Abstract
Neuroinflammation and abnormal immune responses have been implicated in schizophrenia (SCZ). Past studies using positron emission tomography (PET) that examined neuroinflammation in patients with SCZ in vivo using the translocator protein 18kDa (TSPO) target were limited by the insensitivity of the first-generation imaging agent [11C]-PK11195, scanners used, and the small sample sizes studied. Present study uses a novel second-generation TSPO PET radioligand N-acetyl-N-(2-[18F]fluoroethoxybenzyl)-2-phenoxy-5-pyridinamine ([18F]-FEPPA) to evaluate whether there is increased neuroinflammation in patients with SCZ. A cross-sectional study was performed using [18F]-FEPPA and a high-resolution research tomograph (HRRT). Eighteen patients with SCZ with ongoing psychotic symptoms and 27 healthy volunteers (HV) were recruited from a tertiary psychiatric clinical setting and the community, respectively. All participants underwent [18F]-FEPPA PET and magnetic resonance imaging, and PET data were analyzed to obtain [18F]-FEPPA total volume of distribution (V T) using a 2-tissue compartment model with an arterial plasma input function, as previously validated. All subjects were classified as high-, medium- or low-affinity [18F]-FEPPA binders on the basis of rs6971 polymorphism, and genotype information was incorporated into the analyses of imaging outcomes. No significant differences in neuroinflammation indexed as [18F]-FEPPA V T were observed between groups in either gray (F (1,39) = 0.179, P = .674) or white matter regions (F (1,38) = 0.597, P = .445). The lack of significant difference in neuroinflammation in treated patients with SCZ in the midst of a psychotic episode and HV suggests that neuroinflammatory processes may take place early in disease progression or are affected by antipsychotic treatment.
Key words: translocator protein 18kDa (TSPO), inflammation, positron emission tomography, psychosis, rs6971 polymorphism, microglia
Introduction
Several lines of evidence point toward a role of immune response and neuroinflammation in the pathogenesis of schizophrenia (SCZ),1–5 including reports of elevations in peripheral levels of proinflammatory cytokines,1–4 prostaglandin 2,5 and C-reactive protein6,7 in patients with SCZ. Additionally, several studies have shown that anti-inflammatory agents, administered as adjuvants to antipsychotic therapy, can improve positive, cognitive, and global symptoms.8–10 Interactions between the immune system and risk of SCZ starts before birth, with epidemiological studies consistently showing a parallel relationship between exposure to prenatal inflammation and increased risk of developing SCZ in offspring.11
Microglia act as resident macrophages of the central nervous system and first responders mobilizing the inflammatory response to brain insults12 by undergoing activation from the quiescent (“sentry”) state, migrating to the site of neuronal injury and releasing inflammatory cytokines.12–15 Importantly, activated microglia express a mitochondrial translocator protein 18kDa (TSPO),16 previously called peripheral-type benzodiazepine receptor. Microglial activation and the associated elevations in TSPO levels have been recognized as quantifiable indices of neuroinflammation.12 Microglial activation, and neuroinflammation, may also be involved in the structural alterations in brain structure, possibly underlying the gray matter loss observed in a number of disorders,17–20 including SCZ.21–23 While cognitive and depressive symptoms of SCZ in particular have been linked with markers of inflammation,6,10,24,25 to our knowledge, no study has addressed this question in brain in living humans. Furthermore, alterations in white matter tracts and changes in regional connectivity have also been implicated in the pathogenesis of SCZ. Diffusion tensor imaging (DTI) studies have identified differences in fractional anisotropy between patients with SCZ and healthy volunteers (HV) in corpus callosum,26 cingulum bundle,27 superior longitudinal fasciculus (SLF),28 and the posterior limb of the internal capsule (PLIC).29
While 3 early postmortem studies have detected increased presence of microglia in the hippocampal and cortical tissues of patients with SCZ,30–32 other studies have failed to detect any difference33–36 or even reported a decrease in microglial activation.37 Positron emission tomography (PET), though, avoids the limitations of postmortem studies and provides an in vivo index of neuroinflammation in SCZ. Early studies using TSPO radiotracer [11C]-PK11195 have reported increased neuroinflammation in total gray matter38 and the hippocampus39 of antipsychotic-treated patients. The only in vivo study in SCZ using a new generation TSPO ligand, [11C]-DAA1106, reported no difference in tracer binding between patients with SCZ and matched controls, but did find tracer binding to correlate significantly with positive psychotic symptoms and duration of illness. However, this initial study used an older HR+ PET scanner with lower resolution and sensitivity,40 examined a smaller sample size, did not investigate white matter regions or correlation with cognitive deficits, and did not correct for genotype, which is known to significantly affect TSPO-binding outcomes of second-generation radioligands [11C]-DPA-713, [11C]DAA1106, [11C]PBR28, and N-acetyl-N-(2-[18F]fluoroethoxybenzyl)-2-phenoxy-5-pyridinamine ([18F]-FEPPA).41–44
[18F]-FEPPA is a novel radioligand exhibiting optimal chemical, pharmacokinetic, and pharmacodynamic properties for applications in imaging TSPO. Preclinical tracer characterization studies have shown [18F]-FEPPA to exhibit moderate brain uptake, slow washout, and regional distribution that matches previously demonstrated levels of TSPO.45 Recent evaluations in HV have shown that [18F]-FEPPA total distribution volumes (V T) can be quantified with excellent identifiability using a 2-tissue compartment model.46 Thus, in the present study, we used [18F]-FEPPA to examine neuroinflammation in both gray and white matter regions in patients with SCZ with ongoing psychotic symptoms. Moreover, we explored the association between neuroinflammation and gray matter volumes to evaluate the involvement of inflammatory processes in brain regional atrophy. In addition, we explored the associations between neuroinflammation and severity of psychopathology as well as neuropsychological deficits in patients with SCZ, given reported associations between peripheral markers of inflammation and poorer cognitive performance.6,10,24
Methods
Subjects
After 1 subject was excluded for excessive head motion and another participant was determined to be a low-affinity binder (LAB), 16 patients with SCZ currently undergoing antipsychotic therapy (11 male participants; 42.6±14.1 years old) and 27 HV (10 male participants; 43.5±16 years old) between the ages of 18 and 65 were included in this study. Patients met the following inclusion criteria: diagnosis of SCZ, as evaluated with the Structured Clinical Interview for DSM-IV47 with no other Axis I disorders such as depression which may also be associated with neuroinflammation48; with ongoing psychotic symptoms, as confirmed with a minimum score of 5 on 1 item or 4 on 2 items of the Positive and Negative Syndrome Scale (PANSS)49; on stable antipsychotic therapy, and with no use of clozapine or benzodiazepines which have been shown to affect TSPO,50,51 except clonazepam, which exhibits low affinity for TSPO.52,53 HV had no history of psychiatric illness and had no first-degree relatives with a major mental disorder. Exclusion criteria for all subjects included: current diagnosis of substance abuse or a positive urine drug screen; pregnancy or current breastfeeding; clinically significant medical illness; and the presence of metal implants precluding an magnetic resonance imaging (MRI) scan. All subjects provided written consent after being informed of the study procedures. Neurocognitive performance was assessed in patients with SCZ using the Repeatable Battery for the Assessment of Neuropsychological Status.54,55 Clinical status was also assessed with the PANSS, Calgary Depression Scale, Snaith-Hamilton Pleasure Scale, Scale for the Assessment of Negative Symptoms, and Apathy Evaluation Scale, while movement disorders were evaluated with the Barnes Akathisia Rating Scale, and Abnormal Involuntary Movement Scale. Assessments are described in more detail in supplementary methods.
This study was approved by the Research Ethics Board at the Centre for Addiction and Mental Health and the University of Toronto.
MRI Data Acquisition
T1- and proton density (PD)-weighted brain MRIs (T1: echo time [TE] = 5.3–15, repetition time [TR] = 8.9–12, field of view [FOV] = 20cm, matrix = 256×256, slice thickness = 1.5mm, number of excitations (NEx) = 2; proton density: TE = 17, TR = 6000, FOV = 22cm, matrix = 256×256, slice thickness = 2mm, number of excitations = 2) were obtained for each subject using a 1.5 T Signa scanner (General Electric Medical Systems) for 16 of the HV subjects and 12 of the SCZ subjects. MRI images for the remaining 4 SCZ and 11 HV were acquired using the 3 T MR-750 scanner (General Electric Medical Systems), with T1 parameters: slice thickness = 0.9mm, TR = 8.2ms, TE = min full, flip angle = 8°, NEx = 1, acquisition matrix = 256×228, and FOV = 28cm and PD parameters: slice thickness = 2mm, TR = 6000, TE = min full, number of acquisitions = 1, and FOV = 22. MRI images were used for the anatomical delineation of regions and quantification of PET images, as well as determination of regional volumes.46,56 Differences in MRI acquisition parameters did not have a significant effect on [18F]-FEPPA outcome measures (data not shown).
PET Data Acquisition
All [18F]-FEPPA PET scans were performed using a high-resolution CPS-HRRT PET scanner (Siemens Molecular Imaging), which measures radioactivity in 207 1.2-mm thick slices. Following a transmission scan, each subject was administered 177.77±21.43 MBq of [18F]-FEPPA by intravenous bolus injection and scanned for 125 min. The images were reconstructed into 34 time frames: 1 frame of variable length, 5×30, 1×45, 2×60, 1×90, 1×120, 1×210, and 22×300 s.
Input Function Measurement
Arterial blood was collected for the first 22.5min after radiotracer injection at a rate of 2.5 ml/min and blood radioactivity levels were measured using an automatic blood sampling system (Model PBS-101; Veenstra Instruments). Additionally, 7 ml samples were drawn at −5, 2.5, 7, 12, 15, 20, 30, 45, 60, 90, and 120min, relative to time of injection. The relative proportion of radiolabeled metabolites was measured using high-performance liquid chromatography and dispersion- and metabolite-corrected plasma input function were generated as previously described.44,46
Image Analysis
MRI volumes and time-activity curves (TACs) were extracted for hippocampus (HC), medial prefrontal cortex (mPFC), temporal cortex, dorsolateral PFC (DLPFC), and the striatum regions of interest (ROI) using in-house imaging pipeline ROMI.56 All gray matter ROI were delineated using T1 MRI images, except for the striatum which was delineated using PD images.57 Johns Hopkins University DTI atlas in ICBM-152 space was used to define white matter ROIs: corpus callosum, cingulum bundle, SLF, and PLIC, as previously described.58
In addition to the ROI-based analysis, PET images were also analyzed using a voxel-based method by generating parametric images using the Logan graphical analysis. Voxel-wise differences between images of HV and SCZ participants were evaluated using a 2-sample t test in SPM8, including rs6971 polymorphism as a covariate.
Kinetic parameters of [18F]-FEPPA were derived from the TACs using 2-tissue compartment model and plasma input function to obtain the binding outcome measure V T for each ROI, which has been validated for [18F]-FEPPA quantification.46 Kinetic analysis of gray and white matter incorporated a 5% and 2.7% contribution from the blood in the vascular lumen, respectively,59 in the fitting of the 2-compartment kinetic model using PMOD software (PMOD Technologies). PET images were corrected for partial volume effect using the Muller-Gartner approach,60 and the results are presented with and without the partial volume correction.
rs6971 Polymorphism Genotyping
Genomic DNA was obtained from peripheral leukocytes, using high salt extraction method.61 The rs6971 polymorphism, known to affect binding of second-generation TSPO PET radioligands,41–44 was genotyped using a TaqMan assay on demand C_2512465_20 (Applied Biosystems), as previously described. Based on their genotypes, study participants were classified as high-affinity binder, mixed-affinity binder, and LAB, respectively, as previously described.43,44
Statistical Analysis
All statistical analyses were performed using SPSS (version 19.0; IBM). Independent sample t tests were performed to test for differences between the demographic measures of the patient and control groups. A 2-factor ANOVA with ROI as within-subject factor, group as between-subject factor, and controlling for the TSPO genotype was carried out to test for differences in [18F]-FEPPA V T between groups. This analysis was also carried out using age as a covariate. Furthermore, a factorial ANOVA with TSPO genotype as a fixed variable and age as a covariate was used to compare regional differences in [18F]-FEPPA V T between HV and patients with SCZ in individual ROIs. P values below .05 were considered significant and Bonferroni correction was used for multiple comparisons. Partial correlations were used to examine the association between [18F]-FEPPA VTs and MRI volumes for each ROI after normalization to the total intracranial volume (ICV) and controlling for the effects of TSPO (rs6971) polymorphism. A similar approach was used to evaluate correlations between [18F]-FEPPA VTs and duration of untreated psychosis, length of illness, age of disease onset, number of acute crises, chlorpromazine equivalents, clinical presentation, and neuropsychological measures.
Results
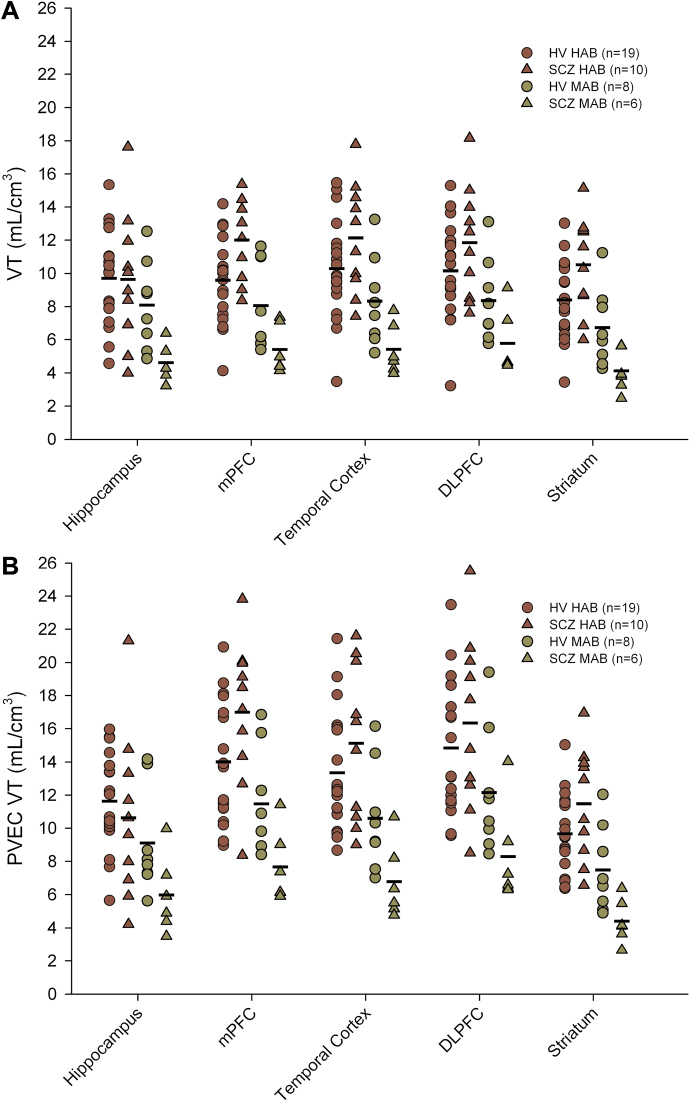
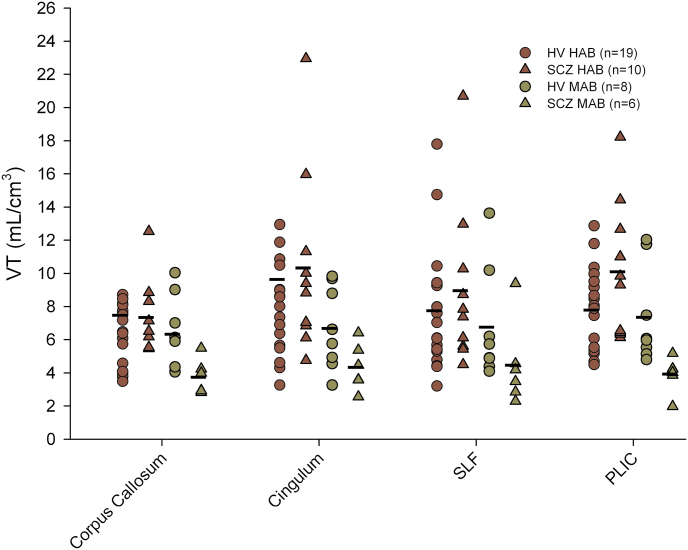
Demographic characteristics of the study population and the clinical characteristics of the SCZ group are presented in table 1. All participants in the SCZ group were on treatment with a single antipsychotic agent, with 14 subjects receiving atypical antipsychotics and 2 receiving typical antipsychotic treatment. PET radiotracer injection parameters did not differ between the 2 groups (all P > .149). Considering the [18F]-FEPPA PET imaging data, no significant effect of clinical group (HV vs SCZ) was detected on [18F]-FEPPA VTs (figure 1) when controlling for genetics (F (1,39) = 0.179, P = .674), as well as with incorporation of age as a covariate (F (1,38) = 0.159, P = .692). Percent differences between the diagnostic groups (HV vs SCZ) were estimated at −15.6%, 3.9%, 0.2%, −1.4%, and −0.54% in the HC, mPFC, DLPFC, temporal cortex, and striatum, respectively. The lack of significant differences between groups was unchanged following correction for partial volume effects (F (1,39) = 0.401, P = .530 and F (1,38) = 0.381, P = .541). Using the same statistical approach, no difference was observed between the clinical groups when considering V T values measured in the white matter tracts (figure 2; F (1,38) = 0.597, P = .445; F (1,37) = 0.574, P = .453 with age added as a covariate). Performing an ANOVA to explore differences between clinical groups in individual white and gray matter yielded similar results (table 2). Furthermore, lack of significant difference between the 2 groups was confirmed by voxel-wise analysis, supporting our observations and suggesting that ROI delineation did not affect the findings.
Table 1.
Mean Demographic Characteristics for Healthy Volunteers (HV) and Patients (SCZ)
| Demographics | HV (n = 27) | SCZ (n = 16) | ||
|---|---|---|---|---|
| Age (years) | 43.5±15.5 | 42.5±14.0 | F = 0.263, P = .611 | |
| Gender | Male/female | 10/17 | 10/6 | Χ 2 = 2.618, P = .106 |
| Genotype | HAB/MAB/LAB | 19/8/0 | 10/6/1 | Χ 2 = 1.904, P = .386 |
| PET measures | Amount injected (mCi) | 4.81±0.36 | 4.79±0.84 | F = 0.027, P = .869 |
| Specific activity (mCi/µmol) | 4071.7±3622.0 | 3589.9±1492.7 | F = 0.426, P = .518 | |
| Mass injected (µg) | 0.91±0.76 | 0.80±0.63 | F = 2.19, P = 0.149 | |
| Age at SCZ onset | 29.1±8.3 | |||
| Number of episodes | 7.3±11.7 | |||
| Length of untreated illness (months) | 43.4±63.0 | |||
| Length of illness (months) | 177.3±105.7 | |||
| CDS | 3.8±2.9 | |||
| AES | 33.8±8.0 | |||
| PANSS | Total | 70.2±9.7 | ||
| Positive | 19.3±2.2 | |||
| Negative | 18.6±5.0 | |||
| General | 31.6±6.3 | |||
| RBANS | Total | 76.3±18.2 | ||
| Immediate memory | 81.9±22.9 | |||
| Visuospatial ability | 79.8±21.5 | |||
| Language | 83.9±20.1 | |||
| Attention | 75.4±14.5 | |||
| Delayed memory | 84.8±19.1 | |||
| Treatment | Typical antipsychotics | 2 | ||
| Atypical antipsychotics | 14 | |||
| Antidepressants | 5 | |||
| Clonazepam | 7 | |||
| Anti-Parkinsonian agents | 2 | |||
| CPZ equivalents | 300.0±237.0 |
Note: AES, Apathy Evaluation Scale; CDS, Calgary Depression Scale; CPZ, chlorpromazine; HAB, high-affinity binder; LAB, low-affinity binder; MAB, mixed-affinity binder; PANSS, Positive and Negative Syndrome Scale; PET, positron emission tomography; RBANS, Repeatable Battery for the Assessment of Neuropsychological Status; SCZ, schizophrenia.
Fig. 1.
Total volumes of distribution (V T) of [18F]-FEPPA in each of the studied ROIs (hippocampus; mPFC, medial prefrontal cortex; temporal cortex; DLPFC, dorsolateral PFC; and striatum). Data are presented as partial volumes uncorrected VTs (A) and partial volume effect-corrected VTs (B). No significant effect of clinical group (HV vs SCZ) was detected on [18F]-FEPPA VTs when controlling for genetics (F (1,39) = 0.179, P = .674). These findings were unchanged following correction for partial volume effects (F (1,39) = 0.401, P = .530). [18F]-FEPPA, N-acetyl-N-(2-[18F]fluoroethoxybenzyl)-2-phenoxy-5-pyridinamine; HV, healthy volunteer; ROI, regions of interest; SCZ, schizophrenia.
Fig. 2.
Total volumes of distribution (V T) of [18F]-FEPPA measured in white matter tracts, with TAC derived from the ROI analysis on corpus callosum, cingulum bundle, superior longitudinal fasciculus (SLF), and the posterior limb of the internal capsule (PLIC). No difference was observed between the clinical groups when considering V T values measured in the white matter tracts (F (1,38) = 0.597, P = .445). [18F]-FEPPA, N-acetyl-N-(2-[18F]fluoroethoxybenzyl)-2-phenoxy-5-pyridinamine; ROI, regions of interest; TAC, time-activity curve.
Table 2.
Regional [18F]-FEPPA VTs in Gray and White Matter ROIs of Patients With Schizophrenia (SCZ) and Healthy Volunteers (HV)
| Gray Matter ROI | HV (n = 27) | SCZ (n = 16) | Model | Diagnostic Effect | Genetic Effect | Age Effect | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adjusted Mean | SE | Adjusted Mean | SE | F (3,39) | P | F (1,39) | P | F (1,39) | P | F (1,39) | P | ||
| V T | Hippocampus | 11.45 | 2.03 | 8.03 | 2.65 | 1.501 | .230 | 1.045 | .313 | 1.801 | .187 | 0.997 | .324 |
| Medial prefrontal cortex | 9.03 | 0.52 | 9.69 | 0.67 | 5.968 | .002 | 0.602 | .442 | 15.606 | <.001 | 1.012 | .321 | |
| Temporal cortex | 9.59 | 0.60 | 9.78 | 0.78 | 5.101 | .004 | 0.037 | .847 | 14.173 | .001 | 0.394 | .534 | |
| Dorsolateral prefrontal cortex | 9.51 | 0.57 | 9.83 | 0.76 | 4.521 | .008 | 0.110 | .742 | 11.472 | .002 | 1.120 | .296 | |
| Striatum | 8.17 | 0.47 | 8.38 | 0.61 | 6.151 | .002 | 0.075 | .786 | 18.443 | <.001 | 0.307 | .583 | |
| V T, corrected for partial volume effect | Hippocampus | 13.08 | 2.02 | 9.19 | 2.63 | 1.570 | .212 | 1.374 | .249 | 2.227 | .144 | 0.473 | .496 |
| Medial prefrontal cortex | 13.10 | 0.77 | 13.76 | 1.00 | 5.460 | .003 | 0.274 | .604 | 15.869 | <.001 | 0.057 | .813 | |
| Temporal cortex | 12.39 | 0.75 | 12.24 | 0.98 | 5.152 | .004 | 0.014 | .907 | 14.878 | <.001 | 0.034 | .854 | |
| Dorsolateral prefrontal cortex | 13.90 | 0.84 | 13.56 | 1.10 | 3.905 | .016 | 0.062 | .804 | 11.397 | .002 | 0.028 | .869 | |
| Striatum | 8.903 | 0.54 | 9.03 | 0.70 | 6.900 | .001 | 0.020 | .888 | 20.652 | <.001 | 0.230 | .634 | |
| White Matter ROI | HV (n = 27) | SCZ (n = 15) | F (3,38) | P | F (3,38) | P | F (3,38) | P | F (3,38) | P | |||
| V T | Corpus callosum | 7.05 | 0.82 | 6.05 | 1.10 | 1.127 | .350 | 0.521 | .475 | 2.071 | .158 | 0.299 | .588 |
| Cingulum | 8.60 | 1.06 | 7.37 | 1.43 | 2.066 | .121 | 0.482 | .492 | 3.414 | .072 | 1.371 | .249 | |
| SLF | 7.37 | 0.63 | 6.52 | 0.85 | 1.624 | .200 | 0.641 | .428 | 2.559 | .118 | 0.911 | .346 | |
| PLIC | 7.57 | 0.56 | 7.22 | 0.74 | 2.314 | .092 | 1.208 | .149 | 6.406 | .016 | 0.400 | .531 | |
Note: Factorial ANOVA comparisons were performed for each ROI to compare differences between diagnostic groups, with genotype (HAB vs MAB) and age added as covariates. White matter data obtained from one subject from the SCZ group was excluded, since no reliable kinetic model fit could be achieved in the white matter ROIs (coefficient of variation [COV] > 50). [18F]-FEPPA, N-acetyl-N-(2-[18F]fluoroethoxybenzyl)-2-phenoxy-5-pyridinamine; HAB, high-affinity binder; MAB, mixed-affinity binder; PLIC, posterior limb of the internal capsule; ROI, regions of interest; SLF, superior longitudinal fasciculus. Significant values are provided in bold.
When considering gray matter regions of all 38 subjects, no correlation was observed between the ROI volumes normalized to ICV and the [18F]-FEPPA V T in respective regions when controlling for the TSPO genotype (all P > .436). Similarly, no correlations were observed when patients with SCZ were considered separately, with or without controlling for age (P > .05). This lack of correlation was confirmed using data corrected for partial volume effect.
None of the disease parameters correlated with regional [18F]-FEPPA V T, except for a negative correlation between striatal V T and number of crises, which appeared to be driven by a single outlier, and neither surpassed Bonferroni correction for multiple comparisons (P < .01). Exploration of correlations between [18F]-FEPPA VTs and measures of psychopathology and cognition did not detect any significant associations (supplementary tables 1–6).
Discussion
Early brain PET imaging of patients with SCZ using prototypical first-generation TSPO radioligand [11C]PK11195 was hindered by the low signal-to-noise ratio, low brain uptake, and high levels of nonspecific binding of the radiotracer.62 These limitations were overcome by the use of the newer TSPO ligand [18F]-FEPPA, which presents optimal chemical, pharmacokinetic, and pharmacodynamic properties for TSPO imaging,45 including 3-fold higher potency compared with PBR28, and is an order of magnitude more potent than DPA713 or PK11195.45
Despite the challenges of using [11C]PK1195, PET imaging studies have reported increased tracer binding potential in the hippocampal tissue of patients with SCZ during psychosis39 and in the whole-brain gray matter of patients with recent-onset SCZ.38 In the current study, despite use of a second-generation TSPO radioligand and scanning patients with ongoing psychotic symptoms in an high-resolution research tomograph (HRRT) scanner, we observed no significant differences in [18F]-FEPPA binding between HV participants and patients with SCZ. While differences were initially expected in the HC and the DLPFC on the basis of past findings,39,63 we also evaluated the [18F]-FEPPA binding in the mPFC, temporal cortex, and the striatum and found no significant effects. Comparing the present findings with other investigations using second-generation TSPO ligands, our results are in line with those of a recent study that demonstrated no differences in [11C]DAA1106 binding in chronic SCZ patients,40 confirming these findings with a larger sample, correcting for genotype, using the HRRT scanner, and including patients with ongoing psychotic symptoms of moderate severity. From a methodological perspective, the present study utilized an F-18 radioligand and the HRRT scanner that has been demonstrated to improve PET quantification64,65
Our findings, however, are in contrast with a recent report of a 16% increase in TSPO binding observed in postmortem tissues using tritium-labeled second-generation TSPO ligand PBR28.63 These differences may result from the unique properties of radioligands used in the studies, or from differences in patient populations, treatment status, and methodology (in vivo vs postmortem). While our current kinetic modeling approach has been previously validated,46 further refinements that would incorporate a possible slow irreversible binding compartment66 are undergoing evaluation for future TSPO-imaging studies with [18F]-FEPPA. It should be noted that the variability in our current study, as with other second-generation TSPO ligands, is relatively high even with the incorporation of the genetic factor. Using the mean and variability of V T from our data, ~21 subjects per group would be needed to detect a 20% difference between the groups using F test ANOVA (alpha = .05 and power = 0.8); however, exposing an additional 5 SCZ patients to radioactivity and the inconvenience of arterial PET studies with no observed trend for an effect with 16 subjects would be unethical and uneconomical.
Alterations in white matter tracts and changes in regional connectivity are increasingly recognized as key factors in the pathogenesis of SCZ. White matter regions assessed in the current study are based on published reports of abnormalities in white matter tracts of patients with SCZ, as detected by measuring fractional anisotropy.29,67 In addition to the diffuse nature of white matter abnormalities in SCZ, the lack of any significant difference in V T values of the white matter tracts may reflect lack of effects of neuroinflammation in these tracts, antipsychotics use or challenges inherent in measuring TSPO binding in these tissues.58
In SCZ, meta-analyses have shown a progressive reduction in cerebral volume and an increase in ventricular volume over the course of the illness, from first-episode21,68 to chronic disease.22 Neuroinflammation, indexed using [18F]-FEPPA V T values in the current study, did not correlate with regional volumes, possibly due to the complicating factor of antipsychotic treatment and the time of assessment (eg, in the chronic stage of disease). Timing of the scan in the course of illness may be relevant, and in our sample only 5 of the 18 participants with SCZ were scanned within the first 5 years of disease onset; of note, excluding these patients did not alter the results (data not shown). The effect of antipsychotic drugs on regional volume is unclear at this time, with some studies showing reductions in gray matter and increased ventricular fluid volume,69 while other groups report increases in gray matter volumes.70,71 Since there is evidence that changes in regional volumes occur at different time points following the initiation of antipsychotic treatment,70 further exploration on how neuroinflammation and regional volume change over the progression of the disease, before and during the course of antipsychotic treatment are warranted. While benzodiazepines may affect [18F]-FEPPA binding to TSPO,50,51 clonazepam does not seem to exhibit affinity.52,53 Importantly, removing the 7 participants who were treated with clonazepam did not alter our findings, but the remaining group size is likely too small to draw conclusions.
In neurochemical brain imaging studies, sample size routinely represents a potential limitation; however, to our knowledge this study is the largest PET study to date investigating neuroinflammation in SCZ, particularly in patients exhibiting psychotic symptoms and controlling for rs6971. Furthermore, although we did not observe an effect of antipsychotic dose in the present study, it is still possible that antipsychotic medications influenced the results. In vitro studies have demonstrated an inhibitory effect for a number of antipsychotic medications on the release of proinflammatory cytokines and nitric oxide by microglia,72,73 thus, antipsychotics may exert inhibitory effects on microglia that could mask any increases in neuroinflammation, accounting for the lack of differences in [18F]-FEPPA binding between groups. Ideally, future studies with antipsychotic-naive patients can address this issue.
In our current study, V T was used as an outcome measure, representing a ratio between the concentration of the radioligand in the tissue and its concentration in the plasma. We did not delineate the individual contributions of the specific binding (V S) and the combination of nonspecific binding and free tracer (V ND) to the total V T, 74 since V T has been previously shown to be highly identifiable (lower coefficient of variance compared with V S and BPND) and a reliable measure of [18F]-FEPPA retention.44 The use of this outcome measure is not likely to affect the findings, since the nonspecific-binding component would not be expected to be altered in patients with SCZ. Finally, the development and characterization of an 18F-labeled tracer provides a TSPO radioligand with a long half-life, providing flexibility to the data acquisition, as well as allowing the tracer to be distributed to centers without a cyclotron.
In conclusion, we found no evidence of increased neuroinflammation as indexed with [18F]-FEPPA binding in the gray and white matter brain regions of patients with SCZ who were experiencing psychotic symptoms despite treatment with antipsychotic medications. No correlations were observed between [18F]-FEPPA binding and psychopathological indices, length of illness, neuropsychological measures, or regional MRI volumes.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
New Investigator Award of the Brain & Behavior Research Foundation (NARSAD); the National Institutes of Health (NIH) award (R01MH-100043).
Supplementary Material
Acknowledgments
The authors would like to thank the staff of the CAMH Research Imaging Centre for their technical assistance. R.M. and M.K. state that they had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1. Theodoropoulou S, Spanakos G, Baxevanis CN, et al. Cytokine serum levels, autologous mixed lymphocyte reaction and surface marker analysis in never medicated and chronically medicated schizophrenic patients. Schizophr Res. 2001;47:13–25. [DOI] [PubMed] [Google Scholar]
- 2. Zhang XY, Zhou DF, Zhang PY, Wu GY, Cao LY, Shen YC. Elevated interleukin-2, interleukin-6 and interleukin-8 serum levels in neuroleptic-free schizophrenia: association with psychopathology. Schizophr Res. 2002;57:247–258. [DOI] [PubMed] [Google Scholar]
- 3. Akiyama K. Serum levels of soluble IL-2 receptor alpha, IL-6 and IL-1 receptor antagonist in schizophrenia before and during neuroleptic administration. Schizophr Res. 1999;37:97–106. [DOI] [PubMed] [Google Scholar]
- 4. van Kammen DP, McAllister-Sistilli CG, Kelley ME, Gurklis JA, Yao JK. Elevated interleukin-6 in schizophrenia. Psychiatry Res. 1999;87:129–136. [DOI] [PubMed] [Google Scholar]
- 5. Kaiya H, Uematsu M, Ofuji M, et al. Elevated plasma prostaglandin E2 levels in schizophrenia. J Neural Transm. 1989;77:39–46. [DOI] [PubMed] [Google Scholar]
- 6. Dickerson F, Stallings C, Origoni A, Boronow J, Yolken R. C-reactive protein is associated with the severity of cognitive impairment but not of psychiatric symptoms in individuals with schizophrenia. Schizophr Res. 2007;93:261–265. [DOI] [PubMed] [Google Scholar]
- 7. Fan X, Goff DC, Henderson DC. Inflammation and schizophrenia. Expert Rev Neurother. 2007;7:789–796. [DOI] [PubMed] [Google Scholar]
- 8. Müller N, Riedel M, Scheppach C, et al. Beneficial antipsychotic effects of celecoxib add-on therapy compared to risperidone alone in schizophrenia. Am J Psychiatry. 2002;159:1029–1034. [DOI] [PubMed] [Google Scholar]
- 9. Akhondzadeh S, Tabatabaee M, Amini H, Ahmadi Abhari SA, Abbasi SH, Behnam B. Celecoxib as adjunctive therapy in schizophrenia: a double-blind, randomized and placebo-controlled trial. Schizophr Res. 2007;90:179–185. [DOI] [PubMed] [Google Scholar]
- 10. Müller N, Riedel M, Schwarz MJ, Engel RR. Clinical effects of COX-2 inhibitors on cognition in schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2005;255:149–151. [DOI] [PubMed] [Google Scholar]
- 11. Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Venneti S, Lopresti BJ, Wiley CA. The peripheral benzodiazepine receptor (Translocator protein 18kDa) in microglia: from pathology to imaging. Prog Neurobiol. 2006;80:308–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Davalos D, Grutzendler J, Yang G, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat Neurosci. 2005;8:752–758. [DOI] [PubMed] [Google Scholar]
- 14. Denes A, Vidyasagar R, Feng J, et al. Proliferating resident microglia after focal cerebral ischaemia in mice. J Cereb Blood Flow Metab. 2007;27:1941–1953. [DOI] [PubMed] [Google Scholar]
- 15. Hanisch UK, Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. [DOI] [PubMed] [Google Scholar]
- 16. Cosenza-Nashat M, Zhao ML, Suh HS, et al. Expression of the translocator protein of 18kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathol Appl Neurobiol. 2009;35:306–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cagnin A, Myers R, Gunn RN, et al. In vivo visualization of activated glia by [11C] ®-PK11195-PET following herpes encephalitis reveals projected neuronal damage beyond the primary focal lesion. Brain. 2001;124:2014–2027. [DOI] [PubMed] [Google Scholar]
- 18. Jaworski T, Lechat B, Demedts D, et al. Dendritic degeneration, neurovascular defects, and inflammation precede neuronal loss in a mouse model for tau-mediated neurodegeneration. Am J Pathol. 2011;179:2001–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gao HM, Zhang F, Zhou H, Kam W, Wilson B, Hong JS. Neuroinflammation and α-synuclein dysfunction potentiate each other, driving chronic progression of neurodegeneration in a mouse model of Parkinson’s disease. Environ Health Perspect. 2011;119:807–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Takahashi K, Funata N, Ikuta F, Sato S. Neuronal apoptosis and inflammatory responses in the central nervous system of a rabbit treated with Shiga toxin-2. J Neuroinflammation. 2008;5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lieberman J, Chakos M, Wu H, et al. Longitudinal study of brain morphology in first episode schizophrenia. Biol Psychiatry. 2001;49:487–499. [DOI] [PubMed] [Google Scholar]
- 22. Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET. Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry. 2000;157:16–25. [DOI] [PubMed] [Google Scholar]
- 23. Lawrie SM, Abukmeil SS. Brain abnormality in schizophrenia. A systematic and quantitative review of volumetric magnetic resonance imaging studies. Br J Psychiatry. 1998;172:110–120. [DOI] [PubMed] [Google Scholar]
- 24. Pedersen A, Diedrich M, Kaestner F, et al. Memory impairment correlates with increased S100B serum concentrations in patients with chronic schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1789–1792. [DOI] [PubMed] [Google Scholar]
- 25. Dickerson F, Stallings C, Origoni A, Vaughan C, Khushalani S, Yolken R. Additive effects of elevated C-reactive protein and exposure to Herpes Simplex Virus type 1 on cognitive impairment in individuals with schizophrenia. Schizophr Res. 2012;134:83–88. [DOI] [PubMed] [Google Scholar]
- 26. Patel S, Mahon K, Wellington R, Zhang J, Chaplin W, Szeszko PR. A meta-analysis of diffusion tensor imaging studies of the corpus callosum in schizophrenia. Schizophr Res. 2011;129:149–155. [DOI] [PubMed] [Google Scholar]
- 27. Yao L, Lui S, Deng W, et al. Association of white matter deficits with clinical symptoms in antipsychotic-naive first-episode schizophrenia: an optimized VBM study using 3T. MAGMA. 2014;27:283–290. [DOI] [PubMed] [Google Scholar]
- 28. Chiapponi C, Piras F, Fagioli S, Piras F, Caltagirone C, Spalletta G. Age-related brain trajectories in schizophrenia: a systematic review of structural MRI studies. Psychiatry Res. 2013;214:83–93. [DOI] [PubMed] [Google Scholar]
- 29. Holleran L, Ahmed M, Anderson-Schmidt H, et al. Altered interhemispheric and temporal lobe white matter microstructural organization in severe chronic schizophrenia. Neuropsychopharmacology. 2014;39:944–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bayer TA, Buslei R, Havas L, Falkai P. Evidence for activation of microglia in patients with psychiatric illnesses. Neurosci Lett. 1999;271:126–128. [DOI] [PubMed] [Google Scholar]
- 31. Radewicz K, Garey LJ, Gentleman SM, Reynolds R. Increase in HLA-DR immunoreactive microglia in frontal and temporal cortex of chronic schizophrenics. J Neuropathol Exp Neurol. 2000;59:137–150. [DOI] [PubMed] [Google Scholar]
- 32. Busse S, Busse M, Schiltz K, et al. Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: further evidence for disease course-related immune alterations? Brain Behav Immun. 2012;26:1273–1279. [DOI] [PubMed] [Google Scholar]
- 33. Wierzba-Bobrowicz T, Lewandowska E, Lechowicz W, Stepień T, Pasennik E. Quantitative analysis of activated microglia, ramified and damage of processes in the frontal and temporal lobes of chronic schizophrenics. Folia Neuropathol. 2005;43:81–89. [PubMed] [Google Scholar]
- 34. Steiner J, Mawrin C, Ziegeler A, et al. Distribution of HLA-DR-positive microglia in schizophrenia reflects impaired cerebral lateralization. Acta Neuropathol. 2006;112:305–316. [DOI] [PubMed] [Google Scholar]
- 35. Arnold SE, Trojanowski JQ, Gur RE, Blackwell P, Han LY, Choi C. Absence of neurodegeneration and neural injury in the cerebral cortex in a sample of elderly patients with schizophrenia. Arch Gen Psychiatry. 1998;55:225–232. [DOI] [PubMed] [Google Scholar]
- 36. Falke E, Han LY, Arnold SE. Absence of neurodegeneration in the thalamus and caudate of elderly patients with schizophrenia. Psychiatry Res. 2000;93:103–110. [DOI] [PubMed] [Google Scholar]
- 37. Kurumaji A, Wakai T, Toru M. Decreases in peripheral-type benzodiazepine receptors in postmortem brains of chronic schizophrenics. J Neural Transm. 1997;104:1361–1370. [DOI] [PubMed] [Google Scholar]
- 38. van Berckel BN, Bossong MG, Boellaard R, et al. Microglia activation in recent-onset schizophrenia: a quantitative ®-[11C]PK11195 positron emission tomography study. Biol Psychiatry. 2008;64:820–822. [DOI] [PubMed] [Google Scholar]
- 39. Doorduin J, de Vries EF, Willemsen AT, de Groot JC, Dierckx RA, Klein HC. Neuroinflammation in schizophrenia-related psychosis: a PET study. J Nucl Med. 2009;50:1801–1807. [DOI] [PubMed] [Google Scholar]
- 40. Takano A, Arakawa R, Ito H, et al. Peripheral benzodiazepine receptors in patients with chronic schizophrenia: a PET study with [11C]DAA1106. Int J Neuropsychopharmacol. 2010;13:943–950. [DOI] [PubMed] [Google Scholar]
- 41. Yoder KK, Nho K, Risacher SL, Kim S, Shen L, Saykin AJ. Influence of TSPO genotype on 11C-PBR28 standardized uptake values. J Nucl Med. 2013;54:1320–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kreisl WC, Lyoo CH, McGwier M, et al. In vivo radioligand binding to translocator protein correlates with severity of Alzheimer’s disease. Brain. 2013;136:2228–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Owen DR, Yeo AJ, Gunn RN, et al. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab. 2012;32:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mizrahi R, Rusjan PM, Kennedy J, et al. Translocator protein (18kDa) polymorphism (rs6971) explains in-vivo brain binding affinity of the PET radioligand [(18)F]-FEPPA. J Cereb Blood Flow Metab. 2012;32:968–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wilson AA, Garcia A, Parkes J, et al. Radiosynthesis and initial evaluation of [18F]-FEPPA for PET imaging of peripheral benzodiazepine receptors. Nucl Med Biol. 2008;35:305–314. [DOI] [PubMed] [Google Scholar]
- 46. Rusjan PM, Wilson AA, Bloomfield PM, et al. Quantitation of translocator protein binding in human brain with the novel radioligand [18F]-FEPPA and positron emission tomography. J Cereb Blood Flow Metab. 2011;31:1807–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. First M, Spitzer R, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders: Patient Edition (SCIDI/P). Version 2.0. New York: Biometric Research, New York State Psychiatric Institute; 1995. [Google Scholar]
- 48. Müller N, Schwarz MJ. A psychoneuroimmunological perspective to Emil Kraepelins dichotomy: schizophrenia and major depression as inflammatory CNS disorders. Eur Arch Psychiatry Clin Neurosci. 2008;258(suppl 2):97–106. [DOI] [PubMed] [Google Scholar]
- 49. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. [DOI] [PubMed] [Google Scholar]
- 50. Yamagishi H, Kawaguchi M. Characterization of central- and peripheral-type benzodiazepine receptors in rat salivary glands. Biochem Pharmacol. 1998;55:209–214. [DOI] [PubMed] [Google Scholar]
- 51. Kalk NJ, Owen DR, Tyacke RJ, et al. Are prescribed benzodiazepines likely to affect the availability of the 18kDa translocator protein (TSPO) in PET studies? Synapse. 2013;67:909–912. [DOI] [PubMed] [Google Scholar]
- 52. Danovich L, Veenman L, Leschiner S, et al. The influence of clozapine treatment and other antipsychotics on the 18kDa translocator protein, formerly named the peripheral-type benzodiazepine receptor, and steroid production. Eur Neuropsychopharmacol. 2008;18:24–33. [DOI] [PubMed] [Google Scholar]
- 53. Krueger KE. Peripheral-type benzodiazepine receptors: a second site of action for benzodiazepines. Neuropsychopharmacology. 1991;4:237–244. [PubMed] [Google Scholar]
- 54. Randolph C. Repeatable Battery for the Assessment of Neuropsychological Status. San Antonio, TX: Psychological Corporation (Harcourt); 1998. [Google Scholar]
- 55. Wilk CM, Gold JM, Humber K, Dickerson F, Fenton WS, Buchanan RW. Brief cognitive assessment in schizophrenia: normative data for the Repeatable Battery for the Assessment of Neuropsychological Status. Schizophr Res. 2004;70:175–186. [DOI] [PubMed] [Google Scholar]
- 56. Rusjan P, Mamo D, Ginovart N, et al. An automated method for the extraction of regional data from PET images. Psychiatry Res. 2006;147:79–89. [DOI] [PubMed] [Google Scholar]
- 57. Mizrahi R, Addington J, Rusjan PM, et al. Increased stress-induced dopamine release in psychosis. Biol Psychiatry. 2012;71:561–567. [DOI] [PubMed] [Google Scholar]
- 58. Suridjan I, Rusjan PM, Kenk M, et al. Quantitative imaging of neuroinflammation in human white matter: a positron emission tomography study with translocator protein 18kDa radioligand, [(18) F]-FEPPA. Synapse. 2014;68:536–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Leenders KL, Perani D, Lammertsma AA, et al. Cerebral blood flow, blood volume and oxygen utilization. Normal values and effect of age. Brain. 1990;113(pt 1):27–47. [DOI] [PubMed] [Google Scholar]
- 60. Müller-Gärtner HW, Links JM, Prince JL, et al. Measurement of radiotracer concentration in brain gray matter using positron emission tomography: MRI-based correction for partial volume effects. J Cereb Blood Flow Metab. 1992;12:571–583. [DOI] [PubMed] [Google Scholar]
- 61. Lahiri DK, Bye S, Nurnberger JI, Jr, Hodes ME, Crisp M. A non-organic and non-enzymatic extraction method gives higher yields of genomic DNA from whole-blood samples than do nine other methods tested. J Biochem Biophys Methods. 1992;25:193–205. [DOI] [PubMed] [Google Scholar]
- 62. Schweitzer PJ, Fallon BA, Mann JJ, Kumar JS. PET tracers for the peripheral benzodiazepine receptor and uses thereof. Drug Discov Today. 2010;15:933–942. [DOI] [PubMed] [Google Scholar]
- 63. Kreisl WC, Jenko KJ, Hines CS, et al. A genetic polymorphism for translocator protein 18kDa affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation. J Cereb Blood Flow Metab. 2013;33:53–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. van Velden FH, Kloet RW, van Berckel BN, et al. HRRT versus HR+ human brain PET studies: an interscanner test-retest study. J Nucl Med. 2009;50:693–702. [DOI] [PubMed] [Google Scholar]
- 65. Leroy C, Comtat C, Trébossen R, Syrota A, Martinot JL, Ribeiro MJ. Assessment of 11C-PE2I binding to the neuronal dopamine transporter in humans with the high-spatial-resolution PET scanner HRRT. J Nucl Med. 2007;48:538–546. [DOI] [PubMed] [Google Scholar]
- 66. Rizzo G, Veronese M, Tonietto M, Zanotti-Fregonara P, Turkheimer FE, Bertoldo A. Kinetic modeling without accounting for the vascular component impairs the quantification of [(11)C]PBR28 brain PET data. J Cereb Blood Flow Metab. 2014;34:1060–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ellison-Wright I, Nathan PJ, Bullmore ET, et al. Distribution of tract deficits in schizophrenia. BMC Psychiatry. 2014;14:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zipursky RB, Lambe EK, Kapur S, Mikulis DJ. Cerebral gray matter volume deficits in first episode psychosis. Arch Gen Psychiatry. 1998;55:540–546. [DOI] [PubMed] [Google Scholar]
- 69. Moncrieff J, Leo J. A systematic review of the effects of antipsychotic drugs on brain volume. Psychol Med. 2010;40:1409–1422. [DOI] [PubMed] [Google Scholar]
- 70. Deng MY, McAlonan GM, Cheung C, et al. A naturalistic study of grey matter volume increase after early treatment in anti-psychotic naïve, newly diagnosed schizophrenia. Psychopharmacology (Berl). 2009;206:437–446. [DOI] [PubMed] [Google Scholar]
- 71. Li M, Chen Z, Deng W, et al. Volume increases in putamen associated with positive symptom reduction in previously drug-naive schizophrenia after 6 weeks antipsychotic treatment. Psychol Med. 2012;42:1475–1483. [DOI] [PubMed] [Google Scholar]
- 72. Hou Y, Wu CF, Yang JY, et al. Effects of clozapine, olanzapine and haloperidol on nitric oxide production by lipopolysaccharide-activated N9 cells. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30:1523–1528. [DOI] [PubMed] [Google Scholar]
- 73. Bian Q, Kato T, Monji A, et al. The effect of atypical antipsychotics, perospirone, ziprasidone and quetiapine on microglial activation induced by interferon-gamma. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:42–48. [DOI] [PubMed] [Google Scholar]
- 74. Innis RB, Cunningham VJ, Delforge J, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab. 2007;27:1533–1539. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.