Abstract
Nicotine improves cognitive functioning in smokers and psychiatric populations, but its cognitive-enhancing effects in healthy nonsmokers are less well understood. Nicotine appears to enhance certain forms of cognition in nonsmokers, but its specificity to subtypes of cognition is not known. This study sought to replicate and extend previous findings on the effects of nicotine on cognitive performance in healthy nonsmokers. Healthy young adults (N=40, 50% female) participated in a placebo-controlled, double-blind, repeated-measures experiment examining the effects of 7mg transdermal nicotine or placebo. Participants completed tests of attention (Attention Network Test), behavioral inhibition (stop signal task, Stroop test), reward responsiveness (signal detection task) and risk-taking behavior (Balloon Analogue Risk Taking task (BART)). Physiological (heart rate, blood pressure) and subjective (Profile of Mood States; POMS, Drug Effects Questionnaire; DEQ) measures were also obtained. Nicotine significantly improved performance only on the Stroop test, but it impaired performance on one aspect of the Attention Network Test, the orienting effect. Nicotine produced its expected effects on physiologic and subjective measures, within the intended time course. The findings of this study contribute to a growing literature indicating that nicotine differentially affects specific subtypes of cognitive performance in healthy nonsmokers.
Keywords: nicotine, nonsmokers, cognition, attention, inhibitory control
Nicotine is a potent, centrally acting cholinergic agonist that has well-documented cognitive-enhancing effects in smokers (Wesnes and Warburton, 1983; Bell et al., 1999), as well as in psychiatric patients including those with Alzheimer’s disease (Sahakian and Jones, 1991), schizophrenia (Levin et al., 1996a; Barr et al., 2008a), and attention-deficit/hyperactivity disorder (ADHD) (Levin et al., 1996b, 2001; Potter and Newhouse, 2004, 2008). These enhancing effects of nicotine are complicated by possible preexisting conditions affecting cognition, including nicotine withdrawal or disease-related impairments. It is less clear whether nicotine also improves cognitive performance in healthy nonsmokers, and whether it affects only certain specific cognitive functions. The present investigation sought to replicate and extend earlier findings that nicotine improved attention in healthy nonsmokers (Levin et al., 1998; Poltavski and Petros, 2006), and to investigate its effects on several other cognitive domains including behavioral inhibition, reward responsiveness and risk-taking.
The current literature suggests that nicotine can reliably improve attention in healthy nonsmokers (see Heishman et al., 2010 and Levin et al., 2006 for reviews). However, attention is a complex cognitive construct comprised of subtypes (e.g., sustained, selective and divided attention) which may be differentially affected by nicotine (Hahn et al., 2009). One valuable schema for separating different components of attention has been proposed by Posner and Peterson (1990), who suggested that attention is comprised of three independent subsystems: the alerting system which helps to maintain an alert and vigilant state; the orienting system which guides and directs attention; and the executive system which resolves conflict and resists distraction (Fan et al., 2002). The three components are assessed in the Attention Network Test (Fan et al., 2002). These components of attention appear to be differentially impaired in different disorders such as schizophrenia or ADHD (Knudson, 2007), suggesting that they may also be differentially affected by a drug such as nicotine. Thus, the present study assessing the effects of nicotine on the Attention Network Test may be useful not only to extend our knowledge of how nicotine acts, but also to pharmacologically separate the different components of attention. To our knowledge only one previous study used the Attention Network Test as an attentional measure in the context of nicotine and nonsmokers. In a sample of 20 nonsmokers, Kleykamp et al. (2005) found that 2 and 4 mg nicotine gum did not affect performance on any of the three attentional measures of the test; however the relatively small sample size and low dose of nicotine may have contributed to their null findings. We employed the Attention Network Test as our primary measure of attention to examine the effect of nicotine on specific components of attention.
Another important domain of cognitive performance is behavioral inhibition, which represents “the ability to delay or refrain from responding due to environmental cues” (Potter and Newhouse, 2004). As with attention, there are thought to be different subtypes of behavioral inhibition such as interference control and motor response inhibition (Nigg, 2000), and nicotine may affect these subtypes differently (Potter and Newhouse, 2004; Barr et al., 2008a). Interference control refers to the ability to suppress competing secondary responses in favor of a primary response and is commonly measured by the Stroop test (Stroop, 1935), whereas motor response inhibition refers to the ability to infrequently inhibit a primary, prepotent motor response. Thus, interference control reflects the ability to protect a consistent behavioral rule from outside distraction, while motor response inhibition reflects the ability to adjust already-initiated behaviors as rules change. Previous studies investigating the effects of nicotine in nonsmokers on the Stroop test have yielded mixed results: Wesnes and Warburton (1983) and Provost and Woodward (1991) reported that nicotine improved Stroop performance in healthy adult nonsmokers, but Foulds et al (1996) failed to see an improvement in a similar sample. Nicotine reportedly improves motor response inhibition in ADHD patients (Potter and Newhouse, 2004), but to our knowledge this measure has not been used to study nicotine in healthy nonsmokers.
In addition to its effects on attention and behavioral inhibition, nicotine may affect motivational dimensions of cognitive performance. In their work on executive function development, Zelazo and Muller (2002) distinguish between more purely cognitive or “cool” aspects of cognition typically associated anatomically with the dorsolateral prefrontal cortex and more affective or “hot” aspects of cognition associated anatomically with the orbital and medial prefrontal cortex. The more cognitive aspects are assessed behaviorally with tasks like the Stroop test and stop task, whereas the more affective aspects are assessed using tasks with more affectively salient stimuli. Although similar dual-mode models have been proposed in the field of cognition (Barrett et al., 2004; Carver et al., 2008), this distinction has not commonly been made in the context of pharmacological research. In one recent study with nonsmokers, Barr et al. (2008b) used a modified signal detection task to show that an acute dose of transdermal nicotine increased the likelihood of responding specifically to rewarded stimuli, suggesting that it increased reward responsiveness. This is one of few studies designed to explore the effects of nicotine on this area of “hot” (emotional) cognition. We sought to replicate their findings on with this modified signal detection task, using a large sample size. Finally, we incorporated a second measure of more motivationally based cognition and decision making called the Balloon Analogue Risk Taking task, to test whether or not an acute dose of nicotine affects risk-taking behavior as measured by this gambling task. This task has not previously been used in this context, and thus further extends our knowledge of the cognitive effects of nicotine in nonsmokers.
In this study, we sought to extend prior research by examining the acute effects of nicotine in healthy nonsmokers on three subtypes of attention, and two subtypes of behavioral inhibition. We also included two tasks measuring motivational aspects of cognition, namely reward responsiveness and risk-taking. First, we hypothesized that nicotine would increase attention as measured by all three components of the Attention Network Test; additionally, we explored whether nicotine differentially affects specific subtypes of attention. Second, we hypothesized that nicotine would improve performance on measures of behavioral inhibition and reward responsiveness and increase risk-taking behavior. The study adds to the existing literature on the cognitive-enhancing effects of nicotine in nonsmokers by using new or rarely used performance tasks, using multiple measures for overlapping theoretical constructs, including a comparatively large sample size and therefore increased statistical power and using a route of nicotine administration that allows for controlled and consistent dosing.
Method
Participants
We recruited 40 healthy nonsmokers (50% female) age 18-40 via online advertisement. Participants were eligible if they had not smoked any form of tobacco product for at least 30 days,1 were fluent in English, had at least high school education equivalency, and were not using any medications other than birth control. Eligible candidates were interviewed by a masters-level assistant in an abbreviated, semi-structured psychiatric interview using DSM-IV criteria (APA, 1994). Medical histories and detailed drug use histories were obtained, and participants completed the Michigan Alcoholism Screening Test (MAST, Selzer 1971), Beck Depression Inventory (BDI, Beck et al. 1961), and a psychiatric symptom checklist (SCL90, Derogatis, 1983). Individuals with any current Axis I Disorder, or who scored more than 5 on the MAST, more than 9 on the BDI, or who drank more than 4 alcoholic drinks per day were excluded. Screening also included a physical examination and an electrocardiogram.
Design and Procedures
The study employed a two-session, within-subject, double-blind design comparing performance after transdermal nicotine (7 mg) or placebo. The orders of both the experimental condition (drug or placebo) as well as the behavioral tasks were counterbalanced across participants. Participants initially took part in a 45-minute orientation session during which they gave written consent and briefly practiced each of the tasks in order to minimize any potential learning effects. They were then scheduled for two study sessions, which were separated by a minimum of 48 hours and no more than 14 days. Each session lasted approximately five hours and was conducted in a period between 8:00am and 5:00pm. Upon arrival in the laboratory, participants provided breath (Blood Alcohol Level and Carbon Monoxide) and urine samples to ensure they were drug and pregnancy free. They then completed baseline mood (Profile of Mood States (POMS); McNair et al., 1971) and blood pressure measures. A patch containing either placebo or nicotine (7 mg Nicoderm) was placed on the participants’ upper arm 15 minutes after arrival. The placebo and nicotine patches were covered with adhesive tape to mask their identity for both the research assistant and the participant. The placebo patch contained a small amount of capsaicin analgesic cream to mimic the tingling sensation of the nicotine patch (Acheson et al., 2006). The transdermal route of administration was chosen because of its steady and sustained release of nicotine across several hours (Fant et al., 2000). Blood pressure readings and self-report questionnaires were administered at several timepoints throughout the session (arrival, 60, 120, 180, 260 and 280 minutes). Participants were allowed to relax for three hours after patch placement to allow for absorption of the nicotine. After exactly three hours from patch placement, participants began the behavioral tasks. In between tasks participants were allowed short breaks if they chose, otherwise they were free to continue. Participants were not allowed to eat at any point during the session, including between behavioral tasks. After the tasks, the patch was removed and participants were free to leave. Participants were paid $150 for their participation.
Behavioral Measures
Attention Network Test
The Attention Network Test (Fan et al., 2002) is a computerized measure of attention based on Posner’s and Petersen’s (1990) formulation of attention, consisting of three functionally and anatomically separate attentional networks: the alerting network maintains a vigilant state; the orienting network selects information from sensory input; and the executive network resolves competing responses. During the task, participants are required to indicate whether a central arrow points to the left or right by pressing the corresponding key on the keyboard. On alerting trials, a warning stimulus predicts an upcoming reaction time trial and the mean difference in reaction time between no-cue and cued trails is the alerting effect. On orienting trials, the warning stimulus also provides spatial information (indicating whether the arrow will appear above or below the fixation point), and the orienting effect is the difference in mean reaction time between no-cue and cued trails. On conflict trials, the presence of additional similar but distracting arrow stimuli (the central arrow is flanked by two arrows on each side which point either in the same or opposite direction) requires conflict resolution, and the conflict effect is the difference in mean reaction time between trials in which additional stimuli are distracting (incongruous) or not (see Fan et al., 2002 for a more detailed description). This task takes approximately 20 minutes to complete.
Stroop Test
The Stroop test (Stroop, 1935) is a widely used measure of interference control. In this experiment we used the card/paper version of the test in which there are three pages, each with five columns of 20 items. In the word condition, the items are simply the words red, blue and green printed in black ink and listed in random sequence. In the color condition, swatches of red, blue and green color are listed in random sequence. In the color-word condition, the words red, blue and green are listed in random sequence and printed in a color of ink incongruous with the meaning of the word (e.g. the word red printed in blue ink). For each condition, the participant has 45 seconds to read out-loud as many of the items on the page as quickly as possible. Importantly, in the color-word condition, participants are instructed to name the color of the ink of each item rather than the word itself. The number of items named was their word score, color score and color-word score respectively. The primary outcome measure in this task is the Stroop effect, which is calculated by subtracting the color-word score from the color score, and is a measure of interference control. This task takes approximately five minutes to complete.
Stop Signal Task
(Logan et al., 1984) This task is a computerized test of motor inhibition. Participants must respond to a visual “go” signal except when it is preceded by an auditory stop signal. By a series of adjusting trials, the task provides a measure of the time needed to inhibit a response once the participant has received the signal to make the response. The primary dependent variable from this task is the stop signal reaction time, which is an average of the time needed for the participant to correctly withhold a response. Go reaction times (i.e. simple reaction time) were also collected. This task takes approximately ten minutes to complete.
Modified Signal Detection Task
(Pizzagalli et al., 2005). This computerized task measures reward responsiveness by determining the bias in responding toward a more frequently rewarded stimulus. Participants choose which of two very similar stimuli (short or long mouth) has been presented on a cartoon face by responding on a keyboard. An asymmetric, probabilistic reinforcer ratio of 3 to 1 is used to elicit a response bias for the long mouth over the short. There are three blocks, each of which contains 80 trials. If an incorrect response is selected, no feedback is given, but if a correct response is given, feedback (“Correct!! You won 5 cents”) is given. The primary outcome variable is response bias, which is an index of the tendency to choose the more rewarded stimulus. Discriminability is a control variable indicating how well the participant could distinguish between the two stimuli. At the end of the session, participants are actually awarded the money they earned. This task takes approximately 15 minutes to complete.
Balloon Analogue Risk Task
(BART; Lejuez et al., 2002; Bornovalova et al., 2009) The BART is a computerized task measuring risk-taking behavior. Participants are presented with a series of trials in which they pump-up a balloon, earning a variable amount of money for each pump (1, 5 or 25 cents), but losing all the money if the balloon explodes. At any time participants are allowed to continue to pump (risky choice) or cash out (safe choice). The primary outcome measures for this task is the average adjusted number of pumps—that is, the number of pumps on the trials in which the balloon did not pop. At the end of the session, participants are actually awarded the money they earned. This task takes approximately ten minutes to complete.
Physiological Measures
Blood pressure and heart rate readings were recorded at each time point to ensure participants’ safety. These readings were also used to ensure that the drug was having a measureable physiological effect, and that this was occurring during the intended time course. For analyses of blood pressure, we used mean arterial pressure.2
Subjective Measures
Profile of Mood State
(McNair et al., 1971). The POMS provides a sensitive measure of mood states, both in response to drugs and in the drug-free state (de Wit & Griffiths, 1991). The version of the POMS used here consists of 72 adjectives commonly used to describe momentary mood states. Eight scales derived using factor analysis are: “Anxiety,” “Depression,” “Anger,” “Vigor,” “Fatigue,” “Confusion,” “Friendliness,” and “Elation.” The primary dependent variables for this measure are derived as follows: Arousal = (Anxiety + Vigor) – (Fatigue + Confusion); Positive Mood = Elation – Depression.
Drug Effects Questionnaire (DEQ)
The DEQ consists of four questions concerning current subjective experience of drug effects. Participants indicate on 100mm lines whether they i) are currently feeling any drug effects (from “none at all” to “a lot”), ii) like the effects they feel (from “not at all” to “very much”), iii) are high (from “not at all” to “very”), and iv) want more of the drug (from “not at all” to “very much”). These measures are used to verify that, in the first place, nicotine has induced a measureable and noticeable effect, and also to compare individual differences in response to the nicotine between participants.
Statistical Analyses
Responses to nicotine and placebo were compared using repeated measures analyses of variance (ANOVA), using SPSS 17 for Windows. Two-way ANOVA’s (drug, time) were used for physiologic and subjective measures, and for the signal detection task (drug, block). Post-hoc tests (t-tests) were conducted when appropriate to determine the significance of individual contrasts. As controls, sex and session order were examined as between-subject factors, and mean-centered BMI was included as a covariate (Delaney and Maxwell, 1981). Additional analyses were performed to determine any potential differences in outcome variables between never-smokers (N=35) and non-naïve participants. Primary variables of interest were not significantly different when analyses were run only for never-smokers, thus we decided to include all participants in our final analyses.
Results
Subjective and physiological measures
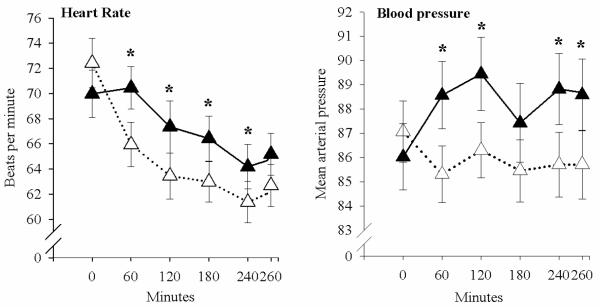
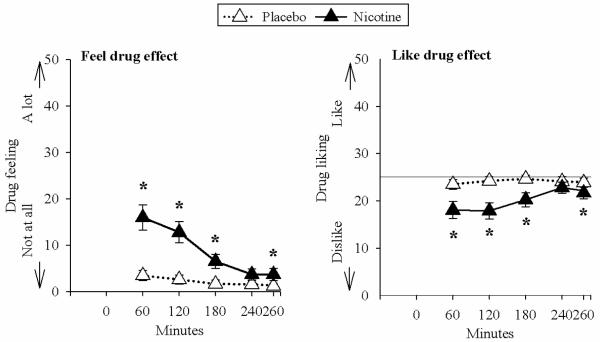
Nicotine increased heart rate compared to placebo (main effect of treatment [F(1,34)=7.45, p=0.01]) (Fig.1). Nicotine also increased mean arterial pressure (treatment and time interaction [F(1,34)=7.56, p=.009]), at 60, 120, 240, and 280 minutes (Fig. 1). Nicotine did not significantly change any of the POMS scales, but it increased DEQ “feel” ratings compared to placebo (treatment and time interaction [F(4,36)=8.10, p<.001]) at 60, 120, 180, and 260 minutes (Fig. 2). Nicotine also decreased DEQ “like” ratings (treatment and time interaction [F(4,36)=2.96, p=.033]) at 60, 120, 180, and 260 minutes (Fig. 2).
Figure 1.
Mean and standard error for heart rate and mean arterial pressure across the session after nicotine (filled symbols) and placebo (open symbols). Times at which nicotine significantly increased heart rate and mean arterial pressure compared to placebo are indicated by asterisks.
Figure 2.
Mean and standard error for subjective effects, feel drug effect and like drug effect, across the session after nicotine and placebo. Times at which nicotine significantly increased drug feeling (“I feel the effects of drug” [not at all to a lot]) and decreased drug liking (“I like the effects of a drug [not at all to a lot]) are indicated with asterisks. It is notable that responses to nicotine were modest and did not approach the maximum ratings of feeling or disliking.
Attention Network Test
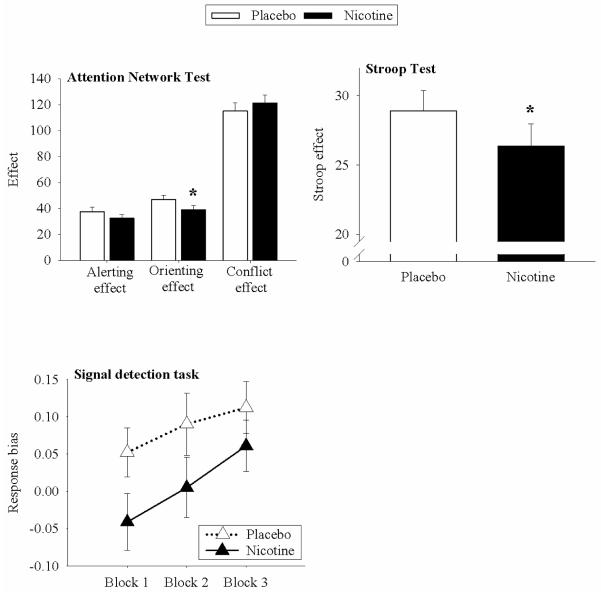
Nicotine did not significantly affect the alerting effect, the conflict effect, or overall accuracy, but did significantly decrease performance on the orienting effect (main effect of treatment [F(1,35)=8.79, p=0.01]) (Fig. 3).
Figure 3.
Mean and standard error for select behavioral outcome variables after nicotine (filled bars) and placebo (open bars). Nicotine significantly decreased ANT orienting effect, a measure of reaction times on directionally-cued conditions. Nicotine also significantly decreased Stroop effect scores, a measure of interference control in the color-word condition. Nicotine also reduced response bias in the signal detection task. RB increased across blocks, but there was no interaction between drug and block.
Stroop Test
Nicotine significantly improved the Stroop effect (main effect of treatment [F(1,35)=6.18, p=.018]) (Fig. 6). This increase in Stroop effect performance was driven mainly by a significant increase in color-word score naming during the nicotine condition (main effect of treatment [F(1,35)=8.13, p=.007]). Nicotine did not change either word score or color score relative to placebo.
Stop Signal Task
Nicotine did not significantly affect either stop signal reaction time or go reaction time.
Modified Signal Detection Task
Relative to placebo, response bias was significantly lower after nicotine (main effect of treatment [F(1,35)=6.08, p=0.019]), but there was no interaction between drug and block. Participants improved significantly across the three blocks, regardless of whether they received drug or placebo (main effect of block [F(1,35)=5.46, p=0.009]) (Fig. 7). Finally, there was no effect of either drug or block on discriminability, indicating that participants did not change in their ability to distinguish the two stimuli either as a function of treatment or time.
BART
Data from only 27 participants were collected for this task. Nicotine did not significantly affect the average adjusted number of pumps. However, regardless of treatment, participants responded significantly less as the reward value increased (main effect of value [F(1,23)=7.00, p=0.004]).
Discussion
This study examined the effects of an acute dose of transdermal nicotine compared to placebo on several measures of cognitive performance in healthy nonsmokers. Nicotine improved performance on the Stroop test, a measure of behavioral inhibition, but did not improve performance on the stop signal task, another measure of inhibition. In contrast, nicotine impaired performance on one component of the Attention Network Test, orienting attention, but had no significant effect on alerting attention or conflict resolution. Furthermore, nicotine decreased reward responsiveness compared to placebo and had no effect on risk-taking.
The effect of nicotine on the tasks was mixed, but the drug produced its expected physiological and subjective effects. Nicotine increased heart rate and mean arterial pressure to levels that are comparable to previous studies (Gehricke et al., 2009), and it increased reports of feeling a drug effect and disliking the drug effect. These measures increased within 60 min, and then decreased over the course of the session (Fig. 1 and 2). The negative subjective effects (i.e., ratings of disliking the drug effect) may have been related to the mild nausea reported by some subjects, and are consistent with previous reports on the subjective effects of nicotine in nonsmokers (Ashare et al., 2010).
Our first goal was to extend earlier findings that nicotine improved attention in healthy nonsmokers. We found that nicotine did not improve performance on any of the sub-measures of the Attention Network Test, and actually decreased performance on the orienting effect, a measure of selective attention. These findings are contrary to previous reports of general attentional improvement using similar doses of nicotine (Levin et al., 1998; Poltavski and Petros, 2006). However, these studies measured attention with the Conners Continuous Performance task, which measures sustained attention (vigilance) but not selective attention. They found improvements on this measure (and behavioral inhibition in the case of Levin et al. (1998)), whereas we found a performance decrease in selective attention (orienting effect) but not in sustained attention (alerting effect) with a different measure of attention (Attention Network Test). The one previous study investigating the effects of nicotine in nonsmokers using the Attention Network Test (Kleykamp et al., 2005) did not show any beneficial effect of nicotine on any of the three subtypes of attention measured by the Attention Network Test, although they used 2 and 4mg doses of nicotine gum and a smaller N (20 participants) which could explain their discrepant findings. While our results confirm some previous reports that nicotine does not improve general attentional functioning in healthy nonsmokers, they also demonstrate the importance of examining specific subtypes of attention, which may be differentially affected by nicotine. Our finding of impairment on the measure of orienting is difficult to explain in light of previous findings, and additional research may reveal that it is a spurious finding.
Nicotine significantly improved one measure of behavioral inhibition, the Stroop test, but did not affect on another measure of inhibition, the stop signal task. Nicotine significantly improved performance on the Stroop effect, a measure of interference control/behavioral inhibition (Fig. 3). This improvement was driven by an increase in color-word score performance during the nicotine session, while the word score and color score conditions remained unaffected. This pattern of findings suggests that nicotine’s cognitive-enhancing effects are due to specifically inhibitory improvements in functioning, rather than a more general increase in arousal or processing speed. In contrast, nicotine did not affect performance on the second measure of inhibition, the stop signal task, which measures the ability to stop a prepotent primary motor response. The sensitivity of the stop signal task to drug effects has been demonstrated in previous studies (de Wit et al., 2002), which showed improved performance after administration of an acute dose of the stimulant d-amphetamine. Thus, there may be subtle differences in the processes underlying these two measures of inhibition (Nigg, 2000). Nigg (2000) notes that the Stroop test measures the “active suppression of a competing (but never intended) response,” whereas the stop task measures the ability to suppress a “primary, intended response to a relevant stimulus.” Thus, nicotine improved the ability to ignore consistently irrelevant stimuli, but not the ability to cancel a response that is normally the correct response. This finding provides evidence for pharmacological specificity of two distinct forms of response inhibition. Of further interest, we initially expected performance on the Stroop test to correlate with performance on the conflict effect portion of the Attention Network Test because both involve error detection and conflict resolution. However, in our study these two measures were unrelated. Future studies using larger samples and more challenging versions of the tasks may elucidate this relationship.
In our study, nicotine reduced response bias in the signal detection task, a measure of reward responsiveness, whereas in a previous study (Barr et al., 2008b), nicotine increased response bias compared to placebo. Possible reasons for the apparent discrepancy include differences in doses (14mg in Barr et al vs. 7mg here), or demographic differences in the participants including mean age (39 vs. 24), history of any past smoking (23% vs. 5%), and sample size (N=30 vs. N=40). Other differences include the use of a short practice session at orientation in our study, as well as the use of the same response key allocation across sessions in our study, both of which may have introduced carry over effects and influenced the results. Notably, the participants in both the Barr et al. (2008) study and the present study reported some unpleasant effects from the drug (nausea in Barr et al. (2008b), disliking in our study), suggesting that the drug produced similar subjective effects across the studies. However, subjective effects of the drug were not related to any behavioral task outcomes in our study, suggesting that aversive feeling states did not influence task performance. In the Barr et al. (2008b) study, response bias improved across the three blocks after nicotine, but not after placebo, while in our study response bias was lower in the nicotine condition right from the first block, and remained significantly lower than placebo through all the three blocks, even though the response bias improved across the three blocks in both conditions (Fig. 3). The reason for the lower response bias in our study compared to Barr et al. (2008b) is difficult to explain, but may be related to the complex and non-linear dose-effect function of nicotine (Picciotto et al., 2008).
Nicotine did not change performance on the BART, relative to placebo, suggesting that it does not increase risk-taking. The absence of a drug effect cannot easily be attributed to insensitivity of the task, since participants were sensitive to changes in the reward value across trials within the sessions. Indeed, the systematic decrease in responding as reward value increased may also be an indicator of sensitivity to changes in reward, and the finding that nicotine did not change this variable suggests that it also did not change sensitivity to reward.
The study had several limitations. First, it would have been of interest to be able to examine the responses to nicotine in relation to participants’ baseline performance. Prior research suggests that, at least in regards to attention, initially under-performing participants are most likely to benefit from nicotine administration (Poltavski and Petros, 2006); however, without an independent assessment of baseline performance we were unable to examine this relationship because of the potential for a regression to the mean.3 Future studies should be designed to more easily determine the response to the drug in relation to baseline performance. A second limitation was that there may have been a ceiling effect on some of the tasks. Participants performed very accurately on almost all of the tasks, and effects of nicotine may only be detected on tasks in which significant improvement is possible. Furthermore, participants spent almost 90 minutes completing tasks, by the end of which time both physiological and subjective effects of nicotine begin to dissipate, making it difficult to detect cognitive-enhancing effects toward the end of the session. There is also some question about the effectiveness of the monetary rewards for the signal detection task and BART. The average amount paid out to participants was $4.80 and $6.20 respectively, which was relatively small compared to the overall compensation they received for the study ($150). Thus, it could be that stronger reinforcers are necessary to detect drug effects on these tasks. Finally, while logistically complicated, future study designs ought to incorporate multiple doses of nicotine in order to more clearly demonstrate potential dose-dependent performance-enhancing or detracting effects of nicotine.
In summary, an acute 7mg dose of transdermal nicotine administered to healthy nonsmokers produced physiological and subjective changes, and improved a specific form of behavioral inhibition, interference control, as measured by the Stroop test. The results are consistent with some other findings, that acute doses of nicotine in nonsmokers produce minimal enhancement of cognitive performance. This makes it less likely that cognitive enhancement is a significant motivating factor in the initiation of smoking. It also suggests that nicotine administration would not provide rapid cognitive improvement for conditions characterized by cognitive impairment. However, the fact that nicotine affected certain specific subtypes of cognition highlights the need for more nuanced cognitive constructs and tasks to be used in similar pharmacology experiments. The present study used a relatively large sample size and multiple measures of specific cognitive constructs, and set the stage for future studies designed to deconstruct the processes by which drugs affect behavior and cognition.
Table 1.
Participant Demographic Information
| N (female) | 40 (20) |
| Mean age (SD) | 23.9 (3.8) |
| Mean BMI (SD) | 23.4 (2.4) |
| Race | |
| African-American | 2 |
| Asian | 5 |
| Caucasian | 26 |
| Hispanic | 5 |
| Other | 2 |
| Mean years of education (SD) | 15.4(1.6) |
| Current drug use | |
| Mean alcoholic drinks per week (SD) | 2(1.5) |
| Mean caffeinated drinks per week (SD) | 4.6 (4.5) |
| Smoking history | |
| Never-smoker | 35 |
| Occasional smoker | 3 |
| Former smoker; quit > 3 years ago | 2 |
Note. No participant had smoked any tobacco product in the past 30 days. Additionally, never-smokers were defined as never having smoked a single cigarette during their lifetime, and occasional smokers were defined as never having been regular smokers.
Table 2.
Means (Standard Deviations) for all Primary Dependent Variables
| Placebo | Nicotine | |
|---|---|---|
| Stroop Test | ||
| Word score | 110.03 (18.99) | 110.80 (18.89) |
| Color score | 82.95 (14.56) | 82.65 (15.91) |
| Color-word score | 53.55 (9.75) | 56.28 (11.43)* |
| Stroop effect | 28.90 (9.17) | 26.38 (10.05)* |
| Attention Network Test | ||
| Alerting effect | 37.57 (22.11) | 32.48 (20.67) |
| Orienting effect | 46.93 (20.02) | 38.98 (20.64)* |
| Conflict effect | 115.00 (41.74) | 121.07 (41.13) |
| Accuracy | 96.85 (2.99) | 96.55 (3.29) |
| Stop Signal Task | ||
| SSRT | 252.36 (38.57) | 258.06 (39.20) |
| GO-RT | 557.95 (148.50) | 548.44 (180.11) |
| Signal Detection Task | ||
| Response bias: block 1 | .05 (.21) | −.04 (.24) |
| Response bias: block 2 | .09 (.26) | .01 (.26) |
| Response bias: block 3 | .11 (.22) | .06 (.22) |
| BART | ||
| Pumps: 5 cents | 40.94 (17.53) | 43.15 (17.61) |
| Pumps: 10 cents | 34.77 (17.72) | 34.50 (14.99) |
| Pumps: 25 cents | 31.45 (19.37) | 29.98 (15.92) |
| Physiologics | ||
| Heart rate | 64.90 (9.82) | 67.14 (9.96)** |
| Mean arterial pressure | 85.86 (7.09) | 88.00 (8.03)** |
| POMS | ||
| Positive mood | 1.47 (1.04) | 1.43 (.96) |
| Arousal | 1.15 (1.18) | 1.05 (1.15) |
| DEQ | ||
| Feel | 2.14 (4.96) | 8.58 (9.66)*** |
| Like | 24.07 (4.41) | 20.14 (7.17)** |
Note.
p<.05,
p<.01,
p<.001
Acknowledgments
This research project was funded by grant DA02812. The authors wish to thank Margaret Wardle for advice regarding data analysis and manuscript preparation.
Footnotes
35 of our participants were never-smokers. Of the remaining five, three reported some lifetime occasional smoking, and two classified themselves as former smokers though both had quit more than three years ago. None of the five had smoked in the past year.
Mean arterial pressure was computed at follows: (systolic + (2 x diastolic))/3 (Klabunde 2005).
Following Baschnagel and Hawk (2008), we used Oldham’s (1962) method to assess change between drug and placebo conditions on the ANT, however, while potentially avoiding the problem of regression to the mean, nicotine still did not appear to improve performance in participants whose baseline performance was initially low.
References
- Acheson A, Reynolds B, Richards JB, de Wit H. Diazepam impairs behavioral inhibition but not delay discounting or risk taking in healthy adults. Experimental and Clinical Psychopharmacology. 2006;14(2):190–198. doi: 10.1037/1064-1297.14.2.190. [DOI] [PubMed] [Google Scholar]
- Ashare RL, Baschnagel JS, Hawk LW. Subjective effects of transdermal nicotine among nonsmokers. Experimental and Clinical Psychopharmacology. 2010;18(2):167–174. doi: 10.1037/a0018864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badcock JC, Michie PT, Johnson L, Combrinck J. Acts of control in Schizophrenia: dissociating the components of inhibition. Psychological Medicine. 2002;32(2):287–297. doi: 10.1017/s0033291701005128. [DOI] [PubMed] [Google Scholar]
- Barkley RA. Behavioral inhibition, sustained attention, and executive functions: constructing a unified theory of ADHD. Psychological Bulletin. 1997;121(1):65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- Barr RS, Culhane MA, Jubelt LE, Mufti RS, Dyer MA, Weiss AP, Deckersbach T, Kelly JF, Freudenreich O, Goff DC, Evins AE. The effects of transdermal nicotine on cognition in nonsmokers with schizophrenia and nonpsychiatric controls. Neuropsychopharmacology. 2008a;33:480–490. doi: 10.1038/sj.npp.1301423. [DOI] [PubMed] [Google Scholar]
- Barr RS, Pizzagalli DA, Culhane MA, Goff DC, Evins AE. A single dose of nicotine enhances reward responsiveness in nonsmokers: implications for development of dependence. Biological Psychiatry. 2008b;63:1061–1065. doi: 10.1016/j.biopsych.2007.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Tugade MM, Engle RW. Individual differences in working memory capacity and dual-process theories of the mind. Psychological Bulletin. 2004;130:553–573. doi: 10.1037/0033-2909.130.4.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Menderson M, Mack J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bell SL, Taylor RC, Singleton EG, Henningfield JE, Heishman SJ. Smoking after nicotine deprivation enhances cognitive performance and decreasing tobacco craving in drug abusers. Nicotine and Tobacco Research. 1999;4(1):3–4. doi: 10.1080/14622299050011141. [DOI] [PubMed] [Google Scholar]
- Bornovalova MA, Cashman-Rolls A, O’Donnell JM, Ettinger K, Richards JB, de Wit H, Lejuez CW. Risk taking differences on a behavioral task as a function of potential reward/loss magnitude and individual differences in impulsivity and sensation seeking. Pharmacology, Biochemistry and Behavior. 2009;93:258–262. doi: 10.1016/j.pbb.2008.10.023. [DOI] [PubMed] [Google Scholar]
- Baschnagel JS, Hawk LW., Jr. The effects of nicotine on the attentional modification of the acoustic startle response in non-smokers. Psychopharmacology. 2008;198:93–101. doi: 10.1007/s00213-008-1094-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, Johnson SL, Joormann J. Serotonergic function, two-mode models of self-regulation, and vulnerability to depression: what depression has in common with impulsive aggression. Psychological Bulletin. 2008;134(6):912–943. doi: 10.1037/a0013740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davranche K, Audiffren M. Effects of a low dose of transdermal nicotine on information processing. Nicotine and Tobacco Research. 2002;4(3):275–285. doi: 10.1080/14622200210141635. [DOI] [PubMed] [Google Scholar]
- Delaney HD, Maxwell SE. On using analysis of covariance in repeated measures designs. Multivariate Behavioral Research. 1981;16(1):105–123. doi: 10.1207/s15327906mbr1601_6. [DOI] [PubMed] [Google Scholar]
- Depatie L, O’Driscoll GA, Holahan AL, Atkinson V, Thavundayil JX, Kin NN, Lal S. Nicotine and behavioral markers of risk for schizophrenia: a double-blind, placebo-controlled, cross-over study. Neuropsychopharmacology. 2002;27(6):1056–70. doi: 10.1016/S0893-133X(02)00372-X. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. SCL-90-R. Administration, scoring and procedures manual. Clinical Psychometric Research; Baltimore, MD: 1983. [Google Scholar]
- de Wit H, Griffiths RR. Testing the abuse liability of anxiolytic and hypnotic drugs in humans. Drug and Alcohol Dependence. 1991;28(1):83–111. doi: 10.1016/0376-8716(91)90054-3. [DOI] [PubMed] [Google Scholar]
- de Wit H, Enggasser JL, Richards JB. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology. 2002;27:813–825. doi: 10.1016/S0893-133X(02)00343-3. [DOI] [PubMed] [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience. 2002;14(3):340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Fant RV, Henningfield JE, Shiffman S, Strahs KR, Reitberg DP. A Pharmacokinetic crossover study to compare the absorption characteristics of three transdermal nicotine patches. Pharmacology, Biochemistry and Behavior. 2000;67:479–482. doi: 10.1016/s0091-3057(00)00399-3. [DOI] [PubMed] [Google Scholar]
- Foulds J, Stapleton J, Swettenham J, Bell N, McSorley K, Russell MAH. Cognitive performance effects of subcutaneous nicotine in smokers and never-smokers. Psychopharmacology. 1996;127:31–38. doi: 10.1007/BF02805972. [DOI] [PubMed] [Google Scholar]
- Gehricke JG, Hong N, Whalen CK, Steinhoff K, Wigal TL. Effects of transdermal nicotine on symptoms, moods, and cardiovascular activity in the everyday lives of smokers and nonsmokers with attention-deficit/hyperactivity disorder. Psychology of Addictive Behaviors. 2009;23(4):644–655. doi: 10.1037/a0017441. [DOI] [PubMed] [Google Scholar]
- Hahn B, Ross TJ, Wolkenberg FA, Shakleya DM, Huestis MA, Stein EA. Performance effects of nicotine during selective attention, divided attention, and simple stimulus detection: an fMRI study. Cerebral Cortex. 2009;19:1990–2000. doi: 10.1093/cercor/bhn226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heishman SJ, Taylor RC, Henningfield JE. Nicotine and smoking: a review of the effects on human performance. Experimental and Clinical Psychopharmacology. 1994;2:345–394. [Google Scholar]
- Heishman SJ, Kleykamp BA, Singleton EG. Meta-analysis of the acute effects of nicotine and smoking on human performance. Psychopharmacology (Berl) 2010;210(4):453–469. doi: 10.1007/s00213-010-1848-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinshaw SP. Impulsivity, emotion regulation, and developmental psychopathology: specificity versus generality of linkages. Annals New York Academy of Sciences. 2003;1008:149–159. doi: 10.1196/annals.1301.016. [DOI] [PubMed] [Google Scholar]
- Klabunde RE. Cardiovascular Physiology Concepts. Lippincott Williams and Wilkins; Philadelphia PA: 2005. [Google Scholar]
- Lejuez CW, Read JP, Kahler CW, Richards JB, Ramsey SE, Stuart GL, Strong DR, Brown RA. Evaluation of a behavioral measure of risk taking: the Balloon Analogue Risk Task (BART) Journal of Experimental Psychology: Applied. 2002;8(2):75–84. doi: 10.1037//1076-898x.8.2.75. [DOI] [PubMed] [Google Scholar]
- Levin ED, Conners CK, Sparrow E, Hinton SC, Erhardt D, Meck WH, Rose JE, March J. Nicotine effects on adults with Attention-Deficit/Hyperactivity Disorder. Psychopharmacology (Berl.) 1996b;123(1):55–63. doi: 10.1007/BF02246281. [DOI] [PubMed] [Google Scholar]
- Levin ED, Wilson W, Rose J, McEvoy J. Nicotine-haloperidol interactions and cognitive performance in schizophrenics. Neuropsychopharmocology. 1996a;15:429–436. doi: 10.1016/S0893-133X(96)00018-8. [DOI] [PubMed] [Google Scholar]
- Levin ED, Conners CK, Silva D, Hinton SC, Meck WH, March J, Rose JE. Transdermal nicotine effects on attention. Psychopharmacology. 1998;140:135–141. doi: 10.1007/s002130050750. [DOI] [PubMed] [Google Scholar]
- Levin ED, McClernon FJ, Rezvani AH. Nicotinic effects on cognitive function: behavioral characterization, pharmacological specification, and anatomic localization. Psychopharmacology. 2006;184:523–539. doi: 10.1007/s00213-005-0164-7. [DOI] [PubMed] [Google Scholar]
- Logan GD, Cowan WB. On the ability to inhibit thought and action: a theory of an act of control. Psychological Review. 1984;91(3):295–327. doi: 10.1037/a0035230. [DOI] [PubMed] [Google Scholar]
- McEwen A, West R, McRobbie H. Motives for smoking and their correlates in clients attending stop smoking treatment service. Nicotine and Tobacco Research. 2008;10(5):843–850. doi: 10.1080/14622200802027248. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Droppleman LF. Manual: Profile of mood states (POMS) Educational and Industrial Testing Service; San Diego: 1971. [Google Scholar]
- Newhouse PA, Potter A, Singh A. Effects of nicotinic stimulation on cognitive performance. Current Opinions in Pharmacology. 2004;4:36–46. doi: 10.1016/j.coph.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Nigg JT. On inhibition/disinhibition in developmental psychopathology: views from cognitive and personality psychology and a working inhibition taxonomy. Psychological Bulletin. 2000;126(2):220–246. doi: 10.1037/0033-2909.126.2.220. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Addy NA, Mineur YS, Brunzell DH. It is not “either/or”: activation and desensitization of nicotinic acetylcholine receptors both contribute to behaviors related to nicotine addiction and mood. Progress in Neurobiology. 2008;84(4):329–42. doi: 10.1016/j.pneurobio.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biological Psychiatry. 2005;57(4):319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poltavski DV, Petros T. Effects of transdermal nicotine on attention in adult non-smokers with and without attentional deficits. Physiology and Behavior. 2006;87:614–624. doi: 10.1016/j.physbeh.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA. Effects of acute nicotine administration on behavioral inhibition in adolescents with attention-deficit/hyperactivity disorder. Psychopharmacology. 2004;176:182–194. doi: 10.1007/s00213-004-1874-y. [DOI] [PubMed] [Google Scholar]
- Potter AS, Newhouse PA. Acute nicotine improves cognitive deficits in young adults with attention-deficit/hyperactivity disorder. Pharmacology, Biochemistry, and Behavior. 2008;88(4):407–417. doi: 10.1016/j.pbb.2007.09.014. [DOI] [PubMed] [Google Scholar]
- Provost SC, Woodward R. Effects of nicotine gum on repeated administration of the Stroop test. Psychopharmacology. 1991;104:536–540. doi: 10.1007/BF02245662. [DOI] [PubMed] [Google Scholar]
- Sacco KA, Bannon KL, George TP. Nicotine receptor mechanisms and cognition in normal states and neuropsychiatric disorders. Journal of Psychopharmacology. 2004;18(4):457–474. doi: 10.1177/0269881104047273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzer ML. The Michigan Alcoholism Screening Test. American Journal of Psychiatry. 1971;127:89–94. doi: 10.1176/ajp.127.12.1653. [DOI] [PubMed] [Google Scholar]
- Stroop J. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Tanskanen A, Viinamaki H, Koivumaa-Honkanen H-T, Jaaskelainen J, Lehtonen J, Heli-Tuulie KH. Smoking among psychiatric patients. European Journal of Psychiatry. 1997;11(3):179–188. [Google Scholar]
- Wesnes K, Warburton DM. Smoking, nicotine, and human performance. Pharmacology and Therapeutics. 1983;21(4):189–208. doi: 10.1016/0163-7258(83)90072-4. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Mueller U. Executive function in typical and atypical development. In: Goswami U, editor. Handbook of Childhood Cognitive Development. Blackwell; 2002. pp. 445–469. [Google Scholar]