Abstract
Background:
Schizophrenia is a multifaceted mental disorder characterized by cognitive, perceptual, and affective symptom dimensions. This heterogeneity at the phenomenological level may be subserved by complex and heterogeneous patterns of structural abnormalities. Thus, delineating such patterns may improve the insight into the variability of disease and facilitate future magnetic resonance imaging-based diagnosis.
Methods:
We aimed to identify structurally complex signatures that directly differentiate patients with predominantly negative (pNEG), positive (pPOS), and disorganized (pDIS) symptoms using Optimally-Discriminative Voxel-Based Analysis (ODVBA). ODVBA is a new analytical framework for group analysis, which showed to have superior sensitivity and specificity over conventional voxel-based morphometric approaches, thus facilitating the identification of subtle neuroanatomical signatures delineating different subgroups.
Results:
pPOS were characterized by pronounced gray matter (GM) volume reductions in the ventromedial prefrontal cortex (vmPFC), which herein is defined to include the orbitofrontal cortex, and in occipitotemporal GM and parts of the lingual gyrus. pNEG was found to have vmPFC reduction but to a lesser degree than pPOS and with a relative sparing of the more medial vmPFC regions, compared to pDIS; it also had significantly less cerebellar GM. pDIS showed relatively highest GM volume preservation among three subtypes.
Conclusions:
Although a common prefronto-perisylvian GM reduction pattern was present at the whole-group level, marked morphometric differences emerged between the three subgroups, including reduced cerebellar GM in pNEG and reduced vmPFC and occipitotemporal GM in pPOS. Besides deepening our insight into the neurobiological underpinnings of clinical heterogeneity, these results also identify important imaging biomarkers that may aid patient stratification.
Key words: volume reduction, ODVBA, voxel-based morphometry, patient stratification
Introduction
The clinical heterogeneity of schizophrenia has seriously impeded efforts to uncover the pathophysiology behind this mental disorder. Hence, it was suggested1–3 that the diverse clinical syndromes of schizophrenia should be subdivided into different nosological entities based on distinct symptom profiles of the disorder. Classical factor analytic studies of schizophrenic symptoms have generally agreed upon a 3-axes model of schizophrenic symptomatology4–6 consisting of negative, positive, and disorganized symptom dimensions, which may provide a valid top-down approach for defining more homogeneous subgroups characterized by distinct neural signatures. Associated neuropsychological studies7,8 also demonstrated that these 3 distinct schizophrenic syndromes are associated with different patterns of neurocognitive performance. Already early neuroimaging studies employing regions of interest (ROI) approaches9–17 measured the associations between circumscribed brain regions and these symptom domains. However, the ROI approaches are limited by high dependency on the prior knowledge and loss of the complex structural information inside the defined regions.
Voxel-based morphometry (VBM),18–20 a neuroimaging technique that does not need to define ROI and is capable of discovering regionally specific alterations in brain volumes, has been extensively applied21–29 in the monistic analysis of schizophrenia based on the hypothesis of a single, unifying pathophysiological process. Additionally, some studies utilized voxel-wise regression analyses30–32 to examine the associations between cerebral alterations and symptoms scores in patients with schizophrenia. Nevertheless, there are only few magnetic resonance imaging (MRI) studies3,33–35 that employed VBM to delineate the neuroanatomical underpinnings of clinically defined subgroups within schizophrenia: Sigmundsson et al3 found mainly prefrontal volumetric alterations in a homogeneous sample of schizophrenic patients with predominant and enduring negative symptoms compared to healthy controls. More recently, 2 studies34,35 aimed at elucidating the neuroanatomical surrogates of the disease’s clinical heterogeneity by comparing a group of healthy controls with 3 patient subgroups, representing the negative, positive, and disorganized symptom dimensions, as determined by factor analytic approaches. Importantly, these studies tried to identify group-level anatomical differences between each psychopathological subgroup and healthy controls using univariate statistical methods, thus not directly addressing the question whether subtle differentiating patterns can be detected when directly comparing psychopathologically defined subgroups of schizophrenia (eg, negative vs positive, negative vs disorganized, positive vs disorganized). Modeling these potentially fine-grained neuroanatomical differences between distinct dimensions of schizophrenic symptomatology using novel multivariate analytical methods would allow us to better understand the heterogeneity of schizophrenia and hence enable us to build more appropriate diagnostic tools for this complex disorder in the future.
Thus, we aimed at identifying structurally distributed signatures that directly differentiate patients with predominant negative, positive, and disorganized schizophrenic symptoms by means of Optimally-Discriminative Voxel-Based Analysis (ODVBA).36 ODVBA is a recently proposed imaging pattern analysis framework for group comparisons that utilizes a spatially adaptive analysis scheme, which accounts for the interrelatedness of spatial information in the brain, and thus provides superior sensitivity and specificity compared to conventional VBM approaches, which employ spatially fixed Gaussian smoothing plus general linear modeling (GLM).37 ODVBA has been extensively validated in both the simulated data, in which the ground truth is known36 and real structural MRI data from various studies.36,38 Thus, ODVBA may provide the level of sensitivity required to detect subtle structural brain patterns that directly differentiate subgroups of patients with schizophrenia. To the best of our knowledge, this is the first voxel-based morphometric study to investigate the direct group differences on measures of brain structures between distinct subgroups within schizophrenia.
Materials and methods
Subjects
The subjects of this study were recruited at the Department of Psychiatry and Psychotherapy at Ludwig-Maximilians University, Munich, Germany, and included 163 patients with an established DSM-IV diagnosis of schizophrenia and 163 matched normal controls (NC).
All participants provided their written informed consent prior to MRI and clinical examination. Patient recruitment was performed by trained clinical investigators and consisted of a structured clinical interview for DSM-IV-axis I disorders (SCID-I), a standardized clinical interview for the assessment of medical and psychiatric history, the review of patients’ records, and the evaluation of disease severity and psychopathology by means of the Positive and Negative Syndrome Scale (PANSS)39 at scanning time. All subjects were diagnosed based on a consensus between 2 experienced psychiatrists who used the DSM-IV criteria and the SCID-I. Participants were excluded if they had other psychiatric and/or neurological diseases, past or present regular alcohol abuse, and/or consumption of illicit drugs, as well as past head trauma with loss of consciousness or electroconvulsive treatment.
Factor Analysis and Subgroup Information
Patterns of co-occurring symptoms were analyzed by employing a maximum-likelihood factor analysis (R software package and the oblique PROMAX rotation) on the PANSS items using a 3-factor model of schizophrenic symptomatology. The 3-dimensional model consisted of negative, positive, and disorganized symptom dimensions. The internal consistency of symptom factors was measured by Cronbach’s α, and interfactor correlations were calculated. Each patient was assigned to one of the 3 symptom dimensions34 according to the maximum individual factor score, resulting in a negative (pNEG, 55 subjects), positive (pPOS, 57 subjects), and disorganized factor (pDIS, 51 subjects) sample. Finally, in order to better put these intergroup comparisons into perspective with respect to the overall structural profile of the patient cohort, we also used 163 matched NC involved in this study.
Demographics and raw psychopathology scores of these subgroups are provided in table 1. The duration of untreated psychosis is defined by the interval between age of disease onset and the first reported prescription of antipsychotic agents. No statistical differences were observed between the samples concerning age, gender, handedness, or education.
Table 1.
Demographics and Clinical Characteristics of the Groups
| Variable | pNEG | pPOS | pDIS | NC | Statistics (p Values) | ||
|---|---|---|---|---|---|---|---|
| (N = 55) | (N = 57) | (N = 51) | (N = 163) | (F Test /T Test) | |||
| pNEG vs pPOS |
pNEG vs pDIS |
pPOS vs pDIS |
|||||
| Age at scan (SD) | 32.21 (9.77) | 31.94 (8.81) | 29.78 (10.39) | 31.2 (9.1) | .446/.879 | .657/.217 | .231/.244 |
| Sex (male/female) | 47/8 | 42/15 | 34/17 | 115/48 | — | — | — |
| Handedness (right/left/ambidextrous) | 54/1 | 51/5/1 | 46/4/1 | 152/10/1 | — | — | — |
| Educational years (SD) | 10.56 (1.91) | 10.60 (2.27) | 10.27 (2.25) | 11.6 (1.6) | .212/.934 | .248/.476 | .950/.461 |
| Duration of illness (SD) | 88.26 (100.62) | 36.91 (60.86) | 39.05 (101.68) | — | .000/.002 | .938/.015 | .000/.896 |
| Age of disease onset (SD) | 24.9 (7.8) | 28.5 (8.4) | 26.7 (7.7) | — | .595/.025 | .943/.242 | .554/.272 |
| Recurrent/first episode | 34/21 | 21/36 | 16/35 | — | .008a (6.987b) | .002a (9.843b) | .550a (0.358b) |
| Duration of untreated psychosis (SD) | 3.57 (6.92) | 1.68 (2.82) | 1.58 (4.44) | — | .000/.081 | .003/.103 | .002/.903 |
| PANSS sum score (SD) | 79.62 (19.43) | 90.46 (35.79) | 80.31 (28.72) | — | .000/.052 | .006/.886 | .115/.110 |
| PANSS positive score (SD) | 12.72 (4.53) | 24.38 (8.18) | 19.41 (5.20) | — | .000/.000 | .328/.000 | .001/.000 |
| PANSS negative score (SD) | 26.50 (7.60) | 19.40 (9.79) | 21.67 (10.63) | — | .065/.000 | .017/.008 | .547/.252 |
| PANSS general score (SD) | 40.29 (10.41) | 46.67 (19.64) | 39.24 (16.65) | — | .000/.035 | .001/.694 | .237/.037 |
| PANSS depression score (SD) | 3.40 (1.48) | 2.95 (1.41) | 1.80 (0.98) | — | .686/.100 | .004/.000 | .010/.000 |
Note: NC = normal controls; pDIS= predominantly disorganized; pNEG= predominantly negative; pPOS = predominantly positive.
aIndicates the p values obtained by Pearson’s χ2 test.
bIndicates the associated Pearson’s χ2 values.
Imaging Protocol and Processing
T1-weighted 3D-magnetization-prepared rapid acquisition with gradient echo sequences (repetition time, 11.6ms; echo time, 4.9ms; field of view, 230mm; matrix, 512 × 512; 126 contiguous axial slices of 1.5mm thickness; voxel size, 0.45 × 0.45 × 1.5mm) were acquired on a 1.5 T Magnetom Vision scanner (Siemens, Erlangen, Germany). The images were first preprocessed by means of the VBM8 toolbox (publically available at http://dbm.neuro.uni-jena.de/vbm8/)—an extension of the Statistical Parametric Mapping software (SPM, publically available at http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) for skull stripping, bias correction, and segmentation.40 The skull-removed partial volume estimation images were then spatially registered to the respective partial volume image of the single-subject Monteal Neurological Institute (MNI) template through a robust method for elastic registration called deformable registration via attribute matching and mutual-saliency weighting.41 The deformation field resulting from this spatial registration was then applied to the segmented images in order to generate mass-preserved volumetric maps (often referred as tissue density maps), named Regional Analysis of Volumes Examined in Normalized Space (RAVENS) maps20 of the gray matter (GM), white matter, and cerebrospinal fluid segments. In these RAVENS maps, the tissue density reflects the amount of tissue present in each subject’s image at a given location after mapping to the standardized template space. For example, a region of decreased density indicates a reduced volume in this structure. In this study, we investigated the brain volume changes in terms of the RAVENS values on the GM images.
Statistical Analysis
ODVBA36 is a novel method based on regional multivariate pattern analysis, aiming to optimally detect the between-group differences in brain volumes by estimating the spatially adaptive filtering of the data prior to statistical analyses. ODVBA thereby transcends the limitations of the mass univariate GLM method that applies a predefined and fixed Gaussian filter (typically 8mm), which lacks of the spatial adaptivity to the shape and the spatial extent of group differences. The framework of ODVBA mainly contains 3 phases: regional nonnegative discriminative projection, determining each voxel’s statistic, and permutation tests.42 The details on implementation of ODVBA can be found in the published article.36 ODVBA has been extensively validated in both simulated data and real data.36,38 It has shown to not only improve sensitivity of detection of the subtle structural abnormities but also to better delineate regions of abnormality. This contrasts with conventional smoothing approaches, such as Gaussian filter,37 which always blur the structure of the true differences and dilute the signal from regions of interest with signals from regions that do not display a group difference.
In this study, the parameter of ODVBA is set as same as those used on the real data in the article.36 We used 5000 permutations to derive statistical significance maps. The resulting maps of significance are partitioned and analyzed according to the Automated Anatomical Labeling package.43 On each anatomical region, we calculated the cluster size and the t statistic (based on means of RAVENS values on the detected area per region). Moreover, to address the multiple comparison problem, we also conducted the cluster-wise family-wise error (FWE) correction based on nonparametric permutation testing.44,45 At the voxel level, p value threshold was defined at .001 uncorrected, and for the cluster-wise analysis, an FWE threshold of p <.05 was used.
Results
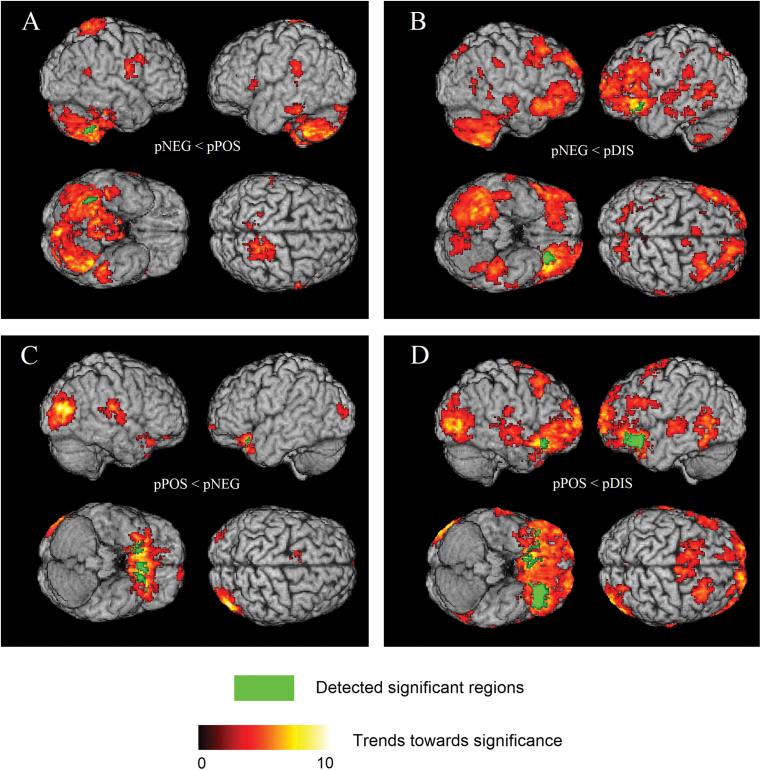
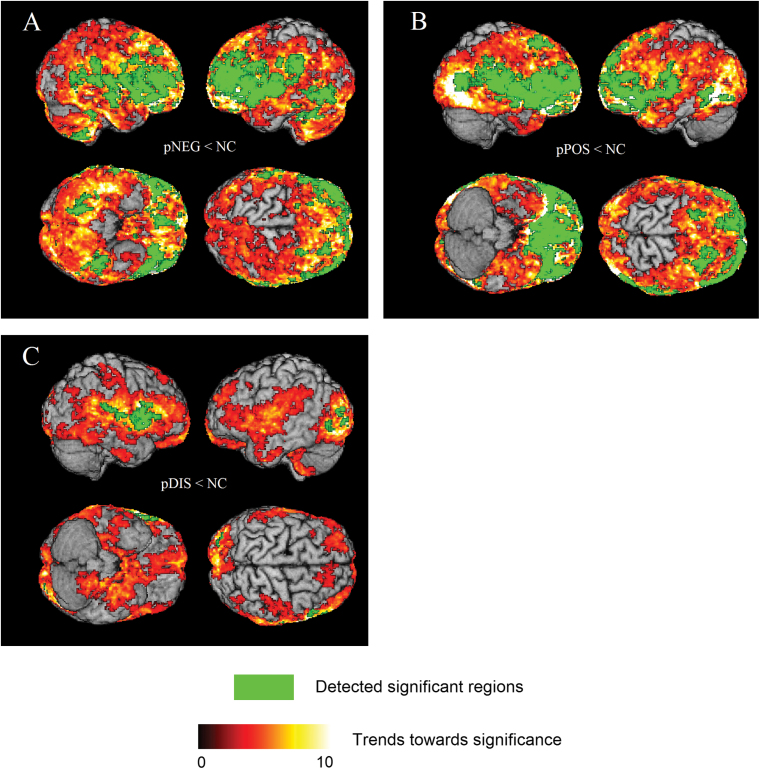
Significant group differences were detected in four comparisons between schizophrenia subgroups: (1) pNEG < pPOS; (2) pNEG < pDIS; (3) pPOS < pNEG; and (4) pPOS < pDIS. The 3D rendering of the results are shown in figure 1. Respective comparisons with controls are shown in figure 2. Anatomical regions and the associated statistical information are listed in table 2. There were no significant differences between groups when we performed the comparisons: (1) pDIS < pNEG; (2) pDIS < pPOS.
Fig. 1.
3D surface renderings of the ODVBA results, obtained from on gray matter (GM) group comparisons of (A) pNEG < pPOS; (B) pNEG < pDIS; (C) pPOS < pNEG; (D) pPOS < pDIS. Hot color represents the trends (uncorrected p < .05) toward significance, indicated by –log(p) value. Green color represents the detected significant regions with FWE corrected p <.05.
Fig. 2.
3D surface renderings of the ODVBA results, obtained from on gray matter (GM) group comparisons of (A) pNEG < NC; (B) pPOS < NC; (C) pDIS < NC. Hot color represents the trends (uncorrected p < .05) toward significance, indicated by –log(p) value. Green color represents the detected significant regions with FWE corrected p <.05.
Table 2.
The Results on the GM Group Comparisons
| Comparisons | Anatomical Regions | Side | GLM | ODVBA | Cluster-wise FWE Correction | ||
|---|---|---|---|---|---|---|---|
| N | t | N | t | ||||
| pNEG < pPOS | Cerebellum | R | \ | \ | 59 | 4.27 | Yes |
| pNEG < pDIS | Inferior orbitofrontal cortex | L | 91 | 4.53 | 198 | 4.98 | Yes |
| Thalamus | R | \ | \ | 110 | 4.08 | No | |
| pPOS < pNEG | Inferior orbitofrontal cortex | L | 57 | 3.83 | 97 | 4.53 | Yes |
| Middle occipital gyrus | R | 62 | 4.54 | 90 | 5.27 | No | |
| Inferior orbitofrontal cortex | R | 60 | 3.77 | 78 | 4.01 | Yes | |
| Gyrus rectus | L | \ | \ | 65 | 4.24 | Yes | |
| pPOS < pDIS | Inferior orbitofrontal cortex | L | 280 | 6.02 | 658 | 6.83 | Yes |
| Inferior orbitofrontal cortex | R | 53 | 4.35 | 164 | 6.56 | Yes | |
| Middle orbitofrontal cortex | L | \ | \ | 105 | 4.26 | Yes | |
| Temporal pole | R | \ | \ | 76 | 5.22 | Yes | |
| Insular cortex | L | \ | \ | 51 | 3.01 | Yes | |
Note: “\” means no significant findings in the voxel level analysis. N denotes the number of significant voxels in each anatomical region. t denotes the t value calculated. “Yes” means the region survives the cluster-wise FWE correction; “No” indicates the contrary. FWE = family-wise error; GM = gray matter; GLM = general linear model; L = left; ODVBA = Optimally-Discriminative Voxel-Based Analysis; pDIS= predominantly disorganized; pNEG= predominantly negative; pPOS = predominantly positive; R = right.
pNEG < pPOS
The patients with negative symptoms showed reduced GM in the cerebellum when compared to the patients with positive symptoms; these differences reached significance on the right side of the cerebellum, after FWE correction, however the trend was pronounced throughout most of the cerebellum.
pNEG < pDIS
The comparison between patients with negative and disorganized symptoms revealed significantly lower GM volumes in the pNEG subgroup, which were predominantly located in the left inferior orbitofrontal cortex, and the right thalamus (no FWE correction).
pPOS < pNEG
The results mainly revealed some regions showing significant volume reduction in patients with positive symptoms vs negative symptoms: the bilateral inferior orbitofrontal cortex, the left gyrus rectus, and an occipitotemporal region (no FWE correction) in the vicinity of the right lingual gyrus.
pPOS < pDIS
Schizophrenia patients with positive symptoms showed significantly reduced volumes relative to those with disorganized symptoms mainly in the bilaterally inferior orbitofrontal cortex, the left middle orbitofrontal cortex, the right temple pole, and the left insular cortex and trends in the right occipitotemporal region (no FWE correction).
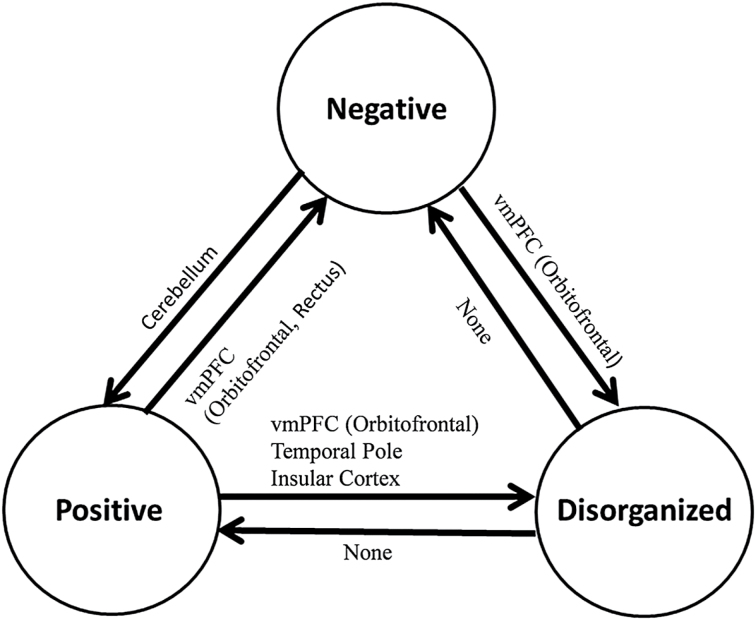
A summary of the ODVBA results on statistically significant differences between the 3 schizophrenia subgroups after FWE correction is shown in figure 3.
Fig. 3.
Summary of the results on significant differences between three subgroups in schizophrenia. A → B means A had lower GM volume than B in some specific regions, and A ← B means B had lower GM volume than A. A and B indicate any two subgroups.
Discussion
Schizophrenia is a heterogeneous mental disorder in which different symptom dimensions could be linked with distinct neural surrogates. Recent neuroimaging studies have suggested specific patterns of structural brain alterations to be related to subgroups/syndromes of schizophrenia. However, the strategy commonly applied so far to study this heterogeneity is based on the univariate VBM comparison between each subgroup of patients and matched health controls. To the best of our knowledge, the present study is the first to investigate the direct differences between psychopathologically defined patient subgroups using an advanced morphometric analysis method. Our results may deepen the insight into the neurobiological architecture of the disorder’s clinical heterogeneity.
More specifically, our results revealed widespread patterns of structural differences exist between pairs of subgroups, which reached statistical significance in certain regions. Interestingly, the pNEG subgroup was characterized by cerebellar volume reductions compared to the pPOS and pDIS subgroups. The cerebellum plays a major role in establishing coordination and motor control but has also been involved46 with higher order cognitive functions as a part of the cortico-subcortico-cerebellar circuitry. Recent literature47 further demonstrated that patients with predominantly negative symptoms have pronounced neuropsychological deficits across multiple cognitive domains, including executive functioning as well as attentional and social cognitive processes. Our cerebellar findings are largely in agreement with previous studies: an early ROI-based neuroimaging study13 showed that negative symptoms were more inversely related to cerebellar size compared to positive and disorganized symptoms. Subsequently, 2 VBM-based correlation analyses31,32 have also reported a significant inverse correlation between cerebellar volume and PANSS negative scores. In a further VBM-based subgroup comparison study,34 patients with negative symptoms were the only subgroup to demonstrate significant volume reductions in the cerebellum compared to healthy controls. Moreover, our cerebellar results agree with studies on cerebellar soft signs,48,49 which revealed that the presence of cerebellar signs in schizophrenia patients was associated with more severe negative symptoms and smaller cerebellar volumes, but not with positive symptoms. More generally, the neurological soft signs (NSS)50,51 have also shown significant correlation with cerebellar volume reduction, and the association between NSS and negative symptoms has been observed52,53 much more consistently across previous studies than links between NSS and positive symptoms.
Relative to disorganized symptoms, negative symptoms seem to be associated with significantly lower volumes in a number of brain regions covering the ventromedial prefrontal cortex (vmPFC) and the orbitofrontal cortex, as well as the thalamus, as parts of the cortico-subcortico-cerebellar circuitry.46 Within this brain network, neurofunctional abnormalities have been shown to be systematically related to negative symptoms by means of positron emission tomography.54,55 Earlier structural MRI studies have also reported associations of negative symptoms with GM volume reductions in this circuit, including the orbitofrontal cortex12,14 and the thalamus.35,56
When comparing the pPOS with the pNEG subgroups, in addition to the aforementioned cerebellar volume reduction of the pNEG subgroup, we found that the pPOS subgroup had significantly less GM volume mainly in the vmPFC (including the bilateral orbitofrontal cortex). The vmPFC mediates a number of higher level functions, including complex sensory integration tasks,57,58 decision making,59 and expectation.60 Some studies have suggested that neural networks in the vmPFC develop during adolescence and young adulthood and regulate emotion through the amygdala.61 The orbitofrontal cortex also seems to mediate the understanding of expected punishments and rewards of an action.62 The dense connections between the vmPFC and various association and limbic areas, as well as subcortical structures, indicate the involvement of this part of the brain in a number of brain systems and higher order cortical functions, many of which may be seriously impaired in patients with pronounced positive symptoms of schizophrenia. Our findings of vmPFC volume reductions in patients with pronounced positive symptoms are in accordance with several previous neuroimaging studies: A specific ROI analysis15 on the orbitofrontal cortex has reported a trend toward significant inverse correlations between the orbitofrontal cortex volumes and positive symptom scores, but negative symptoms scores did not show such a trend. The VBM studies33,34 which investigated group differences between paranoid/positive schizophrenia patients and healthy controls revealed GM volume reductions in the medial and inferior frontal gyrus. Another neuroimaging study63 demonstrated decreased cortical thickness in the left orbitofrontal cortex and other brain regions, which were related to positive, but not negative symptoms. A recent ROI study16 revealed that the volume of the left orbitofrontal cortex was inversely correlated with positive, rather than negative symptom severity in psychotic disorders including schizophrenia, schizoaffective, and psychotic bipolar I disorders. More specifically, hallucinations have also been repeatedly reported64–66 to be significantly associated with orbitofrontal and inferior frontal volume reductions. Interestingly, studies on violent behavior67 also suggested significant association between volume reduction in vmPFC and violent and aggressive behaviors as often encountered in patients with acute psychosis.
The pattern of GM reductions revealed by our analysis is strikingly consistent with the topography of the dopaminergic pathways in the brain, and in particular of the mesolimbic and mesocortical pathways, which include projections from the ventral tegmental area to the limbic, frontal, and insular cortices. The dopaminergic system has been widely implicated in schizophrenia, and in fact core neural “nodes” (eg, basal ganglia) within this system are the main targets of antipsychotics drugs effects and have been shown to be affected very early during the disease process as attenuated psychotic symptoms emerge.68
An interesting observation that emerged from the data is that occipitotemporal brain regions were mainly affected in the pPOS group, as shown both by the intergroup comparisons (figure 1) and the comparison between subgroups and controls (figure 2). These regions have not been widely reported in the literature, albeit previous reports have identified brain volume reductions in Brodmann area 19 and the lingual gyrus, which largely overlap with the regions found herein. The lingual gyrus, in particular, is believed to play an important role in vision and dreaming. Volume reductions in these regions have been previously reported to be involved in positive symptoms like hallucinations.66 Visual memory dysfunction and visuo-limbic disconnection have been shown in cases where the lingual gyrus has been damaged (due to stroke or other traumatic brain injuries). Further, impaired visual memory is related to either damage to this region or disconnections between the lingual gyrus and other brain structures.69
Significant GM volume reduction in patients with pronounced positive symptoms compared to patients with disorganized were mainly located in the orbitofrontal cortex, the temporal pole, and the insular cortex, all of which are major hubs in the paralimbic circuitry.70 The temporal pole in particular may be an important node of this circuit, in which structural abnormalities can lead to disturbances of perceptual integration processes, and have been demonstrated17,71 to be associated with positive symptoms.
Our findings are in agreement with many of previous related studies; however, they are also inconsistent with some previous studies. Regarding the cerebellum, a previous study35 comparing the 3 symptom subgroups to healthy controls reported that the disorganized subgroup had more pronounced alterations in this region than the other subgroups. Regarding the orbitofrontal cortex, earlier studies12,14 showed that lower volume in this region was associated with more severe negative, but not positive symptoms in patients with schizophrenia. These discrepancies may stem from the different experimental strategies and methods used in these studies. However, the novel multivariate analysis technique employed in our study could be more sensitive in identifying the spatial extent and direction of structural differences between these three syndrome subgroups.
Regarding the clinical characteristics of the patients subgroups (table 1), it is worth noting that the pNEG patients had longer illness duration than other groups, as the disease commenced earlier in this subgroup. This observation is in line with previous neuropsychological studies72–75 reporting a pronounced association between an early disease onset and predominant negative symptoms in the clinical phenotype of the disorder. Moreover, there is substantial evidence that patients with predominant negative symptoms are characterized by a higher risk for chronic,76,77 as well as relapsing disease courses and poor disease outcomes.78,79 In agreement with these findings, our clinical data also show that the pNEG group involved a higher proportion of patients with recurrent disease courses compared to the other subgroups (table 1).
In the affective symptoms domain, both pNEG and pPOS patients had significantly higher PANSS depression scores compared to the pDIS group (table 1). For each subgroup contrast, we calculated the correlation between the obtained ODVBA results (means of RAVENS values on the detected area per region) and the PANSS depression scores. According to the results listed in supplementary table 1, generally the volumes in the detected regions were not significantly correlated with depression scores in the group comparisons of pNEG < pPOS and pPOS < pNEG, while the 2 variables were associated in pNEG < pDIS and pPOS < pDIS.
In DSM-V,80 the DSM-IV subtypes of schizophrenia (ie, paranoid, disorganized, catatonic, undifferentiated, and residual types) were eliminated due to their limited diagnostic stability and low reliability. Instead, a dimensional approach was introduced to assess the severity of the core symptoms of schizophrenia across patients. It is worth noting that our 3 subgroups investigated in this paper are different from the DSM-IV subtypes, since they are determined by means of factor analysis: they are distinguishable, but nonetheless to a certain degree co-occurring.34 Importantly, these 3 subgroups have been consistently identified by previous factor analytic studies, thus reflecting the phenotypic heterogeneity of schizophrenia. Thus, identifying neuroanatomical surrogates of this phenotypic heterogeneity may pave the way to more accurate diagnostic and prognostic biomarkers of the disorder’s multifaceted clinical phenotypes. Furthermore, future neuroimaging studies combining the cross-sectional symptom-based decomposition of the disorder with prospective evaluations of illness course may foster biomarker-driven approaches to allow early identification and treatment of patients at risk of enduring negative symptoms and poor disease outcomes.
A limitation of our study, which is common to imaging studies, is that the underlying microstructural abnormalities that lead to changes measured by imaging cannot be uniquely determined. For example, our analysis revealed several regions of reduced GM. However, this finding does not necessarily imply lower number of neurons, but it could be related to differences in myelination or synaptic formation, which might change the amount of tissue perceived in T1-weighted images as GM. Additional analyses we performed to these images indicated that the cerebellar finding in particular might reflect altered MR signal, rather than volumetric differences. Histopathological studies would therefore be necessary to evaluate the macrostructural changes in greater depth. Imaging studies, including ours, can guide such more detailed tissue analysis by directing efforts to specific brain regions.
Besides adding to our knowledge of the biological underpinnings of schizophrenia, these patterns of structural differences between subgroups may serve in the future as early biomarkers of disease subtypes, hence for biomarker-based patient stratification in clinical psychiatry. Part of our future work will investigate the use of these distinctive imaging patterns in conjunction with machine learning algorithms,81,82 to identify disease subtypes on an individual patient basis. Our future work also includes investigating the heterogeneous changes in the volumes of white matter, jointly with the diffusion anisotropy measures, eg, the fractional anisotropy, which can well reflect the degree of myelination in the white matter.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
National Institute of Health (R01AG14971); German Psychiatric Association (to N.K. during his visiting scholarship at University of Pennsylvania).
Supplementary Material
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Carpenter WT, Jr, Heinrichs DW, Wagman AM. Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry. 1988;145:578–583. [DOI] [PubMed] [Google Scholar]
- 2. Crow TJ. Positive and negative schizophrenic symptoms and the role of dopamine. Br J Psychiatry. 1980;137:383–386. [PubMed] [Google Scholar]
- 3. Sigmundsson T, Suckling J, Maier M, et al. Structural abnormalities in frontal, temporal, and limbic regions and interconnecting white matter tracts in schizophrenic patients with prominent negative symptoms. Am J Psychiatry. 2001;158:234–243. [DOI] [PubMed] [Google Scholar]
- 4. Buchanan RW, Carpenter WT. Domains of psychopathology: an approach to the reduction of heterogeneity in schizophrenia. J Nerv Ment Dis. 1994;182:193–204. [PubMed] [Google Scholar]
- 5. Andreasen NC, Arndt S, Alliger R, Miller D, Flaum M. Symptoms of schizophrenia. Methods, meanings, and mechanisms. Arch Gen Psychiatry. 1995;52:341–351. [DOI] [PubMed] [Google Scholar]
- 6. Liddle PF. Schizophrenic syndromes, cognitive performance and neurological dysfunction. Psychol Med. 1987;17:49–57. [DOI] [PubMed] [Google Scholar]
- 7. Liddle PF. The symptoms of chronic schizophrenia. A re-examination of the positive-negative dichotomy. Br J Psychiatry. 1987;151:145–151. [DOI] [PubMed] [Google Scholar]
- 8. Liddle PF, Morris DL. Schizophrenic syndromes and frontal lobe performance. Br J Psychiatry. 1991;158:340–345. [DOI] [PubMed] [Google Scholar]
- 9. Schröder J, Geider FJ, Binkert M, Reitz C, Jauss M, Sauer H. Subsyndromes in chronic schizophrenia: do their psychopathological characteristics correspond to cerebral alterations? Psychiatry Res. 1992;42:209–220. [DOI] [PubMed] [Google Scholar]
- 10. Schröder J, Buchsbaum MS, Siegel BV, Geider FJ, Niethammer R. Structural and functional correlates of subsyndromes in chronic schizophrenia. Psychopathology. 1995;28:38–45. [DOI] [PubMed] [Google Scholar]
- 11. Schroder J, Buchsbaum MS, Siegel BV, et al. Cerebral metabolic activity correlates of subsyndromes in chronic schizophrenia. Schizophr Res. 1996;19:41–53. [DOI] [PubMed] [Google Scholar]
- 12. Baaré WF, Hulshoff Pol HE, Hijman R, Mali WP, Viergever MA, Kahn RS. Volumetric analysis of frontal lobe regions in schizophrenia: relation to cognitive function and symptomatology. Biol Psychiatry. 1999;45:1597–1605. [DOI] [PubMed] [Google Scholar]
- 13. Wassink TH, Andreasen NC, Nopoulos P, Flaum M. Cerebellar morphology as a predictor of symptom and psychosocial outcome in schizophrenia. Biol Psychiatry. 1999;45:41–48. [DOI] [PubMed] [Google Scholar]
- 14. Gur RE, Cowell PE, Latshaw A, et al. Reduced dorsal and orbital prefrontal gray matter volumes in schizophrenia. Arch Gen Psychiatry. 2000;57:761–768. [DOI] [PubMed] [Google Scholar]
- 15. Behere RV, Kalmady SV, Venkatasubramanian G, Gangadhar BN. orbitofrontal lobe volume deficits in antipsychotic-naïve schizophrenia: a 3-Tesla MRI study. Indian J Psychol Med. 2009;31:77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Padmanabhan JL, Tandon N, Haller CS, et al. Correlations between brain structure and symptom dimensions of psychosis in schizophrenia, schizoaffective, and psychotic bipolar I disorders [published online ahead of print June 6, 2014]. Schizophr Bull. 10.1093/schbul/sbu075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Crespo-Facorro B, Nopoulos PC, Chemerinski E, Kim JJ, Andreasen NC, Magnotta V. Temporal pole morphology and psychopathology in males with schizophrenia. Psychiatry Res. 2004;132:107–115. [DOI] [PubMed] [Google Scholar]
- 18. Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11:805–821. [DOI] [PubMed] [Google Scholar]
- 19. Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. [DOI] [PubMed] [Google Scholar]
- 20. Davatzikos C, Genc A, Xu D, Resnick SM. Voxel-based morphometry using the RAVENS maps: methods and validation using simulated longitudinal atrophy. Neuroimage. 2001;14:1361–1369. [DOI] [PubMed] [Google Scholar]
- 21. Wright IC, Ellison ZR, Sharma T, Friston KJ, Murray RM, McGuire PK. Mapping of grey matter changes in schizophrenia. Schizophr Res. 1999;35:1–14. [DOI] [PubMed] [Google Scholar]
- 22. Pol HE, Schnack HG, Mandl RCW, et al. Focal gray matter density changes in schizophrenia. Arch Gen Psychiatry. 2001;58:1118–1125. [DOI] [PubMed] [Google Scholar]
- 23. Job DE, Whalley HC, McConnell S, Glabus M, Johnstone EC, Lawrie SM. Structural gray matter differences between first-episode schizophrenics and normal controls using voxel-based morphometry. Neuroimage. 2002;17:880–889. [PubMed] [Google Scholar]
- 24. Kubicki M, Shenton ME, Salisbury DF, et al. Voxel-based morphometric analysis of gray matter in first episode schizophrenia. Neuroimage. 2002;17:1711–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Davatzikos C, Shen D, Gur RC, et al. Whole-brain morphometric study of schizophrenia revealing a spatially complex set of focal abnormalities. Arch Gen Psychiatry. 2005;62:1218–1227. [DOI] [PubMed] [Google Scholar]
- 26. Jayakumar PN, Venkatasubramanian G, Gangadhar BN, Janakiramaiah N, Keshavan MS. Optimized voxel-based morphometry of gray matter volume in first-episode, antipsychotic-naive schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:587–591. [DOI] [PubMed] [Google Scholar]
- 27. Chua SE, Cheung C, Cheung V, et al. Cerebral grey, white matter and CSF in never-medicated, first-episode schizophrenia. Schizophr Res. 2007;89:12–21. [DOI] [PubMed] [Google Scholar]
- 28. Meda SA, Giuliani NR, Calhoun VD, et al. A large scale (N = 400) investigation of gray matter differences in schizophrenia using optimized voxel-based morphometry. Schizophr Res. 2008;101:95–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Filippi M, Canu E, Gasparotti R, et al. Patterns of brain structural changes in first-contact, antipsychotic drug-naive patients with schizophrenia. AJNR Am J Neuroradiol. 2014;35:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liddle PF, Friston KJ, Frith CD, Hirsch SR, Jones T, Frackowiak RS. Patterns of cerebral blood flow in schizophrenia. Br J Psychiatry. 1992;160:179–186. [DOI] [PubMed] [Google Scholar]
- 31. Bergé D, Carmona S, Rovira M, Bulbena A, Salgado P, Vilarroya O. Gray matter volume deficits and correlation with insight and negative symptoms in first-psychotic-episode subjects. Acta Psychiatr Scand. 2011;123:431–439. [DOI] [PubMed] [Google Scholar]
- 32. Venkatasubramanian G. Neuroanatomical correlates of psychopathology in antipsychotic-naïve schizophrenia. Indian J Psychiatry. 2010;52:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ha TH, Youn T, Ha KS, et al. Gray matter abnormalities in paranoid schizophrenia and their clinical correlations. Psychiatry Res. 2004;132:251–260. [DOI] [PubMed] [Google Scholar]
- 34. Koutsouleris N, Gaser C, Jäger M, et al. Structural correlates of psychopathological symptom dimensions in schizophrenia: a voxel-based morphometric study. Neuroimage. 2008;39:1600–1612. [DOI] [PubMed] [Google Scholar]
- 35. Nenadic I, Sauer H, Gaser C. Distinct pattern of brain structural deficits in subsyndromes of schizophrenia delineated by psychopathology. Neuroimage. 2010;49:1153–1160. [DOI] [PubMed] [Google Scholar]
- 36. Zhang T, Davatzikos C. ODVBA: optimally-discriminative voxel-based analysis. IEEE Trans Med Imaging. 2011;30:1441–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Friston K, Holmes A, Worsley K, Poline J, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1994;2:189–210. [Google Scholar]
- 38. Zhang T, Davatzikos C. Optimally-discriminative voxel-based morphometry significantly increases the ability to detect group differences in schizophrenia, mild cognitive impairment, and Alzheimer’s disease. Neuroimage. 2013;79:94–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 40. Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. [DOI] [PubMed] [Google Scholar]
- 41. Ou Y, Sotiras A, Paragios N, Davatzikos C. DRAMMS: deformable registration via attribute matching and mutual-saliency weighting. Med Image Anal. 2011;15:622–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Holmes AP, Blair RC, Watson JD, Ford I. Nonparametric analysis of statistic images from functional mapping experiments. J Cereb Blood Flow Metab. 1996;16:7–22. [DOI] [PubMed] [Google Scholar]
- 43. Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–289. [DOI] [PubMed] [Google Scholar]
- 44. Nichols TE, Holmes AP. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2002;15:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hayasaka S, Nichols TE. Validating cluster size inference: random field and permutation methods. Neuroimage. 2003;20:2343–2356. [DOI] [PubMed] [Google Scholar]
- 46. Andreasen NC, Paradiso S, O’Leary DS. “Cognitive dysmetria” as an integrative theory of schizophrenia: a dysfunction in cortical-subcortical-cerebellar circuitry? Schizophr Bull. 1998;24:203–218. [DOI] [PubMed] [Google Scholar]
- 47. Ventura J, Hellemann GS, Thames AD, Koellner V, Nuechterlein KH. Symptoms as mediators of the relationship between neurocognition and functional outcome in schizophrenia: a meta-analysis. Schizophr Res. 2009;113:189–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ho BC, Mola C, Andreasen NC. Cerebellar dysfunction in neuroleptic naive schizophrenia patients: clinical, cognitive, and neuroanatomic correlates of cerebellar neurologic signs. Biol Psychiatry. 2004;55:1146–1153. [DOI] [PubMed] [Google Scholar]
- 49. Varambally S, Venkatasubramanian G, Thirthalli J, Janakiramaiah N, Gangadhar BN. Cerebellar and other neurological soft signs in antipsychotic-naïve schizophrenia. Acta Psychiatr Scand. 2006;114:352–356. [DOI] [PubMed] [Google Scholar]
- 50. Mouchet-Mages S, Rodrigo S, Cachia A, et al. Correlations of cerebello-thalamo-prefrontal structure and neurological soft signs in patients with first-episode psychosis. Acta Psychiatr Scand. 2011;123:451–458. [DOI] [PubMed] [Google Scholar]
- 51. Thomann PA, Roebel M, Dos Santos V, Bachmann S, Essig M, Schröder J. Cerebellar substructures and neurological soft signs in first-episode schizophrenia. Psychiatry Res. 2009;173:83–87. [DOI] [PubMed] [Google Scholar]
- 52. Tosato S, Dazzan P. The psychopathology of schizophrenia and the presence of neurological soft signs: a review. Curr Opin Psychiatry. 2005;18:285–288. [DOI] [PubMed] [Google Scholar]
- 53. Bombin I, Arango C, Buchanan RW. Significance and meaning of neurological signs in schizophrenia: two decades later. Schizophr Bull. 2005;31:962–977. [DOI] [PubMed] [Google Scholar]
- 54. Potkin SG, Alva G, Fleming K, et al. A PET study of the pathophysiology of negative symptoms in schizophrenia. Positron emission tomography. Am J Psychiatry. 2002;159:227–237. [DOI] [PubMed] [Google Scholar]
- 55. Galeno R, Molina M, Guirao M, Isoardi R. Severity of negative symptoms in schizophrenia correlated to hyperactivity of the left globus pallidus and the right claustrum. A PET study. World J Biol Psychiatry. 2004;5:20–25. [DOI] [PubMed] [Google Scholar]
- 56. Rao NP, Kalmady S, Arasappa R, Venkatasubramanian G. Clinical correlates of thalamus volume deficits in anti-psychotic-naïve schizophrenia patients: a 3-Tesla MRI study. Indian J Psychiatry. 2010;52:229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nieuwenhuis IL, Takashima A. The role of the ventromedial prefrontal cortex in memory consolidation. Behav Brain Res. 2011;218:325–334. [DOI] [PubMed] [Google Scholar]
- 58. Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci. 2012;16:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123 (Pt 11):2189–2202. [DOI] [PubMed] [Google Scholar]
- 60. Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. [DOI] [PubMed] [Google Scholar]
- 61. Decety J, Michalska KJ. Neurodevelopmental changes in the circuits underlying empathy and sympathy from childhood to adulthood. Dev Sci. 2010;13:886–899. [DOI] [PubMed] [Google Scholar]
- 62. Schoenbaum G, Takahashi Y, Liu TL, McDannald MA. Does the orbitofrontal cortex signal value? Ann N Y Acad Sci. 2011;1239:87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Xiao Y, Lui S, Deng W, et al. Altered cortical thickness related to clinical severity but not the untreated disease duration in schizophrenia [published online ahead of print December 18, 2013]. Schizophr Bull. 10.1093/schbul/sbt177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Martí-Bonmatí L, Lull JJ, García-Martí G, et al. Chronic auditory hallucinations in schizophrenic patients: MR analysis of the coincidence between functional and morphologic abnormalities. Radiology. 2007;244:549–556. [DOI] [PubMed] [Google Scholar]
- 65. van Tol MJ, van der Meer L, Bruggeman R, Modinos G, Knegtering H, Aleman A. Voxel-based gray and white matter morphometry correlates of hallucinations in schizophrenia: the superior temporal gyrus does not stand alone. Neuroimage Clin. 2014;4:249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gaser C, Nenadic I, Volz HP, Büchel C, Sauer H. Neuroanatomy of “hearing voices”: a frontotemporal brain structural abnormality associated with auditory hallucinations in schizophrenia. Cereb Cortex. 2004;14:91–96. [DOI] [PubMed] [Google Scholar]
- 67. Kumari V, Barkataki I, Goswami S, Flora S, Das M, Taylor P. Dysfunctional, but not functional, impulsivity is associated with a history of seriously violent behaviour and reduced orbitofrontal and hippocampal volumes in schizophrenia. Psychiatry Res. 2009;173:39–44. [DOI] [PubMed] [Google Scholar]
- 68. Koutsouleris N, Schmitt GJ, Gaser C, et al. Neuroanatomical correlates of different vulnerability states for psychosis and their clinical outcomes. Br J Psychiatry. 2009;195:218–226. [DOI] [PubMed] [Google Scholar]
- 69. Bogousslavsky J, Miklossy J, Deruaz JP, Assal G, Regli F. Lingual and fusiform gyri in visual processing: a clinico-pathologic study of superior altitudinal hemianopia. J Neurol Neurosurg Psychiatry. 1987;50:607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Mega MS, Cummings JL, Salloway S, Malloy P. The limbic system: an anatomic, phylogenetic, and clinical perspective. J Neuropsychiatry Clin Neurosci. 1997;9:315–330. [DOI] [PubMed] [Google Scholar]
- 71. Jalbrzikowski M, Jonas R, Senturk D, et al. Structural abnormalities in cortical volume, thickness, and surface area in 22q11.2 microdeletion syndrome: relationship with psychotic symptoms. Neuroimage Clin. 2013;3:405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hoff AL, Harris D, Faustman WO, et al. A neuropsychological study of early onset schizophrenia. Schizophr Res. 1996;20:21–28. [DOI] [PubMed] [Google Scholar]
- 73. Häfner H. Onset and early course as determinants of the further course of schizophrenia. Acta Psychiatr Scand. 2000;102:44–48. [PubMed] [Google Scholar]
- 74. Kao YC, Liu YP. Effects of age of onset on clinical characteristics in schizophrenia spectrum disorders. BMC Psychiatry. 2010;10:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. van der Werf M, Köhler S, Verkaaik M, Verhey F, van Os J; GROUP Investigators. Cognitive functioning and age at onset in non-affective psychotic disorder. Acta Psychiatr Scand. 2012;126:274–281. [DOI] [PubMed] [Google Scholar]
- 76. Crow TJ. Positive and negative schizophrenia symptoms and the role of dopamine. Br J Psychiatry. 1981;139:251–254. [DOI] [PubMed] [Google Scholar]
- 77. Addington J, Addington D. Three-year outcome of treatment in an early psychosis program. Can J Psychiatry. 2009;54:626–630. [DOI] [PubMed] [Google Scholar]
- 78. Girón M, Gómez-Beneyto M. Relationship between empathic family attitude and relapse in schizophrenia: a 2-year followup prospective study. Schizophr Bull. 1998;24:619–627. [DOI] [PubMed] [Google Scholar]
- 79. Dyck DG, Short RA, Hendryx MS, et al. Management of negative symptoms among patients with schizophrenia attending multiple-family groups. Psychiatr Serv. 2000;51:513–519. [DOI] [PubMed] [Google Scholar]
- 80. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition: DSM-V. 5th ed. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 81. Fan Y, Shen D, Gur RC, Gur RE, Davatzikos C. COMPARE: classification of morphological patterns using adaptive regional elements. IEEE Trans Med Imaging. 2007;26:93–105. [DOI] [PubMed] [Google Scholar]
- 82. Filipovych R, Resnick SM, Davatzikos C. JointMMCC: joint maximum-margin classification and clustering of imaging data. IEEE Trans Med Imaging. 2012;31:1124–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.