Abstract
Anatomical deficits and resting-state functional connectivity (FC) alterations in prefrontal-thalamic-cerebellar circuit have been implicated in the neurobiology of schizophrenia. However, the effect of structural deficits in schizophrenia on causal connectivity of this circuit remains unclear. This study was conducted to examine the causal connectivity biased by structural deficits in first-episode, drug-naive schizophrenia patients. Structural and resting-state functional magnetic resonance imaging (fMRI) data were obtained from 49 first-episode, drug-naive schizophrenia patients and 50 healthy controls. Data were analyzed by voxel-based morphometry and Granger causality analysis. The causal connectivity of the integrated prefrontal-thalamic (limbic)-cerebellar (sensorimotor) circuit was partly affected by structural deficits in first-episode, drug-naive schizophrenia as follows: (1) unilateral prefrontal-sensorimotor connectivity abnormalities (increased driving effect from the left medial prefrontal cortex [MPFC] to the sensorimotor regions); (2) bilateral limbic-sensorimotor connectivity abnormalities (increased driving effect from the right anterior cingulate cortex [ACC] to the sensorimotor regions and decreased feedback from the sensorimotor regions to the right ACC); and (3) bilateral increased and decreased causal connectivities among the sensorimotor regions. Some correlations between the gray matter volume of the seeds, along with their causal effects and clinical variables (duration of untreated psychosis and symptom severity), were also observed in the patients. The findings indicated the partial effects of structural deficits in first-episode, drug-naive schizophrenia on the prefrontal-thalamic (limbic)-cerebellar (sensorimotor) circuit. Schizophrenia may reinforce the driving connectivities from the left MPFC or right ACC to the sensorimotor regions and may disrupt bilateral causal connectivities among the sensorimotor regions.
Key words: first-episode schizophrenia, causal effect, structural deficits, voxel-based morphometry, Granger causality analysis
Introduction
Dysconnectivity hypotheses postulate that schizophrenia is a disorder with abnormal neuronal connectivity,1–4 including abnormal functional interactions of the prefrontal-thalamic-cerebellar circuit.5–8 Anatomically, this circuit is organized to link into parallel pathways.9,10 For instance, the prefrontal cortex (PFC) is connected to the anterior and dorsomedial thalamus, whereas sensorimotor gyri are linked to the ventral lateral thalamus.9 The cerebellum is connected to the cerebrum through the middle and superior cerebellar peduncles.
Neuroimaging studies have provided broad support for the abnormalities of the prefrontal-thalamic-cerebellar circuit in schizophrenia.8,11–16 Eg, Rusch et al17 observed volumetric reductions of this circuit in patients, including brain regions such as the dorsal PFC, anterior cingulate cortex (ACC), thalamus, right parahippocampal gyrus, and cerebellum. A recent meta-review provided preliminary evidence of gray matter abnormalities in this circuit, including ACC, frontal and temporal gyri, hippocampus, amygdala, thalamus, and insula.18 Diffusion tensor imaging studies have indicated significant decreases in the fractional anisotropy (FA) of this circuit in patients, such as decreased FA in the superior longitudinal fasciculus, fornix, internal capsule, and external capsule.19–21 An extensive collection of functional deficits has also been revealed by resting-state fMRI, including functional deficits in prefrontal, temporal, and parietal cortices in schizophrenia patients.22–26 Using resting-state fMRI, Woodward et al7 revealed decreased prefrontal-thalamic connectivity and increased sensorimotor-thalamic connectivity in a group of chronic schizophrenia patients. They indicated that the prefrontal-thalamic-cerebellar circuit is differentially affected in schizophrenia. A recent study also reported the thalamic-cerebellar disconnection in schizophrenia.8
Although previous studies have provided valuable findings on the dysconnectivity of the prefrontal-thalamic-cerebellar circuit in schizophrenia, 2 issues should be considered when interpreting these findings. First, most previous studies have recruited chronic and medicated patients, which may introduce numerous confounding factors because of medication and long illness duration. Schizophrenia is a progressive mental disorder,27,28 and the duration of untreated psychosis (DUP) may have a neurotoxic effect on the gray matter.29,30 Eg, prolonged DUP is reported to be related to reduced gray matter volume (GMV) in the right superior temporal gyrus (STG)31 and caudate nucleus.32 Exposure to antipsychotic drugs can also significantly confound the findings of both anatomical and functional neuroimaging studies.33,34 Hence, first-episode, drug-naive schizophrenia should be selected as a starting point for neuroimaging studies to avoid possible confounding factors such as long illness duration and medication use, and to provide the naive topological information to the neurobiology of schizophrenia. The second issue refers to the direction of abnormal information flow of the prefrontal-thalamic-cerebellar circuit, which remains unknown in schizophrenia based on previous FC methods.
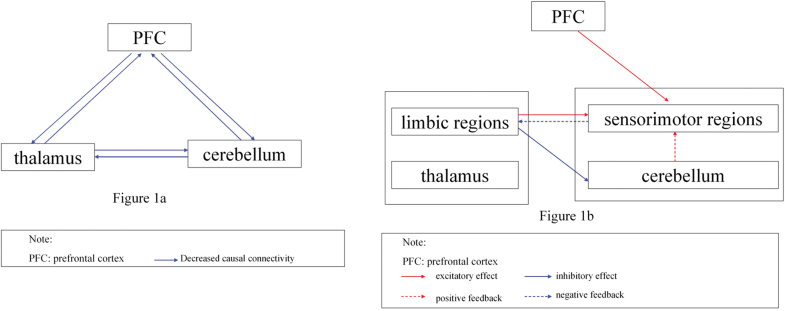
In the present study, a relatively large sample of first-episode, drug-naive schizophrenia patients were examined to thoroughly specify aberrant functional interrelations of the prefrontal-thalamic-cerebellar circuit by estimating anomalous structural deficits. First, the voxel-based morphometry (VBM) method was used to investigate the whole-brain gray matter differences in patients; the brain regions with reduced GMV were selected as seeds. Granger causality analysis (GCA) was then used to detect abnormal causal connectivity between the seeds and other brain regions. GCA is based on the F test for residual in multi-regression analyses.35,36 Given that the F value is always positive, the classical GCA method can only identify the positive effects between brain regions. However, both positive and negative causal effects are essential to maintain normal brain function. To overcome this limitation, a signed regression coefficient β of coefficient-based GCA was used to assess the Granger effect.37–40 A positive β may represent excitatory effect or positive feedback, whereas a negative β may represent inhibitory effect or negative feedback.37,40 This study aims to determine the naive alterations of the causal connectivity between the seeds and other brain regions by recruiting a group of first-episode, drug-naive schizophrenia patients. We hypothesized that the causal relations with the seeds are reduced in schizophrenia patients because of structural deficits according to the general notion that FC is reduced overall in schizophrenia (eg, see reference 41), particularly in the prefrontal-thalamic-cerebellar circuit (see figure 1a). We further examined whether or not decreased GMV of the seeds and/or abnormal causal connectivity are correlated to clinical variables, such as DUP and symptom severity.
Fig. 1.
Hypothesized model and integrated model in first-episode, drug-naive schizophrenia. (a) Overall reduced causal connectivities in prefrontal-thalamic-cerebellar circuit in the hypothesized model. (b) Affected causal connectivities in prefrontal-thalamic (limbic)-cerebellar (sensorimotor) circuit in the integrated model.
Materials and methods
Participants
The participants included 50 right-handed healthy controls and 49 right-handed schizophrenia patients. Schizophrenia was diagnosed through the consensus of 2 experienced clinical psychiatrists using the Structural Clinical Interview for DSM-IV (SCID), patient version.42 Symptom severity was rated by Positive and Negative Symptom Scale (PANSS) at scan time. The patients were at their first episode and were drug naive. The DUP for the patients was less than 3 years. The exclusion criteria were as follows: neurological disorders, severe medical disorders, substance abuse, or any contraindications for MRI. Healthy controls with a first-degree relative who has any psychiatric disorder were also excluded. All participants had more than 9 years of education level and aged from 16 to 30 years.
The local ethics committee of the First Affiliated Hospital of Guangxi Medical University approved the study. Written informed consent was obtained from each participant.
MRI Acquisition
Scanning was conducted on a Siemens 3T scanner. Participants were required to keep still with their eyes closed and remain awake. Each participant received high-resolution T1-weighted volumetric 3D images using a spoiled gradient recall sequence (repetition time = 8.5ms, echo time = 2.98ms, inversion time = 900ms, flip angle = 9°, acquisition matrix = 256×256, field of view = 240 × 240mm3, slice thickness = 1mm, no gap, and 176 slices) and a resting-state fMRI scan using a gradient-echo echo-planar imaging (EPI) sequence (repetition time/echo time = 2000ms/30ms, 30 slices, 64×64 matrix, 90° flip angle, 24cm field of view, 4mm slice thickness, 0.4mm gap, and 250 volumes).
Anatomical Data Analyses
Each scan was visually inspected for artifacts. The anatomical scans were processed using the VBM toolbox (VBM8, http://dbm.neuro.uni-jena.de/vbm) of the Statistical Parametric Mapping software package (SPM8, http://www.fil.ion.ucl.ac.uk/spm). First, anatomical scans were registered to the same template by assessing the 12-parameter affine transformation. The scans were then segmented for signal intensity and prior probability information. Segmented scans were spatially normalized and resampled to 1.5 × 1.5 × 1.5mm3. Intensity modulation was performed on optimally normalized segmented gray matter images by increasing or reducing voxel intensity, depending on whether or not the surrounding voxels compressed or expanded after normalization. Finally, the obtained gray matter scans were smoothed with an 8mm full-width at half-maximum (FWHM) Gaussian kernel.
Two-sample t tests were applied to compare the differences of anatomical data between groups. Age and sex ratios were used as covariates in the analyses to minimize the potential effect of these factors, although both variables showed no significant difference between groups. A corrected significance level of P < .005 was acquired using Gaussian random field (GRF) theory (min z > 2.807, cluster significance: P < .005).
Functional Data Preprocessing
Data Processing Assistant for Resting-State fMRI43 was used to preprocess functional data in Matlab. Participants should have no more than 2mm of maximal translation of x, y, or z and 2° of maximal rotation after the correction of slice timing and head movement. The obtained scans were spatially normalized to the standard Montreal Neurological Institute EPI template in SPM8 and resampled to 3 × 3 × 3mm3. The images were then smoothed with an 8mm FWHM Gaussian kernel, band-pass filtered (0.01–0.08 Hz), and linearly detrended. Several spurious covariates, along with their temporal derivatives, including 6 head motion parameters obtained by rigid body correction, signal from a ventricular region of interest, and signal from a region centered in the white matter, were removed from analyses with linear regression.44,45 However, the global signal was not removed in the present study because regressing out the global signal in preprocessing resting-state FC data would be controversial.44,46–48
GCA Processing
Based on the obtained anatomical data map, regions with decreased GMV were selected as seeds. The peak voxel of each seed was chosen as a 6 mm-radius sphere seed for GCA. Voxel-wise coefficient GCA was conducted in the whole brain using REST software.49 Vector autoregressive models were used to estimate Granger causality to determine whether or not the past value of a time series can correctly forecast the present value of another. When the combination of the past value of the time series X and Y can estimate the current value of Y more accurately than the past value of Y alone, the time series X has causal effect on time series Y. Coefficient-based GCA assessed the Granger effect with coefficient β in vector autoregressive models.37–40 A positive value of β may imply excitatory effect or positive feedback, whereas a negative β may imply inhibitory effect or negative feedback. In the present study, we applied bivariate coefficient GCA to explore the causal effect between the seeds and other voxels of the whole brain. Each seed was examined by seed-to-whole-brain and whole-brain-to-seed analyses. The seed-to-whole-brain analysis was applied to estimate the driving effect (excitatory or inhibitory effects) from the seed to other voxels of the whole brain, whereas the whole-brain-to-seed analysis was used to estimate the feedback effect (positive or negative feedback) from the other voxels of the whole brain to the seed. Two-sample t tests were conducted on the causal effects between patients and controls with a GRF-corrected significance level of P < .005 (min z > 2.807, cluster significance: P < .005). Age and sex ratio were applied as covariates in the 2-sample t tests. The framewise displacement (FD) values for each participant was computed as described in a previous study.50 The mean FD was also applied as a covariate in the group comparisons.
Correlation Analyses
To examine the correlations between altered GMV and causal effect or clinical variables, mean z values of GMV and causal effect were extracted from the seeds and brain regions with abnormal causal effect, respectively. After assessing the normality of the data, Pearson correlations were performed between these variables.
Results
Characteristics of Participants
The patients and controls have no significant differences in age, sex ratio, education level, and FD values (table 1).
Table 1.
Characteristics of Participants
| Patients (n = 49) | Controls (n = 50) | P Value | |
|---|---|---|---|
| Sex (male/female) | 30/19 | 23/27 | .13a |
| Age (years) | 22.69 ± 4.62 | 23.48 ± 2.49 | .30b |
| Years of education (years) | 10.94 ± 2.40 | 11.46 ± 1.78 | .22b |
| FD (mm) | 0.06 ± 0.05 | 0.05 ± 0.02 | .20b |
| DUP (months) | 22.45 ± 6.71 | ||
| PANSS | |||
| Positive scores | 22.27 ± 5.33 | ||
| Negative scores | 22.82 ± 6.86 | ||
| Total scores | 91.31 ± 10.98 | ||
Note: DUP, duration of untreated psychosis; FD, framewise displacement; PANSS, Positive and Negative Symptom Scale.
aThe P value for gender distribution was obtained by chi-square test.
bThe P values were obtained by 2-sample t tests.
Anatomical Differences Between Groups
Compared with the controls, the patients showed significantly reduced GMV in the left medial PFC (MPFC), right ACC, left STG, left inferior temporal gyrus (ITG), left fusiform gyrus, and right lingual gyrus (supplementary figure S1 and table 2), which were selected as seeds for the subsequent GCA. No significantly increased GMV was found for the patients relative to the controls.
Table 2.
Regions With Decreased Gray Matter Volume in Patients
| Cluster location | Peak (MNI) | Number of Voxels | T Value | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Left medial prefrontal cortex | −4.5 | 33 | −15 | 49 | −3.9796 |
| Right anterior cingulate cortex | 10.5 | 28.5 | 25.5 | 42 | −4.4521 |
| Left superior temporal gyrus | −45 | −36 | 18 | 56 | −4.3831 |
| Left inferior temporal gyrus | −57 | −54 | −15 | 41 | −4.5943 |
| Left fusiform gyrus | −28.5 | −40.5 | −15 | 143 | −5.3703 |
| Right lingual gyrus | 16.5 | −67.5 | −10.5 | 46 | −4.1553 |
Note: MNI, Montreal Neurological Institute.
Voxel-wise GCA: Seed-to-Whole-Brain Analysis
As shown in supplementary figures S2–S6 and table 3, the driving effects from the left MPFC and the right ACC to the frontal, temporal, and parietal lobes, as well as the driving effects from the left fusiform gyrus to the occipital gyrus, were increased in patients compared with those in healthy controls. However, the driving effects from the right ACC to the cerebellum vermis 4, 5 decreased in patients. By contrast, the driving effects from the left ITG and left STG to the right middle frontal gyrus and left ITG decreased in patients.
Table 3.
Regions With Abnormal Causal Effect With the Seeds in Patients
| Cluster Location | Peak (MNI) | Number of Voxels | T Valuea | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Seed-to-whole-brain effect | |||||
| Seed: Left medial prefrontal cortex | |||||
| Left middle temporal gyrus | −51 | −15 | −21 | 105 | 3.8529 |
| Right angular gyrus | 42 | −48 | 24 | 128 | 4.4152 |
| Seed: Right anterior cingulate cortex | |||||
| Cerebellum vermis 4,5 | 3 | −54 | −21 | 76 | −4.0020 |
| Right supramarginal gyrus | 51 | −27 | 18 | 61 | 4.0283 |
| Left superior occipital gyrus | −15 | −96 | 24 | 110 | 4.8257 |
| Right superior occipital gyrus | 27 | −84 | 27 | 134 | 3.7840 |
| Right supramarginal gyrus | 69 | −42 | 24 | 54 | 4.6773 |
| Right superior frontal gyrus | 27 | 0 | 66 | 51 | 3.3449 |
| Seed: Left superior temporal gyrus | |||||
| Left inferior temporal gyrus | −36 | −3 | −33 | 80 | −4.0702 |
| Seed: Left inferior temporal gyrus | |||||
| Right middle frontal gyrus | 45 | 45 | 30 | 61 | −3.8534 |
| Seed: Left fusiform gyrus | |||||
| Left lingual gyrus | −12 | −75 | −3 | 153 | 4.0313 |
| Right calcarine cortex | 33 | −54 | 15 | 40 | 3.7471 |
| Left superior occipital gyrus | −15 | −90 | 24 | 64 | 3.6024 |
| Seed: Right lingual gyrus | |||||
| None | |||||
| Whole-brain-to-seed effect | |||||
| Seed: Left medial prefrontal cortex | |||||
| None | |||||
| Seed: Right anterior cingulate cortex | |||||
| Left middle occipital gyrus | −36 | −93 | 15 | 47 | −3.5923 |
| Right middle occipital gyrus | 30 | −84 | 24 | 68 | −4.0408 |
| Seed: Left superior temporal gyrus | |||||
| None | |||||
| Seed: Left inferior temporal gyrus | |||||
| Left cerebellum Crus1 | −30 | −69 | −33 | 56 | 3.9539 |
| Left supramarginal gyrus | −57 | −21 | 21 | 64 | −4.0336 |
| Bilateral supplementary motor area | 9 | −6 | 63 | 115 | −4.3348 |
| Seed: Left fusiform gyrus | |||||
| Right angular gyrus | 42 | −48 | 24 | 41 | −3.695 |
| Seed: Right lingual gyrus | |||||
| Right inferior temporal gyrus | 51 | −57 | −21 | 81 | 4.7053 |
| Left superior parietal gyrus | −27 | −57 | 51 | 72 | 4.6021 |
Note: Abbreviation is explained in the first footnote to table 2.
aA positive T value represents an increased causal effect and a negative T value represents a decreased causal effect.
Voxel-wise GCA: Whole-Brain-to-Seed Analysis
Between-group comparisons exhibited negative feedback from the bilateral middle occipital gyrus to the right ACC, from the left supramarginal gyrus and bilateral supplementary motor area to the left ITG, and from the right angular gyrus to the left fusiform gyrus. By contrast, the causal effects from the left cerebellum Crus I to the left ITG, as well as from the right ITG or left superior parietal gyrus to the right lingual gyrus increased in patients (supplementary figures S7–S10 and table 3).
Correlations Between Abnormal GMV, Causal Effects, and Clinical Variables in Patients
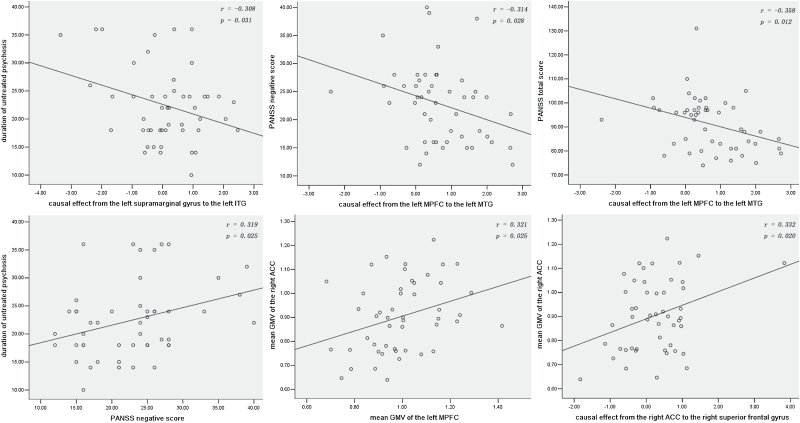
As shown in figure 2, negative correlations were found between the DUP and causal effect from the left supramarginal gyrus to the left ITG (r = −.308, P = .031) and between the PANSS negative/total score and causal effect from the left MPFC to the left middle temporal gyrus (MTG) in the patient group (r = −.314, P = .028; r = −.358, P = .012). Positive correlations were found between the DUP and PANSS negative score (r = .319, P = .025), between the mean GMV of the right ACC and the left MPFC (r = .321, P = .025), and between the mean GMV of the right ACC and causal effect from the right ACC to the right superior frontal gyrus (r = .332, P = .020) in patients. No correlation was found between the GMV or causal effect of seeds and age or education level in the patient group.
Fig. 2.
Correlations between abnormal GMV, causal effects, and clinical variables in schizophrenia patients. GMV, gray matter volume; ITG, inferior temporal gyrus; PANSS, Positive and Negative Symptom Scale; MTG, middle temporal gyrus; MPFC, medial prefrontal cortex; ACC, anterior cingulate cortex.
Discussion
Based on the similar function and intimate connections between the thalamus and the limbic regions, as well as between the cerebellum and sensorimotor regions,18,22,24,51–53 we integrated our findings to the prefrontal-thalamic-cerebellar circuit as the prefrontal-thalamic (limbic)-cerebellar (sensorimotor) circuit to interpret our results clearly (figure 1b). The predominant finding of this study indicates that the causal connectivity of the integrated circuit is partly affected by structural deficits in first-episode, drug-naive schizophrenia. The main results include: (1) unilateral prefrontal-sensorimotor connectivity abnormalities (increased driving effect from the left MPFC to the sensorimotor regions), (2) bilateral limbic-sensorimotor connectivity abnormalities (increased driving effect from the right ACC to the sensorimotor regions and decreased feedback from the sensorimotor regions to the right ACC), and (3) bilateral increased and decreased causal connectivities among the sensorimotor regions. We also observed certain correlations between the GMV of the seeds, along with their causal effect and clinical variables (DUP and PANSS scores) in patients, suggesting that GCA has important functional implications for the neurobiology of schizophrenia.
The combination of increased driving effects from the left MPFC or right ACC to the sensorimotor regions and increased bilateral causal connectivities among the sensorimotor regions is the most remarkable finding of the present study, which appears inconsistent with our hypothesis and general consideration that schizophrenia has overall reduced FC at first glance (eg, see the reference 41). However, the present results provide supporting information for the neurondevelopmental model of schizophrenia. Significant differences of the prefrontal-thalamic-cerebellar circuit were observed between healthy children, adolescents, and adults by a seed-based FC study.54 The connectivity of this circuit can represent an inverted U-curve with maximal connectivity in adolescents. Specifically, the prefrontal-thalamic connectivity is absent in healthy children. Our participants aged from 16 to 30 years, the developmental period from adolescents to adults. The development of the prefrontal-thalamic (limbic)-cerebellar (sensorimotor) circuit may be disrupted by schizophrenia in our patient group; the connectivity of this circuit may remain at a relatively high position of the inverted U-curve in the present patient group. Therefore, our patients can exhibit increased connectivity of the prefrontal-thalamic (limbic)-cerebellar (sensorimotor) circuit. However, a precise time-point of developmental changes is difficult to determine because of limited evidence from a single cross-sectional study. Interestingly, the causal connectivity between the PFC and the thalamic or limbic regions is unaffected in our patient group. Considering that prefrontal-thalamic connectivity is absent in healthy children and increases with age in healthy adolescents, the prefrontal-thalamic connectivity develops from childhood to adolescence and reaches the adult level in healthy adolescents.54 Developmental disruption by schizophrenia can occur after the prefrontal-thalamic connectivity fully matured in our patient group. However, the correlation between age and prefrontal-thalamic connectivity is not present in our sample of patients, which seems inconsistent with the findings from healthy subjects (including healthy children, adolescents, and adults).54 The inconsistency may be due to the homogeneity of our sample of patients with the age range from 16 to 30 years. The prefrontal-thalamic connectivity of our sample of patients may reach at a relatively high position of the inverted U-curve suggested by the previous study.54 Therefore, the combination of only including adult patients and concentration of prefrontal-thalamic connectivity contributes to the result of no correlation observed between age and prefrontal-thalamic connectivity in our sample of patients.
The increased causal connectivity is also informed when considering the physiological meaning of FC. Previously, increased FC is commonly explained as compensatory reallocation or dedifferentiation.55–57 From this viewpoint, we speculated that increased causal connectivity may be a compensatory effort for structural deficits. Both the left MPFC and the right ACC exhibited increased driving connectivity with the sensorimotor regions. In addition, a positive correlation was observed between the mean GMV of the left MPFC and the right ACC, indicating that the 2 regions may coactivate with each other to reinforce the connectivity caused by structural deficits.
Our findings are comparable with the results of Woodward et al7, which revealed decreased prefrontal-thalamic connectivity and increased thalamic-sensorimotor connectivity in chronic, medicated schizophrenia patients using 6 regions as seeds: PFC, motor cortex or supplementary motor area, somatosensory cortex, temporal lobe, posterior parietal cortex, and occipital lobe. Although the 2 studies can not be compared directly, we found that the prefrontal-thalamic connectivity decreased with illness duration. Previously, functional abnormalities in frontoparietal connectivity normalize after the patients achieve clinical remission from antipsychotic treatment,34 suggesting that medication is not attributed to the abnormal FC in schizophrenia. Therefore, the 2 studies indicate that progressively decreased prefrontal-thalamic connectivity may be a chronic characteristic of the prolonged illness duration in schizophrenia.
A negative correlation was found between the DUP and negative feedback from the left supramarginal gyrus to the left ITG, indicating that a longer DUP in patients will reflect a lower negative feedback from the left supramarginal gyrus to the left ITG. The supramarginal gyrus is a portion of the parietal lobe that promotes language perception and processing. Prolonged DUP may have a destructive effect on the feedback from the left supramarginal gyrus to the left ITG. Negative correlations were also observed between the PANSS negative/total score and the increased driving connectivity from the left MPFC to the left MTG in patients. Increased frontal connectivity is previously reported to be negatively correlated with the severity of negative symptoms in schizophrenia.58 Consistent with this study58 and the general notion that negative symptoms indicate poor treatment outcome, these correlations revealed that a greater PANSS negative/total score reflects a lower driving connectivity from the left MPFC to the left MTG. A positive correlation was also found between the DUP and PANSS negative score. Therefore, our correlation results are significant for the core symptoms in schizophrenia.
Interestingly, the cerebellum is recruited to participate in abnormal connectivities in the present study, which has a positive feedback to the left ITG and a negative feedback to the right ACC. The cerebellum has long been considered to promote motor coordination. This notion has been challenged by the fact that the cerebellum functions in more extensive deficits.8,21,59,60 In the present study, the cerebellum may provide feedback through the prefrontal-thalamic-cerebellar circuit. As supporting information of this circuit, decreased blood flow was observed in the frontal-thalamic-cerebellar regions in schizophrenia.2
The present study has certain limitations. First, the current report is a cross-sectional study. Whether or not the abnormalities of the prefrontal-thalamic (limbic)-cerebellar (sensorimotor) circuit are altered by illness duration and medication remains unknown. A longitudinal investigation is needed to understand the abnormal trajectories of the prefrontal-thalamic (limbic)-cerebellar (sensorimotor) circuit in schizophrenia. Second, neuropsychological assessment was not conducted in the present study. Thus, the relationship between the causal effect and neuropsychological parameters was not determined. Finally, the present results were acquired at rest. Hence, we can only deduce that the causal effect in schizophrenia in the present study is related to the general pathophysiology of schizophrenia but not with a particular pattern of task or activity.
Despite the limitations, the present study is the first to examine causal connectivity caused by structural deficits in schizophrenia at rest. The findings establish partial effects of structural deficits in first-episode, drug-naive schizophrenia on the prefrontal-thalamic (limbic)-cerebellar (sensorimotor) circuit. Schizophrenia may reinforce the driving connectivities from the left MPFC or right ACC to the sensorimotor regions and may disrupt bilateral causal connectivities among the sensorimotor regions.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
Natural Science Foundation of China (81260210 and 30900483); Natural Science Foundation of Guangxi Province (2013GXNSFAA019107); Natural Science Foundation of Shandong Province (ZR2012HM065); Ministry of Health of China (201002003).
Supplementary Material
Acknowledgments
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Bullmore ET, Frangou S, Murray RM. The dysplastic net hypothesis: an integration of developmental and dysconnectivity theories of schizophrenia. Schizophr Res. 1997;28:143–156. [DOI] [PubMed] [Google Scholar]
- 2. Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24:203–218. [DOI] [PubMed] [Google Scholar]
- 3. Friston KJ. The disconnection hypothesis. Schizophr Res. 1998;30:115–125. [DOI] [PubMed] [Google Scholar]
- 4. Stephan KE, Friston KJ, Frith CD. Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull. 2009;35:509–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Swerdlow NR. Integrative circuit models and their implications for the pathophysiologies and treatments of the schizophrenias. Curr Top Behav Neurosci. 2010;4:555–583. [DOI] [PubMed] [Google Scholar]
- 6. Jones EG. Cortical development and thalamic pathology in schizophrenia. Schizophr Bull. 1997;23:483–501. [DOI] [PubMed] [Google Scholar]
- 7. Woodward ND, Karbasforoushan H, Heckers S. Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry. 2012;169:1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen YL, Tu PC, Lee YC, Chen YS, Li CT, Su TP. Resting-state fMRI mapping of cerebellar functional dysconnections involving multiple large-scale networks in patients with schizophrenia. Schizophr Res. 2013;149:26–34. [DOI] [PubMed] [Google Scholar]
- 9. Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. [DOI] [PubMed] [Google Scholar]
- 10. Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26:317–330. [DOI] [PubMed] [Google Scholar]
- 11. Shenton ME, Dickey CC, Frumin M, McCarley RW. A review of MRI findings in schizophrenia. Schizophr Res. 2001;49:1–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Glahn DC, Laird AR, Ellison-Wright I, et al. Meta-analysis of gray matter anomalies in schizophrenia: application of anatomic likelihood estimation and network analysis. Biol Psychiatry. 2008;64:774–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Andreasen NC. The role of the thalamus in schizophrenia. Can J Psychiatry. 1997;42:27–33. [DOI] [PubMed] [Google Scholar]
- 14. Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66:811–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Andreasen NC, Pierson R. The role of the cerebellum in schizophrenia. Biol Psychiatry. 2008;64:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Welsh RC, Chen AC, Taylor SF. Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in schizophrenia. Schizophr Bull. 2010;36:713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rüsch N, Spoletini I, Wilke M, et al. Prefrontal-thalamic-cerebellar gray matter networks and executive functioning in schizophrenia. Schizophr Res. 2007;93:79–89. [DOI] [PubMed] [Google Scholar]
- 18. Shepherd AM, Laurens KR, Matheson SL, Carr VJ, Green MJ. Systematic meta-review and quality assessment of the structural brain alterations in schizophrenia. Neurosci Biobehav Rev. 2012;36:1342–1356. [DOI] [PubMed] [Google Scholar]
- 19. Kuswanto CN, Teh I, Lee TS, Sim K. Diffusion tensor imaging findings of white matter changes in first episode schizophrenia: a systematic review. Clin Psychopharmacol Neurosci. 2012;10:13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guo W, Liu F, Liu Z, et al. Right lateralized white matter abnormalities in first-episode, drug-naive paranoid schizophrenia. Neurosci Lett. 2012;531:5–9. [DOI] [PubMed] [Google Scholar]
- 21. Liu H, Fan G, Xu K, Wang F. Changes in cerebellar functional connectivity and anatomical connectivity in schizophrenia: a combined resting-state functional MRI and diffusion tensor imaging study. J Magn Reson Imaging. 2011;34:1430–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gur RE, Gur RC. Functional magnetic resonance imaging in schizophrenia. Dialogues Clin Neurosci. 2010;12:333–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brown GG, Thompson WK. Functional brain imaging in schizophrenia: selected results and methods. Curr Top Behav Neurosci. 2010;4:181–214. [DOI] [PubMed] [Google Scholar]
- 24. van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol. 2010;20:519–534. [DOI] [PubMed] [Google Scholar]
- 25. Guo W, Su Q, Yao D, et al. Decreased regional activity of default-mode network in unaffected siblings of schizophrenia patients at rest. Eur Neuropsychopharmacol. 2014;24:545–552. [DOI] [PubMed] [Google Scholar]
- 26. Guo W, Xiao C, Liu G, et al. Decreased resting-state interhemispheric coordination in first-episode, drug-naive paranoid schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2014;48:14–19. [DOI] [PubMed] [Google Scholar]
- 27. Asami T, Bouix S, Whitford TJ, Shenton ME, Salisbury DF, McCarley RW. Longitudinal loss of gray matter volume in patients with first-episode schizophrenia: DARTEL automated analysis and ROI validation. Neuroimage. 2012;59:986–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chan RC, Di X, McAlonan GM, Gong QY. Brain anatomical abnormalities in high-risk individuals, first-episode, and chronic schizophrenia: an activation likelihood estimation meta-analysis of illness progression. Schizophr Bull. 2011;37:177–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Perkins DO, Gu H, Boteva K, Lieberman JA. Relationship between duration of untreated psychosis and outcome in first-episode schizophrenia: a critical review and meta-analysis. Am J Psychiatry. 2005;162:1785–1804. [DOI] [PubMed] [Google Scholar]
- 30. Marshall M, Lewis S, Lockwood A, Drake R, Jones P, Croudace T. Association between duration of untreated psychosis and outcome in cohorts of first-episode patients: a systematic review. Arch Gen Psychiatry. 2005;62:975–983. [DOI] [PubMed] [Google Scholar]
- 31. Guo X, Li J, Wei Q, et al. Duration of untreated psychosis is associated with temporal and occipitotemporal gray matter volume decrease in treatment naïve schizophrenia. PLoS One. 2013;8:e83679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Crespo-Facorro B, Roiz-Santiáñez R, Pelayo-Terán JM, et al. Caudate nucleus volume and its clinical and cognitive correlations in first episode schizophrenia. Schizophr Res. 2007;91:87–96. [DOI] [PubMed] [Google Scholar]
- 33. Lieberman JA, Tollefson GD, Charles C, et al. ; HGDH Study Group. Antipsychotic drug effects on brain morphology in first-episode psychosis. Arch Gen Psychiatry. 2005;62:361–370. [DOI] [PubMed] [Google Scholar]
- 34. Lui S, Li T, Deng W, et al. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Arch Gen Psychiatry. 2010;67:783–792. [DOI] [PubMed] [Google Scholar]
- 35. Morgan VL, Rogers BP, Sonmezturk HH, Gore JC, Abou-Khalil B. Cross hippocampal influence in mesial temporal lobe epilepsy measured with high temporal resolution functional magnetic resonance imaging. Epilepsia. 2011;52:1741–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Szaflarski JP, DiFrancesco M, Hirschauer T, et al. Cortical and subcortical contributions to absence seizure onset examined with EEG/fMRI. Epilepsy Behav. 2010;18:404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chen G, Hamilton JP, Thomason ME, Gotlib IH, Saad ZS, Cox RW. Granger Causality via Vector Auto-Regression Tuned for FMRI Data Analysis. Proc Intl Soc Mag Reson Med. 2009;17:1718. [Google Scholar]
- 38. Guye M, Régis J, Tamura M, et al. The role of corticothalamic coupling in human temporal lobe epilepsy. Brain. 2006;129:1917–1928. [DOI] [PubMed] [Google Scholar]
- 39. Hamilton JP, Chen G, Thomason ME, Schwartz ME, Gotlib IH. Investigating neural primacy in Major Depressive Disorder: multivariate Granger causality analysis of resting-state fMRI time-series data. Mol Psychiatry. 2011;16:763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ji GJ, Zhang Z, Zhang H, et al. Disrupted causal connectivity in mesial temporal lobe epilepsy. PLoS One. 2013;8:e63183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Karlsgodt KH, Sun D, Jimenez AM, et al. Developmental disruptions in neural connectivity in the pathophysiology of schizophrenia. Dev Psychopathol. 2008;20:1297–1327. [DOI] [PubMed] [Google Scholar]
- 42. First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders (SCID). Washington, DC: American Psychiatric Press; 1997. [Google Scholar]
- 43. Yan C, Zang Y. DPARSF: A MATLAB toolbox for “pipeline” data analysis of resting-state fMRI. Front Syst Neurosci. 2010;4:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102:9673–9678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Liu F, Guo W, Fouche JP, et al. Multivariate classification of social anxiety disorder using whole brain functional connectivity [published online ahead of print September 27, 2013]. Brain Struct Funct. 10.1007/s00429-013-0641-4. [DOI] [PubMed] [Google Scholar]
- 46. Murphy K, Birn RM, Handwerker DA, Jones TB, Bandettini PA. The impact of global signal regression on resting state correlations: are anti-correlated networks introduced? Neuroimage. 2009;44:893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Saad ZS, Gotts SJ, Murphy K, et al. Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect. 2012;2:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Liu F, Hu M, Wang S, et al. Abnormal regional spontaneous neural activity in first-episode, treatment-naive patients with late-life depression: a resting-state fMRI study. Prog Neuropsychopharmacol Biol Psychiatry. 2012;39:326–331. [DOI] [PubMed] [Google Scholar]
- 49. Song XW, Dong ZY, Long XY, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6:e25031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2012;59:2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Ren W, Lui S, Deng W, et al. Anatomical and functional brain abnormalities in drug-naive first-episode schizophrenia. Am J Psychiatry. 2013;170:1308–1316. [DOI] [PubMed] [Google Scholar]
- 52. Lui S, Deng W, Huang X, et al. Association of cerebral deficits with clinical symptoms in antipsychotic-naive first-episode schizophrenia: an optimized voxel-based morphometry and resting state functional connectivity study. Am J Psychiatry. 2009;166:196–205. [DOI] [PubMed] [Google Scholar]
- 53. Jiang T, Zhou Y, Liu B, Liu Y, Song M. Brainnetome-wide association studies in schizophrenia: the advances and future. Neurosci Biobehav Rev. 2013;37:2818–2835. [DOI] [PubMed] [Google Scholar]
- 54. Fair DA, Bathula D, Mills KL, et al. Maturing thalamocortical functional connectivity across development. Front Syst Neurosci. 2010;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage. 2002;17:1394–1402. [DOI] [PubMed] [Google Scholar]
- 56. Grady CL, McIntosh AR, Craik FI. Task-related activity in prefrontal cortex and its relation to recognition memory performance in young and old adults. Neuropsychologia. 2005;43:1466–1481. [DOI] [PubMed] [Google Scholar]
- 57. Guo W, Liu F, Liu J, et al. Is there a cerebellar compensatory effort in first-episode, treatment-naive major depressive disorder at rest? Prog Neuropsychopharmacol Biol Psychiatry. 2013;46:13–18. [DOI] [PubMed] [Google Scholar]
- 58. Mingoia G, Wagner G, Langbein K, et al. Default mode network activity in schizophrenia studied at resting state using probabilistic ICA. Schizophr Res. 2012;138:143–149. [DOI] [PubMed] [Google Scholar]
- 59. Honey GD, Pomarol-Clotet E, Corlett PR, et al. Functional dysconnectivity in schizophrenia associated with attentional modulation of motor function. Brain. 2005;128:2597–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schmahmann JD. The role of the cerebellum in cognition and emotion: personal reflections since 1982 on the dysmetria of thought hypothesis, and its historical evolution from theory to therapy. Neuropsychol Rev 2010;20:236–260. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.