Abstract
Objectives/Hypothesis
To determine speech, eating, aesthetics, social disruption, and overall quality-of-life outcomes over a year period in patients who underwent transoral robotic surgery as part of carcinoma of unknown primary diagnosis and treatment.
Study Design
Observational prospective study.
Methods
Twenty-two patients who underwent transoral robotic surgery for the management of carcinoma of unknown primary were included. Patients prospectively completed the Head and Neck Cancer Inventory during a preoperative visit, and at 3-week, 3-month, 6-month, and 12-month postoperative visits. Patients’ demographic, pathological, and follow-up information were also collected.
Results
The mean follow-up time was 19.8 months. There were overall declines in all quality of life scores during treatment period, which was followed by a continuous recovery. The scores immediately after transoral robotic surgery (3 weeks) were significantly higher than the scores after conclusion of adjuvant therapy (3 months) in multiple domains (P <.05) and the 6-month scores in speech (P = .02) and eating (P = .008) domains. All scores, except for eating (P = .01) returned to pre-treatment levels at 1 year. Patients with detected primaries displayed similar quality-of-life scores compared to patients with occult primaries. Human papillomavirus status and type of adjuvant treatment had no significant impact on quality of life.
Conclusions
Transoral robotic surgery is a promising, minimally invasive procedure for the surgical management of carcinoma of unknown primary. Patients maintain high functional and quality-of-life status at 1 year after surgery.
Keywords: Transoral robotic surgery, carcinoma of unknown primary, quality of life, transoral robotic surgery, carcinoma of unknown primary, neck metastasis
INTRODUCTION
Carcinoma of unknown primary (CUP) constitutes approximately 2% to 4% percent of the squamous cell carcinoma (SCC) of the head and neck.1,2 Currently, the recommended approach to CUP involves identification of primary tumor site and management of cancer according to the National Comprehensive Cancer Network (NCCN) guidelines for the diagnosed primary.2–5 With advanced diagnostic options, positron-emission tomography/computerized tomography (PET/CT), panendoscopy, and directed biopsies, only 59.6% of primary tumors are identified.6 Most common primary sites identified in the workup of CUP are palatine tonsils and base of tongue.5 The remaining true CUP cases are treated with either wide-field primary irradiation or chemoradiation therapy with or without neck dissection. Even with recent advances in radiation technology, there is significant morbidity associated with wide-field primary irradiation.7–9
Transoral robotic surgery (TORS) has been advocated as an effective alternative tool for both diagnosis and treatment of CUP.10,11 Compared to traditional methods, the daVinci robotic system, with its three-dimensional magnified view and wristed instruments, allows a more detailed oropharyngeal examination and excision of the suspicious sites resulting in increased detection of primary tumor sites.10,12 According to the limited published series, TORS was found to have 90% primary tumor identification rate.11 Although TORS has been shown to have significant potential in diagnosis and treatment of CUP, long-term functional outcomes have not been described yet in this selected population. To our knowledge, this is the first study that reports the long-term quality-of-life (QOL) outcomes and functional status of patients with unknown primary tumors treated with TORS.
MATERIALS AND METHODS
Ethical Considerations
Institutional review board approval was obtained from The Ohio State University Office of Responsible Research Practices.
Patients and Setting
This prospective study was conducted at Arthur G. James Cancer Hospital, The Ohio State University, a tertiary care referral center. Between June 2009 and December 2012, a total of 22 patients were enrolled in the study at their first head and neck cancer clinic visits.
Patient selection criteria included 1) documented SCC metastasis to cervical lymph nodes; 2) unidentified primary tumor site despite comprehensive history and physical exam, fiberoptic laryngopharyngeal examination, and radiological imaging; 3) eligibility for TORS; and 4) consent for the study.
Exclusion criteria were 1) detected primary tumor prior to TORS; 2) distant metastasis; 3) lymphoma, melanoma, or adenocarcinoma; and 4) previous history of SCC of the head and neck.
Treatment Details
All patients were evaluated with PET/CT prior to surgery. Under operative conditions, panendoscopy of the upper aerodigestive tract and biopsies from suspicious sites were performed. Biopsy specimens were sent for frozen section analysis. The daVinci Si Surgical System (Intuitive Surgical Inc., Sunnyvale, CA) was then used for a detailed laryngopharyngeal examination and surgical resection of the occult primary. Transoral robotic resection was planned according to a positive frozen section result or a highly suspicious lesion identified in PET/CT, panendoscopy, or robotic exam. Bilateral transoral robotic lingual and palatine tonsillectomies (if not previous performed) were done in all patients. Multiple directed biopsies from nasopharynx and pyriform sinuses were performed only if the primary site was not pathologically confirmed following TORS. After completion of the robotic part, all patients (except one patient with a history of excisional lymph node biopsy) underwent neck dissection. Type and level of neck dissection was determined based on clinical, radiological, and intraoperative findings. Panendoscopy with biopsies, robotic procedures, and neck dissection were performed under general anesthesia in the same surgical session. Gastrostomy tubes (G-tubes) were placed during radiation therapy only if necessary.
Data Collection
Clinical data collected included patient age, gender, smoking history, extent of neck dissection, intraoperative complications, type of adjuvant therapy, G-tube dependence, and follow-up time. Tumor stage, TNM classification, site of the lesion, tissue diagnosis, high-risk types of human papillomavirus (HPV) and p16INKa (p16) status, surgical margin status, extracapsular spread, and tumor size were collected upon completion of pathological review of the specimens. Chromogenic in situ hybridization technique was used for identification of HPV types 16 and 18. p16 protein expression was determined based on immunohistochemical staining of cancer cells. Nuclear or cytoplasmic staining of at least 70% of tumor cells was required to conclude p16 positivity.
QOL Outcomes
Long-term QOL scores of patients were collected preoperatively (baseline), at 3 weeks, 3 months, 6 months, and 12 months postoperatively via the Head and Neck Cancer Inventory (HNCI). The HNCI is a previously validated 30-item survey that measures patient reported QOL status in four domains: speech, eating, aesthetics, and social disruption.13,14 The patient’s functional (ability to perform task) and attitudinal (satisfaction with his or her performance) scores are measured separately for each domain. The last item in the questionnaire provides the overall QOL score. Each item in the questionnaire is graded on a five-point ordinal scale, from 1 (no/not at all/never/very poor) to 5 (extremely/always/excellent). For ease of interpretation, scores were converted to a 0 to 100 scale, and the mean scores for each domain were grouped as high (70–100), intermediate (31–69), and low (0–30) scores.
Statistical Analysis
Statistical analysis was achieved using SPSS 16.0 software (SPSS Inc., Chicago, IL). Descriptive statistics were used to summarize the data. QOL scores at different time intervals were analyzed by paired sample t test and Wilcoxon signed rank test. Mann-Whitney U test and independent samples t test were used to compare the mean QOL domain scores among categorical variables. A P value <.05 was considered statistically significant.
RESULTS
In this clinical trial, 22 patients underwent TORS for CUP between 2009 and 2012. There were two female (9%) and 20 male (91%) patients, with a mean age of 57.2 years (range, 36.7–71.5 years). Fifteen patients (68%) had smoking history (mean, 22.5 pack/years; range, 5–105 pack/years). All patients were alive throughout the study period, and the average follow-up time was 19.8 months (range, 6.2–47.5 months).
Together with traditional diagnostic approach and TORS, SCC was detected in palatine tonsil in 12 patients (54.5%) and base of tongue in four patients (18.2%). However, no primary tumor was identified in six patients (27.3%). The mean tumor size was 1.7 ± 1.3 cm. High-risk types of HPV positivity and p16 protein expression were identified in 17 (80.9%) and 20 patients (95.2%), respectively. These tests were performed on cancer cells obtained either from tumor specimens or neck metastasis. Multifocal disease was determined in four patients (18.2%): ipsilateral palatine tonsil and base of tongue in one, bilateral tonsils in two, and bilateral tongue base in one patient. Table I summarizes the clinical and pathological information of patients.
TABLE I.
Clinical and Pathological Information.
| Variable | No. of Patients (%) |
|---|---|
| Gender, n = 22 | |
| Female | 2 (9) |
| Male | 20 (91) |
| T stage, n = 22 | |
| Tx | 6 (27.3) |
| T1 | 10 (45.5) |
| T2 | 5 (22.7) |
| T3 | 1 (4.5) |
| T4 | 0 (0) |
| N stage, n = 22 | |
| N1 | 3 (13.6) |
| N2 | 14 (63.7) |
| N3 | 5 (22.7) |
| Site of origin, n = 22 | |
| Tonsil | 12 (54.5) |
| Base of tongue | 4 (18.2) |
| Occult primary | 6 (27.3) |
| HPV status, n = 21 | |
| Positive | 17 (80.9) |
| Negative | 4 (19.1) |
| p16 status, n = 21 | |
| Positive | 20 (95.2) |
| Negative | 1 (4.8) |
| ECS, n = 22 | |
| Positive | 9 (40.9) |
| Negative | 13 (59.1) |
ECS = extracapsular spread; HPV = human papillomavirus; p16 = p16Ink4A.
Complete tumor removal with negative margins was achieved in 12 of 16 patients (75%). There were no perioperative complications. The average panendoscopy, TORS setup, and operative times were 7.3, 21.6, and 28 minutes, respectively. All patients were able to sustain regular oral diet in the immediate postoperative period. However, 10 patients (45.5%) needed temporary G-tubes during radiotherapy. Among those, one patient continued to be G-tube dependent. None of the patients required tracheostomy tube placement. During follow-up, all patients were clinically free of disease. Treatment and follow-up details are summarized in Table II.
TABLE II.
Treatment Details.
| Variable | No. of Patients (%) |
|---|---|
| Overall stage, n = 22 | |
| III | 2 (9.1) |
| IV | 20 (90.9) |
| Surgical margin, n = 16 | |
| Positive | 4 (25) |
| Negative | 12 (75) |
| Neck dissection, n = 21 | |
| Unilateral | 18 (85.7) |
| Bilateral | 3 (14.3) |
| Adjuvant therapy, n = 22 | |
| Radiotherapy | 10 (45.5) |
| Chemoradiation therapy | 12 (54.5) |
| G-tube use, n = 22 | |
| Yes | 10 (45.5) |
| No | 12 (54.5) |
| Permanent G-tube, n = 22 | 1 (4.5) |
G-tube = gastrostomy tube.
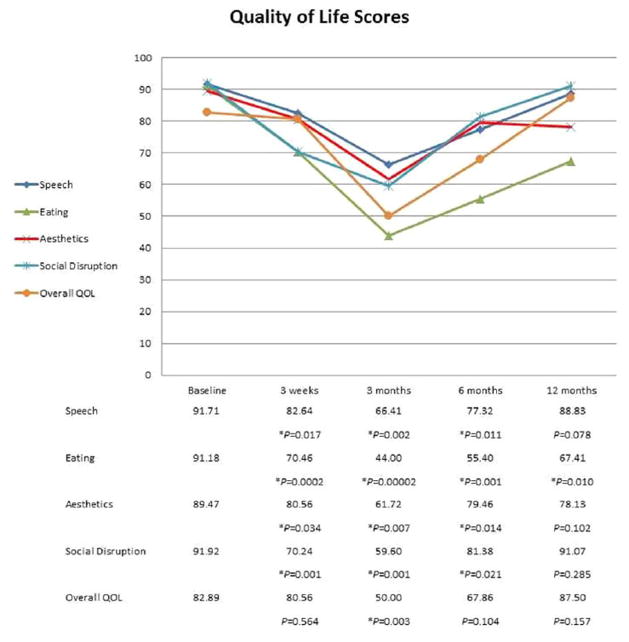
Nineteen patients (86%) completed the HNCI prior to surgery (baseline), 18 (82%) at 3 weeks, 16 (73%) at 3 months, 14 (64%) at 6 months, and eight (36%) at 12 months after TORS. Although high levels of speech, eating, aesthetics, social disruption, and overall QOL scores were achieved after TORS, there was a gradual decrease in all scores during treatment. The lowest scores were identified at 3 months (just after the completion of adjuvant radiotherapy), which was followed by a continuous recovery at 6 and 12 months in all domains (Fig. 1). The declines in speech, eating, aesthetics, and social disruption domain scores at 3 weeks, 3 months, and 6 months were significant compared to baseline (P <.05). After 12 months, speech, aesthetics, and social disruption scores returned to baseline levels. However, eating scores were still significantly lower than the preoperative levels at 1-year follow-up (P = .010). A similar pattern was observed in overall QOL scores.
Fig. 1.
The mean quality of life scores over a 1-year period. *Statistically significant compared to matching baseline score (P <.05). QOL = quality of life. [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
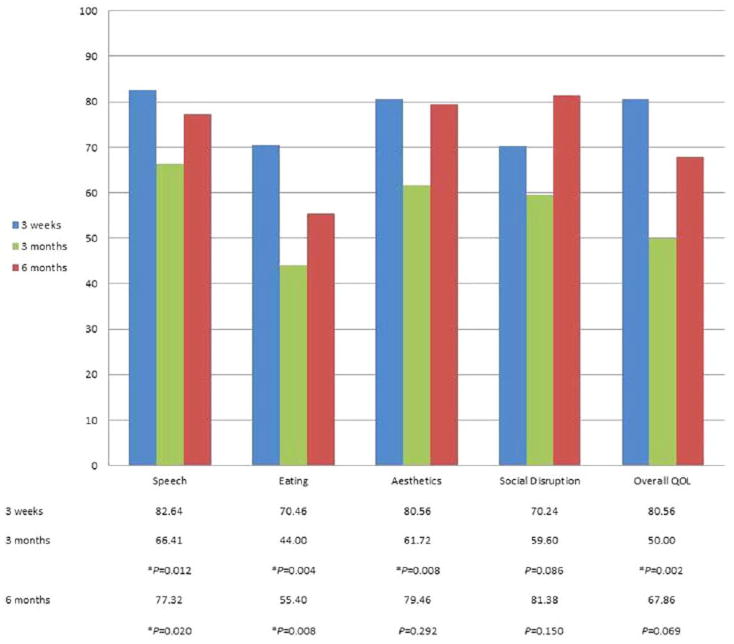
Statistical analysis of QOL outcomes at 3 months (end of adjuvant therapy) in comparison to 3 weeks (after TORS) showed significant declines in speech, eating, aesthetics domains, and overall QOL item (P <.05). Speech (P = .02) and eating (P = .008) scores at 6 months were also significantly lower than the matching scores immediately after TORS (Fig. 2).
Fig. 2.
Comparison of 3-week quality of life scores with 3 and 6-month scores. *Statistically significant compared to matching 3-week score (P <.05). QOL = quality of life. [Color figure can be viewed in the online issue, which is available at www.laryngoscope.com.]
Distribution of QOL scores according to primary tumor status (detected or occult), type of adjuvant treatment, and HPV status are given in Table III. Patients with detected primaries displayed similar QOL scores compared to patients with occult primaries; except baseline eating (P= .046), 3-month aesthetics (P = .008), and baseline social disruption scores (P= .036) were significantly higher in the former. There were no statistically significant differences in QOL scores of patients receiving adjuvant radiotherapy versus adjuvant chemoradiotherapy. HPV status did not have any significant impact on QOL outcomes.
TABLE III.
Association Between Quality-of-Life Scores and Clinical Variables.
| Detected Primary | Occult Primary | P Value | TORS + RT | TORS + CRT | P Value | HPV+ | HPV− | P Value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Speech | Baseline | 94 | 86 | .831 | 88 | 95 | .497 | 92 | 89 | .079 |
| 3 weeks | 85 | 76 | .314 | 80 | 85 | .640 | 81 | 92 | .311 | |
| 3 months | 72 | 41 | .060 | 65 | 69 | .784 | 64 | 85 | .226 | |
| 6 months | 79 | 73 | .638 | 77 | 78 | .921 | 75 | 89 | .290 | |
| 12 months | 88 | 93 | .675 | 86 | 91 | .631 | 88 | — | * | |
| Eating | Baseline | 95 | 82 | .046† | 87 | 95 | .178 | 91 | 93 | .897 |
| 3 weeks | 75 | 58 | .112 | 71 | 70 | .921 | 73 | 70 | .767 | |
| 3 months | 47 | 32 | .244 | 43 | 45 | .863 | 44 | 51 | .628 | |
| 6 months | 59 | 41 | .170 | 49 | 61 | .259 | 53 | 72 | .469 | |
| 12 months | 67 | 69 | .930 | 63 | 72 | .607 | 67 | — | * | |
| Aesthetics | Baseline | 97 | 73 | .072 | 81 | 98 | .113 | 88 | 94 | .959 |
| 3 weeks | 89 | 58 | .075 | 72 | 88 | .573 | 79 | 91 | .474 | |
| 3 months | 72 | 17 | .008† | 64 | 59 | .790 | 64 | 67 | .896 | |
| 6 months | 83 | 67 | .252 | 84 | 75 | .452 | 79 | 83 | .766 | |
| 12 months | 81 | 69 | .527 | 81 | 75 | .718 | 78 | — | * | |
| Social disruption | Baseline | 97 | 81 | .036† | 92 | 92 | .278 | 92 | 90 | .327 |
| 3 weeks | 73 | 65 | .458 | 76 | 65 | .204 | 72 | 67 | .646 | |
| 3 months | 64 | 39 | .209 | 65 | 53 | .467 | 67 | 40 | .184 | |
| 6 months | 82 | 79 | .775 | 84 | 79 | .209 | 84 | 81 | .807 | |
| 12 months | 90 | 95 | .720 | 98 | 84 | .181 | 91 | — | * | |
| Overall QOL | Baseline | 85 | 79 | .579 | 78 | 88 | .315 | 94 | 75 | .442 |
| 3 weeks | 81 | 80 | .945 | 78 | 83 | .662 | 79 | 88 | .484 | |
| 3 months | 52 | 42 | .614 | 50 | 50 | 1.0 | 48 | 58 | .625 | |
| 6 months | 66 | 75 | .677 | 68 | 68 | 1.0 | 63 | 83 | .358 | |
| 12 months | 92 | 75 | .315 | 94 | 81 | .390 | 88 | — | * |
HPV+ means positive for high-risk types of human papillomavirus; HPV− means negative for high-risk types of human papillomavirus.
No statistics were computed because of absent data.
Statistically significant (P <.05).
CRT = chemoradiation therapy; HPV = human papillomavirus; QOL = quality of life; RT = radiotherapy; TORS = transoral robotic surgery.
Speech, eating, aesthetics, and social disruption domain scores with overall functional and attitudinal scores at 3 months were analyzed to identify any significant difference among clinical outcome variables (Table IV). Females and younger patients tended to have higher scores in most domains (P >.05). Smokers avoided social contact significantly (P = .045). No significant difference was identified between tonsil and base of tongue cancers regarding QOL outcomes. However, patients with occult primary lesions had lower aesthetic (P = .008) and overall attitude scores (P = .019). The only variable with a significant negative impact on eating function was G-tube dependence (P = .037). It was also correlated with lower overall function scores (P = .020).
TABLE IV.
Three-Month Quality-of-Life Scores Comparing Clinical Outcome Variables.
| Variable | No. | Speech | Eating | Aesthetics | Social Disruption | Overall Function | Overall Attitude |
|---|---|---|---|---|---|---|---|
| Gender | |||||||
| Female | 2 | 91 | 40 | 100 | 84 | 58 | 83 |
| Male | 14 | 63 | 44 | 56 | 56 | 51 | 58 |
| P value | .162 | .844 | .101 | .238 | .647 | .134 | |
| Age, yr | |||||||
| 55> | 7 | 60 | 41 | 54 | 48 | 49 | 53 |
| 55< | 9 | 71 | 46 | 68 | 68 | 54 | 68 |
| P value | .439 | .662 | .433 | .299 | .584 | .172 | |
| Smoking | |||||||
| Smoker | 9 | 62 | 41 | 58 | 46 | 47 | 55 |
| Nonsmoker | 7 | 73 | 49 | 66 | 77 | 58 | 70 |
| P value | .435 | .607 | .678 | .045* | .277 | .179 | |
| Site of origin | |||||||
| Tonsil/BOT | 13 | 72 | 47 | 72 | 64 | 56 | 67 |
| Unknown | 3 | 41 | 32 | 17 | 39 | 36 | 36 |
| P value | .060 | .244 | .008* | .209 | .127 | .019* | |
| G-tube | |||||||
| G-tube | 7 | 53 | 33 | 55 | 47 | 39 | 51 |
| No G-tube | 9 | 77 | 54 | 67 | 69 | 62 | 69 |
| P value | .142 | .037* | .542 | .210 | .020* | .093 | |
Statistically significant (P <.05).
BOT = base of tongue; G-tube = gastrostomy tube.
DISCUSSION
Current standard of diagnosis and treatment of CUP involves PET/CT, panendoscopy with biopsies, unilateral or bilateral tonsillectomy, with or without neck dissection, and wide-field radiation therapy.1–8 Despite immense advances in radiation technology, the morbidity associated with radiation is significant.1,7–9 Studies shows that morbidity and mortality outcomes are significantly improved when the occult primary is detected and treatment is shifted toward the detected primary.2–5 Accordingly, there is significant interest in evolving technologies that result in better detection rates and decreased morbidity and mortality.
Synopsis of Findings
Although high levels of speech, eating, aesthetics, social disruption, and overall QOL scores were achieved after TORS, this study demonstrated an initial gradual decline in all domains during the completion of the entire treatment regimen when compared to baseline scores. The lowest scores were detected at 3 months, followed by progressive improvement toward the end of 1 year. All QOL scores returned to preoperative levels at 12 months except eating scores, which were significantly low (P= .010).
The postoperative visit at 3 weeks coincided with recovery immediately after surgery but prior to starting radiation, and the 3-month visit represented the period immediately after completion of adjuvant radiation therapy. Comparative analysis of the 3-week to 3-month postoperative visit showed a significant worsening in scores after adjuvant radiation.
Decline in eating domain scores were more significant when compared to baseline (P = .0002 at 3 weeks, P = .00002 at 3 months, P = .001 at 6 months, and P = .010 at 1 year). It is postulated that a large area of oropharyngeal resections with TORS contributes to dysphagia, which results in lower eating function and attitude scores at 3 weeks.15 However, in the current study, further decline and relatively slow recovery in eating scores after 3 months is attributed to the effects of oropharyngeal and neck irradiation. Temporary G-tube use during adjuvant treatment was also a significant contributor of lower eating scores (P = .037), because these patients received the lowest possible scores regardless of their oral intake.
Comparison With Other Studies
Hurtuk et al. confirmed a similar pattern in QOL outcomes of 64 patients who underwent TORS for a variety of benign or malignant head and neck tumors. There was an overall deterioration from baseline in speech, eating, aesthetics, social disruption domains, and overall QOL item after surgery. At the end of 1 year, all domain scores returned to high levels (70–100) except for eating, which was still in intermediate score range (31–69).15 Parallel results were identified by Dziegielewski et al. in 81 patients with oropharynx cancers after TORS resection. In addition to eating (function and attitude) scores, speech function scores at 1 year were also found to be significantly lower than baseline.16 In our study, 12-month speech scores remained at high levels (mean score, 88.83 ± 1.28), and were not significantly different than baseline (P = .078).
Prior studies demonstrated that degree of dysphagia is significantly correlated with the use of radio-sensitizing chemotherapy and the radiation dose received by pharyngeal constrictor muscles.17–20 According to the study of Leonhardt et al., adjuvant treatment had significant negative influence on swallowing function; nevertheless, TORS alone did not result in any significant decline in eating function after 6 months.20 Genden et al. revealed that TORS resulted in significantly superior eating and diet scores after 2 weeks and a faster recovery in swallowing function compared to primary chemoradiation.21 In our study, all patients received either adjuvant intensity-modulated radiotherapy alone or concurrent chemoradiation concordant with the NCCN guidelines. In our patients, given the presence of adverse features, a median dose of 65.4 Gy (range, 60–70.2 Gy) radiation was delivered to the oropharynx and neck, which is higher than the recommended 44- to 60-Gy radiation dose for intermediate- or low-risk oropharyngeal cancers.22 This probably deteriorated dysphagia and may explain the increased G-tube use and decreased eating scores during radiation. In high-risk patients, the recommended approach is to clear the positive margins surgically to avoid chemotherapy and reduce radiation dose. In four patients with positive margins, re-resection of the deep margin was not performed because of the inability to precisely localize the positive margin in the healed surgical site and concerns over the additional morbidity of a second resection. Therefore, adjuvant chemoradiation was given. Nevertheless, in the current study, type of adjuvant treatment did not result any significant differences in QOL scores.
Strengths and Limitations of the Study
To our knowledge, this is the first study that analyzes the long-term QOL and functional outcomes of patients who underwent TORS for the management of CUP. According to the study, TORS plus adjuvant therapy resulted in promising long-term outcomes in the treatment of CUP. Although the patient population is small, results are concordant with similar oropharyngeal studies.15–17,20 This study presents preliminary data for future randomized, controlled, prospective clinical trials comparing the clinical outcomes of TORS plus radiation versus primary chemoradiation therapy in CUP cases.
In the current study, HPV status was not found to have any significant effect on QOL outcomes, and there is a 15% discrepancy in HPV/p16 results. These are probable consequences of small sample size. Large prospective controlled studies are required to explore the true association between HPV/p16 status and QOL outcomes.
CONCLUSION
TORS is a promising minimally invasive procedure for the surgical management of carcinoma of unknown primary. Patients maintain long-term and highly functional QOL status.
Acknowledgments
Clinical robotics research grant from Intuitive Surgical, Inc. supported a research assistant.
Footnotes
Level of Evidence: 4
Accepted for presentation at the 5th World Congress, IFNOS and Annual AHNS Meeting, New York, New York, U.S.A., July 26-30, 2014.
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
BIBLIOGRAPHY
- 1.Nieder C, Ang KK. Cervical lymph node metastases from occult squamous cell carcinoma. Curr Treat Options Oncol. 2002;3:33–40. doi: 10.1007/s11864-002-0039-7. [DOI] [PubMed] [Google Scholar]
- 2.Karni RJ, Rich JT, Sinha P, Haughey BH. Transoral laser microsurgery: a new approach for unknown primaries of the head and neck. Laryngoscope. 2011;121:1194–1201. doi: 10.1002/lary.21743. [DOI] [PubMed] [Google Scholar]
- 3.Strojan P, Ferlito A, Medina JE, et al. Contemporary management of lymph node metastases from an unknown primary to the neck: I. A review of diagnostic approaches. Head Neck. 2013;35:123–132. doi: 10.1002/hed.21898. [DOI] [PubMed] [Google Scholar]
- 4.Strojan P, Ferlito A, Langendijk JA, et al. Contemporary management of lymph node metastases from an unknown primary to the neck: II. a review of therapeutic options. Head Neck. 2013;35:286–293. doi: 10.1002/hed.21899. [DOI] [PubMed] [Google Scholar]
- 5.Cianchetti M, Mancuso AA, Amdur RJ, et al. Diagnostic evaluation of squamous cell carcinoma metastatic to cervical lymph nodes from an unknown head and neck primary site. Laryngoscope. 2009;119:2348–2354. doi: 10.1002/lary.20638. [DOI] [PubMed] [Google Scholar]
- 6.Waltonen JD, Ozer E, Hall NC, Schuller DE, Agrawal A. Metastatic carcinoma of the neck of unknown primary origin: evolution and efficacy of the modern workup. Arch Otolaryngol Head Neck Surg. 2009;135:1024–1029. doi: 10.1001/archoto.2009.145. [DOI] [PubMed] [Google Scholar]
- 7.Balaker AE, Abemayor E, Elashoff D, St John MA. Cancer of unknown primary: does treatment modality make a difference? Laryngoscope. 2012;122:1279–1282. doi: 10.1002/lary.22424. [DOI] [PubMed] [Google Scholar]
- 8.Nieder C, Gregoire V, Ang KK. Cervical lymph node metastases from occult squamous cell carcinoma: cut down a tree to get an apple? Int J Radiat Oncol Biol Phys. 2001;50:727–733. doi: 10.1016/s0360-3016(01)01462-6. [DOI] [PubMed] [Google Scholar]
- 9.Chen AM, Li BQ, Farwell DG, Marsano J, Vijayakumar S, Purdy JA. Improved dosimetric and clinical outcomes with intensity-modulated radiotherapy for head-and-neck cancer of unknown primary origin. Int J Radiat Oncol Biol Phys. 2011;79:756–762. doi: 10.1016/j.ijrobp.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 10.Abuzeid WM, Bradford CR, Divi V. Transoral robotic biopsy of the tongue base: A novel paradigm in the evaluation of unknown primary tumors of the head and neck. Head Neck. 2013;35:E126–E130. doi: 10.1002/hed.21968. [DOI] [PubMed] [Google Scholar]
- 11.Mehta V, Johnson P, Tassler A, et al. A new paradigm for the diagnosis and management of unknown primary tumors of the head and neck: a role for transoral robotic surgery. Laryngoscope. 2013;123:146–151. doi: 10.1002/lary.23562. [DOI] [PubMed] [Google Scholar]
- 12.Hurtuk A, Agrawal A, Old M, Teknos TN, Ozer E. Outcomes of transoral robotic surgery: a preliminary clinical experience. Otolaryngol Head Neck Surg. 2011;145:248–253. doi: 10.1177/0194599811402172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Funk GF, Karnell LH, Christensen AJ, Moran PJ, Ricks J. Comprehensive head and neck oncology health status assessment. Head Neck. 2003;25:561–575. doi: 10.1002/hed.10245. [DOI] [PubMed] [Google Scholar]
- 14.Funk GF, Karnell LH, Smith RB, Christensen AJ. Clinical significance of health status assessment measures in head and neck cancer: what do quality-of-life scores mean? Arch Otolaryngol Head Neck Surg. 2004;130:825–829. doi: 10.1001/archotol.130.7.825. [DOI] [PubMed] [Google Scholar]
- 15.Hurtuk AM, Marcinow A, Agrawal A, Old M, Teknos TN, Ozer E. Quality-of-life outcomes in transoral robotic surgery. Otolaryngol Head Neck Surg. 2012;146:68–73. doi: 10.1177/0194599811421298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dziegielewski PT, Teknos TN, Durmus K, et al. Transoral robotic surgery for oropharyngeal cancer: long-term quality of life and functional outcomes. JAMA Otolaryngol Head Neck Surg. 2013;139:1099–1108. doi: 10.1001/jamaoto.2013.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sinclair CF, McColloch NL, Carroll WR, Rosenthal EL, Desmond RA, Magnuson JS. Patient-perceived and objective functional outcomes following transoral robotic surgery for early oropharyngeal carcinoma. Arch Otolaryngol Head Neck Surg. 2011;137:1112–1116. doi: 10.1001/archoto.2011.172. [DOI] [PubMed] [Google Scholar]
- 18.Levendag PC, Teguh DN, Voet P, et al. Dysphagia disorders in patients with cancer of the oropharynx are significantly affected by the radiation therapy dose to the superior and middle constrictor muscle: a dose-effect relationship. Radiother Oncol. 2007;85:64–73. doi: 10.1016/j.radonc.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Logemann JA, Pauloski BR, Rademaker AW, et al. Swallowing disorders in the first year after radiation and chemoradiation. Head Neck. 2008;30:148–158. doi: 10.1002/hed.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leonhardt FD, Quon H, Abrahao M, O’Malley BW, Jr, Weinstein GS. Transoral robotic surgery for oropharyngeal carcinoma and its impact on patient-reported quality of life and function. Head Neck. 2012;34:146–154. doi: 10.1002/hed.21688. [DOI] [PubMed] [Google Scholar]
- 21.Genden EM, Kotz T, Tong CC, et al. Transoral robotic resection and reconstruction for head and neck cancer. Laryngoscope. 2011;121:1668–1674. doi: 10.1002/lary.21845. [DOI] [PubMed] [Google Scholar]
- 22.National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology. [Accessed February 11, 2014];Head and neck cancers Version 2.2013. 2013 May 29; Available at: http://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf.