specific effects of the fMRI design (beta-values of the first-level analysis), we used positive PANSS score and age as covariates because of the between-group differences in these parameters (see Results section) and because younger patients have been shown to experience VH more frequently.25 Direct group comparisons were further evaluated using post hoc t tests. In contrast to standard statistical procedures, the second-level analysis was performed separately for each voxel and therefore required appropriate corrections to account for the multiple comparisons (see below).
Anatomical Labeling and Thresholding Strategy
Anatomical labeling of significant activation clusters was performed using the Talairach Client database (http://www.talairach.org/). For activation, clusters localized outside the cerebral cortex, particularly those located in the midbrain, anatomical labeling was performed by an experienced neuroradiologist in reference to a 7-Tesla T2-weighted normalized MRI centered on the clusters of interest (figure 1).
Correction of the statistical maps to address the multiple comparisons problem relied on a previously described dual process26: (a) voxel-wise thresholding using a FDR approach27 was applied to the FC maps at the q <.05 level and (b) cluster-size thresholding was applied via iterative Monte Carlo simulations to objectively discard small and/or isolated voxels.
Results
Demographic and Behavioral Measures
The demographic and clinical data of the three groups are compared in table 1. No significant differences were found among groups in gender, handedness ratio, and antipsychotic dosage. A significant between-group difference was found in age, which was thus included as a first covariate in the subsequent ANCOVA.
Similarly, positive PANSS scores statistically differed among groups. Therefore, the positive PANSS score was introduced as second covariate in the ANCOVA. The difference in SAPS-it #1 score for AH severity was not significant between the VAH and the AH groups (P = .87).
Results of the Multisubject RFX-GLM
The results of the multisubject RFX-GLM are shown in figure 1. Several brain structures appeared functionally connected to the NAcc, including the bilateral VTAs, putamen, hippocampi, fusiform gyri, and bilateral prefrontal areas. As defined in the Materials and Methods section, VTAs were characterized visually, using a 7-Tesla morphological MRI, after normalization in Talairach space.
Results of the RFX-ANCOVA
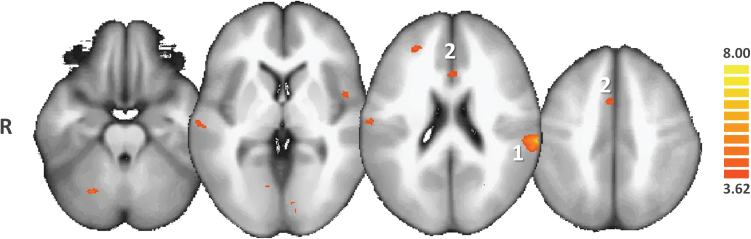
Specific contrasts of the RFX-ANCOVA were explored through post hoc t test comparisons. Overall, the post hoc t tests revealed that in AH patients, in contrast with NoH patients, the NAcc was significantly more connected to the left bilateral superior temporal gyrus, the left inferior parietal lobule, both anterior and posterior cingulates, the medial frontal gyrus, the bilateral lingual gyrus, and the VTA (see figure 2 and supplementary table 2).
Fig. 2.
Post hoc ANCOVA comparison between patients with auditory hallucinations (AH) and patients with no hallucinations. Only the regions of increased functional connectivity of the nucleus accumbens (NAcc) in AH are shown in the red-to-yellow color code (q(FDR) < .05). AH patients exhibit greater functional connectivity of the NAcc with left superior temporal gyrus (1), ie, the main auditory association area and with the anterior cingulate cortex (2).
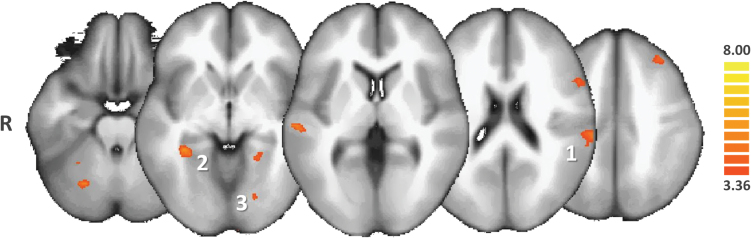
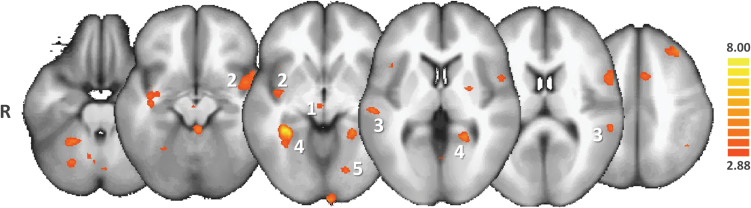
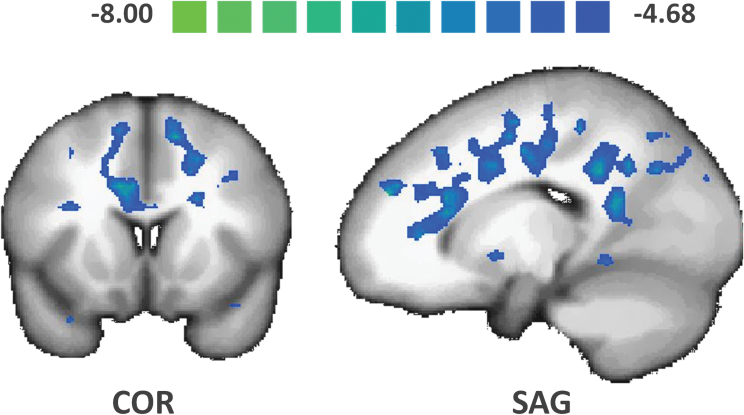
In pooled hallucinated patients (VAH + AH), in contrast with NoH patients, the NAcc was significantly more functionally connected with the bilateral parahippocampal regions, the left inferior frontal gyrus, the left inferior parietal lobule, and the midbrain (see figure 3 and supplementary table 3). In VAH patients, in contrast with NoH patients and in complement to the brain areas mentioned in the previous contrast, the NAcc was significantly more connected with the bilateral parahippocampal gyri (see figure 4 and supplementary table 4). In VAH patients, in contrast with AH patients, the NAcc was significantly more connected with the bilateral parahippocampal gyri, the bilateral insula, the bilateral putamen, and the VTA (see figure 5 and supplementary table 5). We also found that in (VAH + AH) patients, by comparison with the NoH patients, a large bilateral anterior-posterior crescent moon zone pattern was significantly less connected with the NAcc (see figure 6 and supplementary table 6).
Fig. 3.
Post hoc ANCOVA comparison between patients with hallucinations (AH + VAH groups) and patients with no hallucinations (NoH). Only the regions of increased functional connectivity of the nucleus accumbens (NAcc) in AH + VAH subjects compared with NoH subjects are shown in the red-to-yellow color code (q(FDR) < .05]. Patients with hallucinations exhibited greater functional connectivity of the NAcc with the superior temporal gyrus (1), the bilateral parahippocampal regions (2), and association visual areas (3). AH, auditory hallucinations; VAH, visuoauditory hallucinations.
Fig. 4.
Post hoc ANCOVA comparison between patients with visuoauditory hallucinations (VAH) and patients with no hallucinations (NoH). Only the regions of increased functional connectivity of the nucleus accumbens (NAcc) in VAH compared with NoH patients are shown in the red-to-yellow color code (q(FDR) < .05). VAH patients exhibited greater functional connectivity of the NAcc with the ventral tegmental area (1), the insulae (2), the superior temporal gyri (3), the parahippocampal gyri (4), and associative visual areas (5).
Fig. 5.
Post hoc ANCOVA comparison between patients with visuoauditory hallucinations (VAH) and patients with pure auditory hallucinations (AH). Only the regions of increased functional connectivity of the nucleus accumbens (NAcc) in VAH compared with AH patients are shown in the red-to-yellow color code (q(FDR) < .05). VAH patients exhibited greater functional connectivity of the NAcc with parts of the mesolimbic system, ie, the bilateral ventral tegmental areas (1), the bilateral insulae (2), and the bilateral putamen (3). The bilateral parahippocampal regions (4) and associative visual areas were also more connected with the NAcc in the VAH group.
Fig. 6.
Post hoc ANOVA comparison between patients with hallucinations (AH and VAH) and patients with no hallucinations (NoH). Only the regions of decreased functional connectivity of the nucleus accumbens (NAcc) in VAH and AH patients compared with NoH patients are shown in the green-to-blue color code. These regions formed a large anterior-posterior crescent moon shape which notably included several bilateral prefrontal and cingular areas (q(FDR) < .05). AH, auditory hallucinations; VAH, visuoauditory hallucinations.
Discussion
Our study aimed to demonstrate that the strength in the rs-FC between the NAcc and rest of the mesolimbic pathway differed according to the sensory modality of hallucinations. This is the first report using fMRI to explore the rs-FC of the NAcc in schizophrenia patients who differed in the nature and complexity of their hallucinatory experiences. Importantly, the current findings are derived from group comparisons including a patient control group without hallucinations. The inclusion of healthy subjects as controls would not have been appropriate here because the observed differences could also have been attributed to schizophrenia or even to antipsychotic medications, which are known to potentially affect rs-FC processes in the brain.28 Because all the experimental groups consisted of schizophrenia patients, who were assessed and matched for antipsychotic dosages, this comparison scheme enabled the potential effects of medication on the results to be minimized. The correction parameters included in the data preprocessing procedures (ie, correction for head movements and cerebrospinal fluid) also reduced the risk of false-positive results. The relevance of head movement correction methods has been addressed recently,29 but the lack of power was emphasized rather than the potential for false positives.
The NAcc rs-FC has been previously investigated in healthy subjects. In a meta-analytic connectivity modeling study, Cauda and colleagues demonstrated that the NAcc was preferentially connected to the prefrontal cortex, globus pallidus, thalamus, midbrain, amygdala, and insula.24 To address the relevance of the brain areas identified by the RFX-GLM procedure, we projected the obtained GLM map on the findings from Cauda’s meta-analysis (figure 1). Visual inspection of the superimposed rs-FC maps revealed overlapping areas at the level of the midbrain, vStr, and frontal areas. All of these structures belong to striato-pallido-thalamo-cortical (STPC) circuits.30 The NAcc is also part of this circuit.31 This functional network is supported by a complex anatomical network that has been mainly investigated in rodents. Studies indicate that ascending DAergic and possibly glutamatergic fibers project from the VTA onto the vStr and prefrontal cortex,31,32 whereas descending glutamatergic axons connect back to the NAcc and VTA from the prefrontal cortex.33 In contrast, significant differences were observed in the rs-FC of the NAcc between healthy subjects and patients. In schizophrenia, sustained NAcc rs-FC was observed in the prefrontal cortex and occipital areas (figure 1). Conversely, meta-analysis findings in healthy subjects revealed increased NAcc rs-FC with the dorsal anterior cingulate cortex (ACC) and associative striatum compared to patients with schizophrenia. Even if indirect and potentially biased, this comparison helped in confirming that the chosen seeds correctly targeted the NAcc and its conventional functional network. As previously argued, a more direct comparison would be biased because of the schizophrenia factor. Moreover, differences in medications, age, and gender among these samples could not be addressed in this visual comparison, although substantial new and highly controlled information was provided by the RFX-ANCOVA and matched the main objective of the study.
Compared with the NoH group, the bilateral NAcc in AH patients was functionally more connected to the inferior parietal lobules and the superior temporal gyri (figure 2), which represent speech areas34 and are known to be involved in both psychotic and nonpsychotic AH.35 Consequently, structures known to be associated with the occurrence of AH were particularly connected with the NAcc in subjects experiencing AH. Importantly, the VTA was also significantly more connected with the NAcc in the AH group in comparison with the NoH group (figure 2). Additionally, the right ACC was found to be hyper-connected to the NAcc in the AH group compared with NoH. Although myriad potential roles are attributed to the multimodal ACC, this region has been repeatedly reported in AH trait studies in schizophrenia.36 Importantly, the ACC may be involved in attribution errors related to the foreign sources of speech accompanying AH.37,38 Similarly, the bilateral posterior cingulate cortex (PCC) was more functionally connected to the NAcc in the AH group compared with NoH patients. In addition, a thicker layer of PCC gray matter has been linked to both schizophrenia and AH.39 Moreover, the PCC is considered to be a key node of the default mode network (DMN),40 and increased PCC functional fluctuations have been correlated with the severity of AH in chronic hallucinators with schizophrenia.41
Taken together, these findings involving speech areas, the ACC and PCC, indicate that the functional network of NAcc in AH patients may be congruent with the symptomatology of AH patients, which supports the mesolimbic hypothesis in the AH subjects.
Similarly, when compared with the NoH group, VAH subjects exhibited preferential NAcc rs-FC with several areas involved in AH, ie, the inferior parietal lobules and the inferior frontal gyri (figure 4 and supplementary table 4). This finding underlines the fact that visual hallucinatory experiences in schizophrenia are most often associated with an auditory component, whether simultaneously or not. The parahippocampal cortex also appeared significantly connected with the NAcc in the VAH vs NoH comparison (figure 4 and supplementary table 4) and in the (VAH + AH) vs NoH comparisons (figure 3 and supplementary table 3). A previous meta-analysis of the AH state indicated hippocampal complex involvement in AH occurrence.4 Of note, the hippocampi were not found to be more functionally connected with the NAcc in the AH group when compared with NoH patients. Moreover, transnosographic data have linked the hippocampal complex to VH, notably as shown in Parkinson’s disease.42 Our team recently supported this hypothesis using multimodal connectivity analyses of AH and VAH in schizophrenia.6 Nevertheless, we must acknowledge a partial overlap among subjects across the previous and present studies. Here, we propose that the involvement of the hippocampal complexes may reflect the visual dimension of hallucinations in subjects with VAH. This involvement appears consistent with the findings from the VAH vs AH post hoc comparison. Moreover, the VTA was also significantly more connected with the NAcc in the VAH group in comparison with the NoH group.
Interestingly, we found that the bilateral VTAs were functionally more connected to the NAcc in the VAH group than in the AH group (figure 3 and supplementary table 3). Moreover, the bilateral insula and putamen were hyper-connected to the NAcc in the VAH group. As noted above, these structures belong to the STPC loops.43 Similarly, the parahippocampal regions are part of the mesolimbic pathway.30 These findings suggest that there was an increased crosstalk between the NAcc and the rest of the mesolimbic system in patients with VAH rather than AH. Because positive PANSS scores were included as a covariate in the ANCOVA, these differences cannot be explained by the symptom severity, particularly that of positive symptoms. Moreover, recent findings indicate that the activity of the vStr is unlikely to underlie the severity of positive symptoms.44 Because the VAH group involves 2 sensory modalities, the current results suggest that the connectivity strength between the NAcc and the rest of the mesolimbic pathway might depend more on the complexity of the hallucinatory experiences than the overall disorder severity, as measured by individual positive PANSS scores added as covariates in the analysis.
The FC patterns between the NAcc and mesolimbic structures in the VAH group supported the hypothesis that increased DA network recruitment produces a wider range of hallucinatory experiences, in terms of the sensory modalities potentially involved. Moreover, the anterior insula is a multimodal structure involved in the emotional processing of interoceptive awareness.45 Beyond schizophrenia (which involves an impairment of interoceptive awareness), dysfunction of the anterior insula has been linked to the triggering of hallucinations.46 Furthermore, the anterior insula is a component of the salience network (SN),47 which is intimately linked to DA transmission.14 The anterior insula and SN have been implicated in modulating large-scale network activity in healthy subjects, including the DMN.48 Interestingly, the intrinsic instability of the DMN during complex hallucinations was described recently.23,49 Moreover, a bilateral volume reduction of the anterior insula was evidenced in patients with positive symptoms.50 The observed increase in the FC strength between the NAcc and the SN in VAH patients compared with AH patients suggests that the more these structures are connected, the more patients will exhibit aberrant salience. Indeed, such mesolimbic hyperconnectivity appears to be linked with the number of hallucinatory modalities involved. Considering the physiological interactions between insula and the DMN, an impairment of the SN, anchored in the anterior insula, may be at the root of the spontaneous disengagement of this resting-state network,49 and the subsequent overactivation of modality-dependent association cortices during hallucinations, which in turn might lead to the misinterpretation of erroneous percepts as hallucinations.
We must acknowledge that this interpretation of our results requires caution, notably in reference to the DAergic system. In its basis on the rs-FC of the NAcc, the present study highlights the involvement of the mesolimbic network in hallucinations. DAergic transmission plays an important, albeit nonexclusive, role in the mesolimbic pathway. However, because fMRI failed to directly demonstrate DAergic system impairments, these findings do not exclude the involvement of other neurotransmitter systems, such as glutamatergic transmission, which is also involved in the mesolimbic pathway.34 Therefore, molecular imaging is a promising method for future studies of the psychopharmacological correlates of AH and VAH in schizophrenia. Additional evidence to support this hypothesis would require a comparison of groups that differ with respect to the number of sensory modalities in which hallucinations are reported. This design would help assess whether engaging more sensory modalities (ie, more than 2) further increases the interconnection of mesolimbic structures.
Another possible limitation of our findings is that all participants with hallucinations presented treatment-resistant hallucinations. Therefore, examining treatment-naive patients with hallucinations may be interesting, as there is currently no evidence that treatment-resistant hallucinations are neurobiologically similar to other types. Moreover, additional studies are required to determine whether the differences between AH and VAH in schizophrenia parallel those in other disorders, eg, Parkinson’s disease or drug-induced hallucinations. Last, in these group-group comparisons, we mainly focused on increased NAcc rs-FC. Reverse contrasts, as highlighted in figure 6, revealed large anterior-posterior crescent moon shape set of areas that were significantly less connected with the NAcc in patients with hallucinations. These regions notably included several bilateral prefrontal and cingular areas (see figure 6 and supplementary table 6).
In conclusion, the results of the present study suggest an involvement of the mesolimbic pathway in both AH and VH of schizophrenia. Moreover, the functional connectivity between the NAcc and other mesolimbic structures appears more related to increasing number of modalities of hallucinations, rather than with clinical severity. The progressive implication of sensory modalities in these hallucinatory experiences could result from the increasing disruption of the SN. More generally, the present findings suggest that both clinical and neurobiological studies of hallucinations could benefit from a fine-scale evaluation of the symptoms, assessing not only their global severity but also their sensorial patterns.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
Fondation Pierre Houriez (hosted by the Fondation de France) to R.J. (#FdF-200462/2009-02).
Supplementary Material
Acknowledgments
The authors thank Dr Franco Cauda for kindly providing his personal data. R.J. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors designed the study; B.R., A.A., E.P., and R.J. recruited the participants; E.P. and R.J. acquired the MRI data; B.R., A.A., and R.J. performed the analyses; and all authors helped write the manuscript. The authors declare no competing financial interests.
References
- 1. van Os J, Kapur S. Schizophrenia. Lancet. 2009;374:635–645. [DOI] [PubMed] [Google Scholar]
- 2. Andreasen NC, Flaum M. Schizophrenia: the characteristic symptoms. Schizophr Bull. 1991;17:27–49. [DOI] [PubMed] [Google Scholar]
- 3. Waters F, Collerton D, Ffytche D, et al. Visual hallucinations in the psychosis spectrum and comparative information from neurodegenerative disorders and eye disease. Schizophr Bull. 2014;40:s233–s245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jardri R, Pouchet A, Pins D, Thomas P. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. Am J Psychiatry. 2011;168:73–81. [DOI] [PubMed] [Google Scholar]
- 5. Behrendt RP. Contribution of hippocampal region CA3 to consciousness and schizophrenic hallucinations. Neurosci Biobehav Rev. 2010;34:1121–1136. [DOI] [PubMed] [Google Scholar]
- 6. Amad A, Cachia A, Gorwood P, et al. The multimodal connectivity of the hippocampal complex in auditory and visual hallucinations. Mol Psychiatry. 2014;19:184–191. [DOI] [PubMed] [Google Scholar]
- 7. Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. 2009;35:549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stevens JR. An anatomy of schizophrenia? Arch Gen Psychiatry. 1973;29:177–189. [DOI] [PubMed] [Google Scholar]
- 9. Meltzer HY, Stahl SM. The dopamine hypothesis of schizophrenia: a review. Schizophr Bull. 1976;2:19–76. [DOI] [PubMed] [Google Scholar]
- 10. Crow TJ, Deakin JF, Longden A. The nucleus accumbens–possible site of antipsychotic action of neuroleptic drugs? Psychol Med. 1977;7:213–221. [DOI] [PubMed] [Google Scholar]
- 11. Jones CA, Watson DJ, Fone KC. Animal models of schizophrenia. Br J Pharmacol. 2011;164:1162–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Epstein J, Stern E, Silbersweig D. Mesolimbic activity associated with psychosis in schizophrenia. Symptom-specific PET studies. Ann N Y Acad Sci. 1999;877:562–574. [DOI] [PubMed] [Google Scholar]
- 13. Raij TT, Valkonen-Korhonen M, Holi M, Therman S, Lehtonen J, Hari R. Reality of auditory verbal hallucinations. Brain. 2009;132:2994–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heinz A, Schlagenhauf F. Dopaminergic dysfunction in schizophrenia: salience attribution revisited. Schizophr Bull. 2010;36:472–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. González-Maeso J, Sealfon SC. Psychedelics and schizophrenia. Trends Neurosci. 2009;32:225–232. [DOI] [PubMed] [Google Scholar]
- 16. Kometer M, Schmidt A, Jäncke L, Vollenweider FX. Activation of serotonin 2A receptors underlies the psilocybin-induced effects on a oscillations, N170 visual-evoked potentials, and visual hallucinations. J Neurosci. 2013;33:10544–10551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. González-Maeso J, Weisstaub NV, Zhou M, et al. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53:439–452. [DOI] [PubMed] [Google Scholar]
- 18. Manganelli F, Vitale C, Santangelo G, et al. Functional involvement of central cholinergic circuits and visual hallucinations in Parkinson’s disease. Brain. 2009;132:2350–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 20. Andreasen NC. Methods for assessing positive and negative symptoms. Mod Probl Pharmacopsychiatry. 1990;24:73–88. [DOI] [PubMed] [Google Scholar]
- 21. Gardner DM, Murphy AL, O’Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. Am J Psychiatry. 2010;167:686–693. [DOI] [PubMed] [Google Scholar]
- 22. Talairach J, Tournoux P. Co-Planar Stereotaxic Atlas of the Human Brain: 3-Dimensional Proportional System– An Approach to Cerebral Imaging. New York, NY: Thieme Medical Publishers, 1988. [Google Scholar]
- 23. Sommer IE, Clos M, Meijering AL, Diederen KM, Eickhoff SB. Resting state functional connectivity in patients with chronic hallucinations. PLoS ONE. 2012;7:e43516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cauda F, Cavanna AE, D’agata F, Sacco K, Duca S, Geminiani GC. Functional connectivity and coactivation of the nucleus accumbens: a combined functional connectivity and structure-based meta-analysis. J Cogn Neurosci. 2011;23:2864–2877. [DOI] [PubMed] [Google Scholar]
- 25. David CN, Greenstein D, Clasen L, et al. Childhood onset schizophrenia: high rate of visual hallucinations. J Am Acad Child Adolesc Psychiatry. 2011;50:681–686.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Goebel R, Esposito F, Formisano E. Analysis of functional image analysis contest (FIAC) data with brainvoyager QX: From single-subject to cortically aligned group general linear model analysis and self-organizing group independent component analysis. Hum Brain Mapp. 2006;27:392–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15:870–878. [DOI] [PubMed] [Google Scholar]
- 28. Surguladze SA, Chu EM, Marshall N, et al. Emotion processing in schizophrenia: fMRI study of patients treated with risperidone long-acting injections or conventional depot medication. J Psychopharmacol (Oxford). 2011;25:722–733. [DOI] [PubMed] [Google Scholar]
- 29. Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ikemoto S. Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res Rev. 2007;56:27–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stuber GD, Hnasko TS, Britt JP, Edwards RH, Bonci A. Dopaminergic terminals in the nucleus accumbens but not the dorsal striatum corelease glutamate. J Neurosci. 2010;30:8229–8233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hnasko TS, Hjelmstad GO, Fields HL, Edwards RH. Ventral tegmental area glutamate neurons: electrophysiological properties and projections. J Neurosci. 2012;32:15076–15085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tzschentke TM, Schmidt WJ. Functional relationship among medial prefrontal cortex, nucleus accumbens, and ventral tegmental area in locomotion and reward. Crit Rev Neurobiol. 2000;14:131–142. [PubMed] [Google Scholar]
- 34. Hickok G. The functional neuroanatomy of language. Phys Life Rev. 2009;6:121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Diederen KM, Daalman K, de Weijer AD, et al. Auditory hallucinations elicit similar brain activation in psychotic and nonpsychotic individuals. Schizophr Bull. 2012;38:1074–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kühn S, Gallinat J. Quantitative meta-analysis on state and trait aspects of auditory verbal hallucinations in schizophrenia. Schizophr Bull. 2012;38:779–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mechelli A, Allen P, Amaro E, Jr, et al. Misattribution of speech and impaired connectivity in patients with auditory verbal hallucinations. Hum Brain Mapp. 2007;28:1213–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Vercammen A, Knegtering H, Bruggeman R, Aleman A. Subjective loudness and reality of auditory verbal hallucinations and activation of the inner speech processing network. Schizophr Bull. 2011;37:1009–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nenadic I, Smesny S, Schlösser RG, Sauer H, Gaser C. Auditory hallucinations and brain structure in schizophrenia: voxel-based morphometric study. Br J Psychiatry. 2010;196:412–413. [DOI] [PubMed] [Google Scholar]
- 40. Leech R, Kamourieh S, Beckmann CF, Sharp DJ. Fractionating the default mode network: distinct contributions of the ventral and dorsal posterior cingulate cortex to cognitive control. J Neurosci. 2011;31:3217–3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rotarska-Jagiela A, van de Ven V, Oertel-Knöchel V, Uhlhaas PJ, Vogeley K, Linden DE. Resting-state functional network correlates of psychotic symptoms in schizophrenia. Schizophr Res. 2010;117:21–30. [DOI] [PubMed] [Google Scholar]
- 42. Kalaitzakis ME, Christian LM, Moran LB, Graeber MB, Pearce RK, Gentleman SM. Dementia and visual hallucinations associated with limbic pathology in Parkinson’s disease. Parkinsonism Relat Disord. 2009;15:196–204. [DOI] [PubMed] [Google Scholar]
- 43. Bentivoglio M, Morelli M. The organization and circuits of mesencephalic dopaminergic neurons and the distribution of dopamine receptors in the brain. In: Dunett SB, Bentivoglio M, Björklund A, Hökfelt T. eds. Handbook Chemical Neuroanatomy vol. 21: Dopamine. Amsterdam, the Netherlands: Elsevier science; 2005:1–108. [Google Scholar]
- 44. Sorg C, Manoliu A, Neufang S, et al. Increased intrinsic brain activity in the striatum reflects symptom dimensions in schizophrenia. Schizophr Bull. 2013;39:387–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Craig AD. How do you feel–now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. [DOI] [PubMed] [Google Scholar]
- 46. Wylie KP, Tregellas JR. The role of the insula in schizophrenia. Schizophr Res. 2010;123:93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jardri R, Thomas P, Delmaire C, Delion P, Pins D. The neurodynamic organization of modality-dependent hallucinations. Cereb Cortex. 2013;23:1108–1117. [DOI] [PubMed] [Google Scholar]
- 50. Palaniyappan L, Mallikarjun P, Joseph V, White TP, Liddle PF. Reality distortion is related to the structure of the salience network in schizophrenia. Psychol Med. 2011;41:1701–1708. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.