Abstract
Ionizing radiation (IR) triggers mitochondrial overproduction of H2O2 and accumulation of lipid hydroperoxides leading to the induction of apoptotic and necroptotic cell death pathways. Given the high catalytic efficiency of the seleno-enzyme glutathione peroxidase (Gpx) toward reduction of lipid hydroperoxides and H2O2, we tested the potential of mitochondria-targeted derivatives of ebselen to mitigate the deleterious effects of IR. We report that 2-[[2-[4-(3-oxo-1,2-benzoselenazol-2-yl)phenyl]acetyl]amino]ethyl-triphenyl-phosphonium chloride (MitoPeroxidase 2) was effective in reducing lipid hydroperoxides, preventing apoptotic cell death, and, when administered 24 h postirradiation, increased the survival of mice exposed to whole body γ-irradiation.
Keywords: Ebselen, mitochondria, H2O2, radiation, mitigators, apoptosis
Exposure of eukaryotic cells to ionizing radiation (IR) results in a burst of species with high energy (eaq–; H•, HO•, and O2–•) and indiscriminate reactivity with biomolecules, including DNA, on the millisecond time scale.1 Studies by Patt, Bacq, and others have established that aminothiols such as cysteine and cysteamine protect mice from short-lived radiolytic intermediates.2−4 Humans, however, do not tolerate the doses of cysteine and cysteamine, which would be required for analogous protection, and the early hope that these compounds might be useful as radiation protectors (RPs) has not been realized. These initial observations have been followed by a research program under the auspice of the Walter Reed Army Research Institute, which has led to the synthesis of 4400 aminothiols and their assessment as RPs.5 Structural variables in the synthesis of RPs were the length and branching of the carbon chain that linked NH2, SH, and OH functional groups. From this chemical library, only amifostine (H2O3P–S–(CH2)2–NH–(CH2)3–NH2) has found clinical applications.6
The immediate burst of radicals upon exposure to IR is followed by a dose-dependent and continuous mitochondrial overproduction of reactive oxygen species.7,8 These primary lethal effects of IR, realized predominantly in rapidly proliferating hematopoietic cells and gut epithelial cells, initiate secondary effects whereby activated inflammatory cells massively generate reactive oxygen species (ROSs) thus producing “friendly fire” and inducing new waves of oxidation reactions culminating in apoptotic and nonapototic cell death.9 Among these secondary oxidants, H2O2 and lipid hydroperoxides (LOOH) are most prominent in setting-up the “peroxide tone” and perpetuating enzymatic and nonenzymatic oxidations of critical biomolecules with signaling functions. In particular, peroxidase functions of mitochondrial intermembrane space hemoprotein, cytochrome c (cyt c), toward a mitochondria-specific phospholipid, cardiolipin (CL), has been associated with the accumulation of CL hydroperoxides required for the execution of mitochondrial apoptosis.10 In addition, a group of cytosolic nonheme iron proteins, lipoxygenases (LOX), catalyze oxygenation reactions yielding hydroperoxides of free polyunsaturated fatty acids thus inducing necroptotic and ferroptotic cell death signals.11 Hence, regulation of the levels of ROSs is important for the control of the major radiation-induced cell death pathways.12
The central regulator of LOOH is a seleno-enzyme, glutathione peroxidase 4 (GPx4), whose deficiency leads to cell death.12 GPx has been shown to impede mitochondrial apoptosis via clearance of hydroperoxides of CL,13,14 while overexpression of mitochondrial catalase has been found to increase radioresistance in vitro and in vivo.15
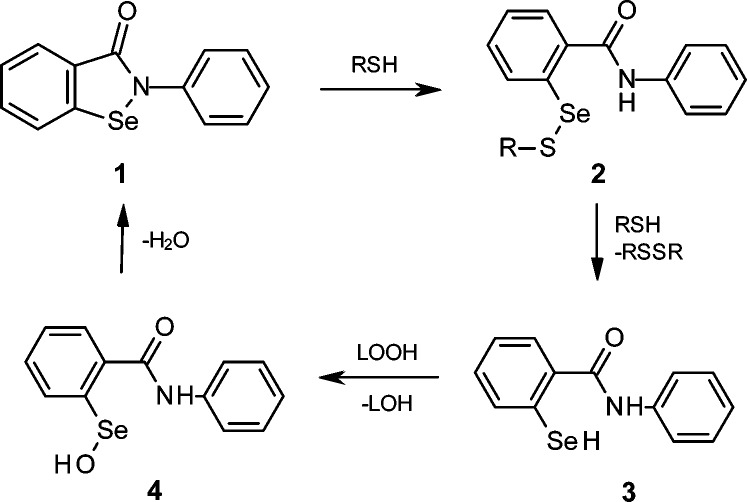
Recently, Tak and Park have reported that 2-phenyl-1,2-benzoselenazol-3-one (ebselen; Scheme 1, 1), when administered for 14 days prior to radiation, provides substantial protection against killing and oxidative damage to mice exposed to whole-body irradiation (WBI).16 Ebselen is a multifunctional drug that accumulates in the endoplasmic reticulum (ER),17 reacts with cellular thiols, and mimics the activity of glutathione peroxidase (GPx) by clearing H2O2 and LOOH (Scheme 1).18 Following recent clinical trials for the prevention and treatment of cardiovascular diseases, arthritis, stroke, and atherosclerosis,18 ebselen has been included in the National Institutes of Health Clinical Collection,19 a chemical library of bioavailable drugs considered clinically safe.
Scheme 1. Ebselen Exhibits GPx-Like Activity.
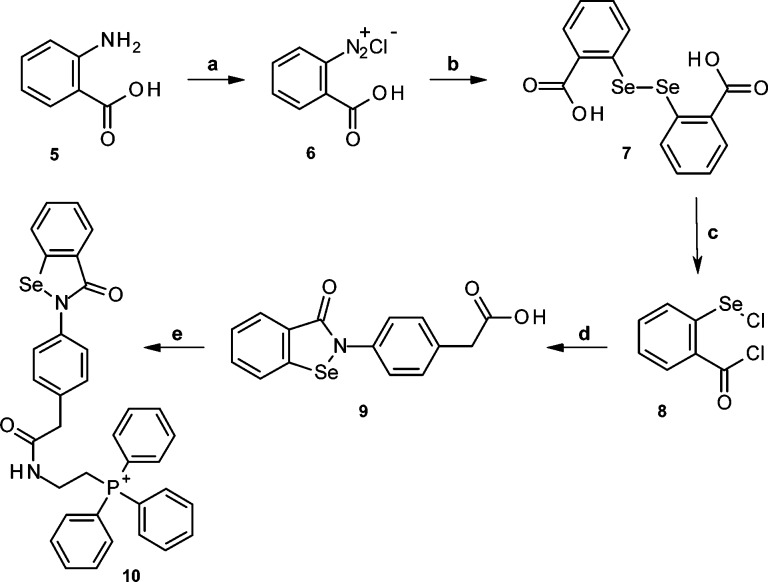
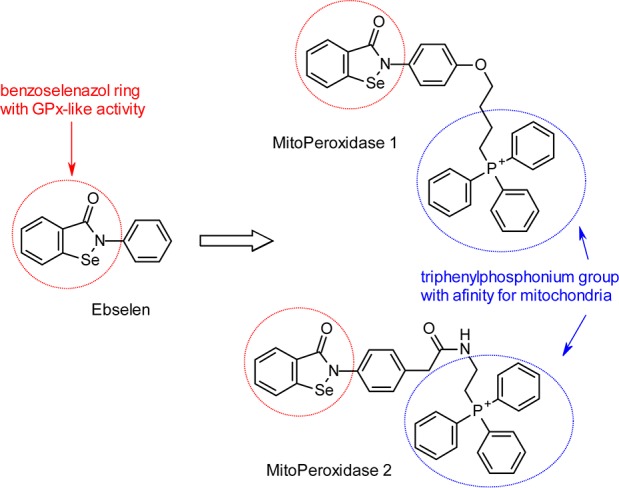
We have assessed the potential of the derivatives of ebselen 4-[4-(3-oxo-1,2-benzoselenazol-2-yl)phenoxy]butyl-triphenyl-phospho-nium iodide (MitoPeroxidase 1; synthesized as reported in ref (20); Scheme 2) and 2-[[2-[4-(3-oxo-1,2-benzoselenazol-2-yl)phenyl]acetyl]amino]ethyl-triphenyl-phosphonium chloride (MitoPeroxidase 2; Scheme 3, 10) to act as radiation mitigators (RMs). Because under conditions of oxidative stress sufficient concentrations of antioxidants at the sites of generation of reactive metabolites are critical to protection from oxidative damage, we have targeted the synthesis of ebselen derivatives that selectively compartmentalize to mitochondria (Scheme 2).
Scheme 2. Design of Mitochondria-Specific Ebselen Derivatives.
Scheme 3. Synthesis of MitoPeroxidase 2.
Reagents and conditions: (a) aq. NaNO2, HCl (18%), 0 °C; (b) Na2Se2, aq. NaOH, 40 °C (2 h), 55–63%; (c) SOCl2, DMF, reflux (1 h), >95%; (d) 2-(4-aminophenyl)acetic acid, Et3N, CH3CN, 0 °C (1 h) to 25 °C (3 h), 72–76%; (e) (Ph)3P+CH2CH2NH2, DCC, 0 °C (1 h) to 25 °C (4 h), 80–85%.
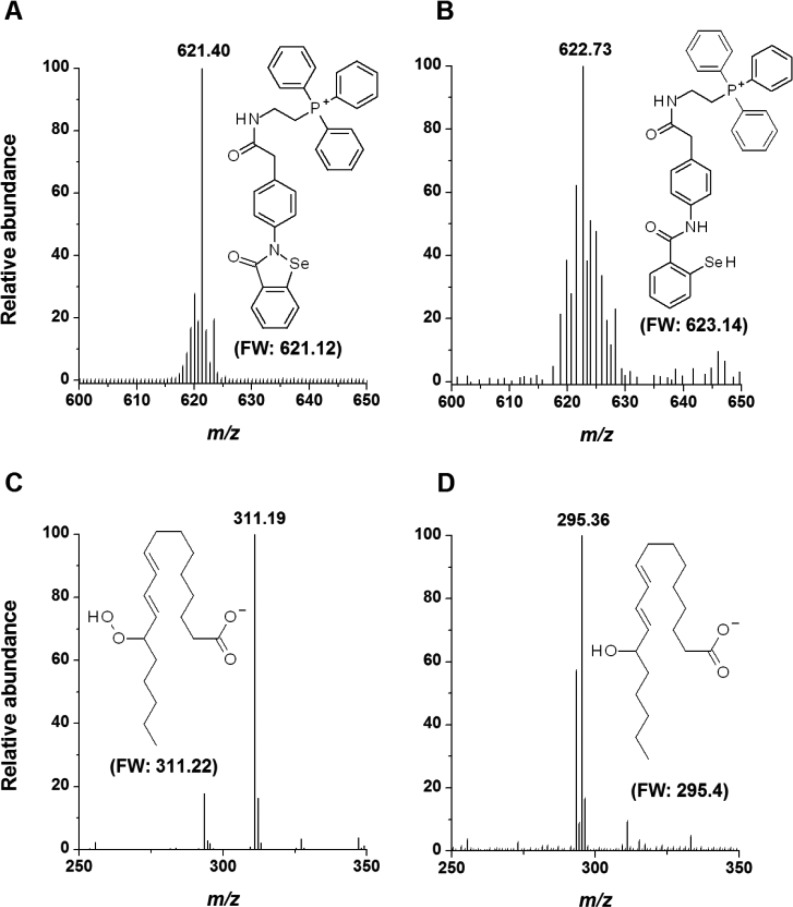
To date, several methods for delivery of drugs into mitochondria have been developed, including their derivatization with certain peptides or with a triphenylphosphonium group ((Ph)3P+).21 Since mitochondria maintain a negative potential, the positive charge of the (Ph)3P+ group drives attached molecules inside the matrix and toward a diffusion equilibrium, thus affording up to a thousand-fold accumulation of the drug in mitochondria vs cytosol. The accumulation of organic cations in mitochondria may also be mediated by proteins such as the 2-oxoglutarate carrier.22 Previous studies have shown that MitoPeroxidase 1 is taken up by isolated mitochondria, catalyzes the breakdown of H2O2 in the presence of thiols, and inhibits apoptosis induced by oxidants.20 In agreement with the reactions presented in Scheme 1, incubation (5 min; t = 25 °C) of MitoPeroxidase 2 (15 μM; Figure 1A) and 6,8-bis(sulfanyl)octanoic acid (dihydrolipoic acid; DHLA; 15 μM) in He-deaerated methanol led to the formation of a selanyl-benzamide (Figure 1B), which readily reduced 13S-hydroperoxy-9Z,11E-octadecadienoic acid (10 μM; ROOH; Figure 1C) to the corresponding alcohol (ROH; tincubation = 5 min; 25 °C; Figure 1D; MS/MS spectra of both ROOH and ROH are included in Supporting Information). Similarly, the selanyl-benzamide reduced hydroperoxides of CL (Supporting Information).
Figure 1.
Mass-spectral analysis of the thiol-dependent reduction of ROOH by MitoPeroxidase 2. DHLA reduced MitoPeroxidase 2 (A) to selanyl-benzamide (B), which reacted with ROOH (C) to afford ROH (D). The mass spectra in A and B exhibit isotopic distribution characteristics for the Se isotopes.20
Though the benzoselenazol ring is a common pharmacophore for ebselen and MitoPeroxidases 1 and 2, several factors may differentiate the pharmacological effect of these compounds. In contrast to ebselen, which accumulates in the ER, MitoPeroxidases 1 and 2 are expected to compartmentalize to mitochondria to reach millimolar concentrations.23 In addition, the conjugated aromatic system of MitoPeroxidase 1 is extended to the phenolic oxygen, and thus, its Se–N bond is more polarized. This is likely to facilitate the conversion of MitoPeroxidase 1 to a selenenyl sulfide (reaction 1 → 2), which may result in an increased toxicity due to enhanced oxidation of cellular thiols.24 However, weakening of the Se···O=C< interaction in 3 by the electron-withdrawing effect of the phenolic oxygen may increase the GPx activity of the parent benzoselenazol.25,26
Figure 2A depicts the toxicity of ebselen and MitoPeroxidases 1 and 2 in mouse embryonic cells (MEC). In contrast to ebselen and MitoPeroxidase 2, the toxicity of MitoPeroxidase 1 sharply increased in the concentration range of 10 to 20 μM. Comparable toxicity with MitoPeroxidase 2 was observed at ∼40 μM concentration, while ebselen did not exhibit any significant toxicity in this concentration range.
Figure 2.
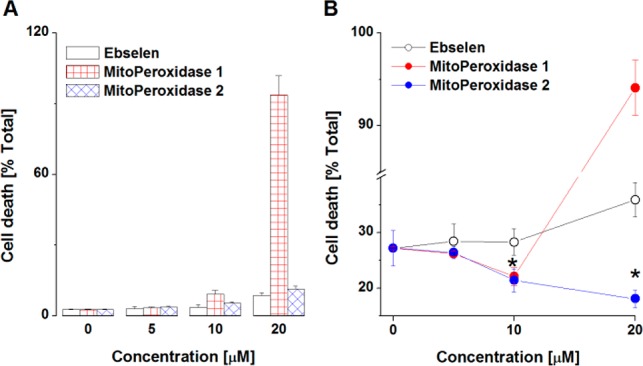
Toxicity (A) and radiomitigative properties (B) of ebselen and MitoPeroxidases 1 and 2. (A,B) Cell death was assessed flow-cytometrically by analysis of the externalization of phosphatidylserine. (B) Cells were exposed to IR and then treated with selenazols. The results represent the mean ± SD (n = 3; *p < 0.05).
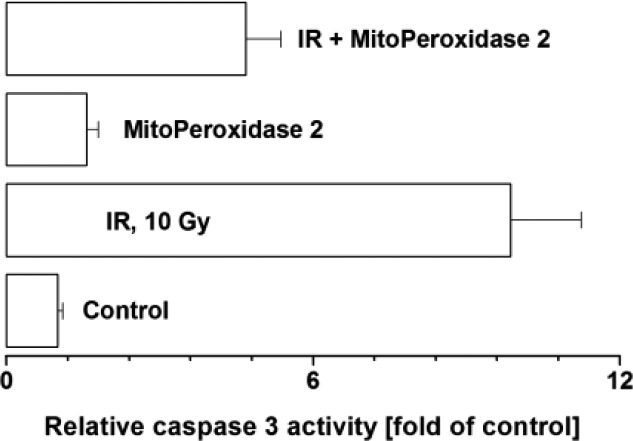
We next assessed the radio-mitigative properties of the three benzoselenazols. Exposure of MEC to γ-irradiation (10 Gy) resulted in ∼28% cell death (Figure 2B), whereas treatment with MitoPeroxidases 1 and 2 (but not ebselen), prior to or 30 min postirradiation, afforded radioprotection/mitigation. MitoPeroxidase 1 exerted ∼25% radioprotection/mitigation, while at concentrations higher than 10 μM its toxicity prevailed the radioprotective effect. In contrast, 20 μM MitoPeroxidase 2 afforded ∼50% radioprotection/mitigation. In this model system, MitoPeroxidase 2 acted as a potent inhibitor of radiation-induced activation of caspase 3 (Figure 3), an executioner caspase in apoptosis. Treatment of irradiated MEC with 3-hydroxypropyl(triphenyl)phosphonium chloride, which structurally mimics the triphenylphosphonium moieties of MitoPeroxidases 1 and 2, did not afford any radiomitigation (data not shown).
Figure 3.
MitoPeroxidase 2 impedes the activity of caspase 3 in MEC. Cells were exposed to IR (10 Gy) and then treated with 20 μM MitoPeroxidase 2 as indicated in Figure 2. Caspase 3 activity was determined by EnzChek Caspase 3 Assay Kit (Z-DEVD-AMC substrate; Life Technologies, Grand Island, NY). The results represent the mean + SD (n = 3).
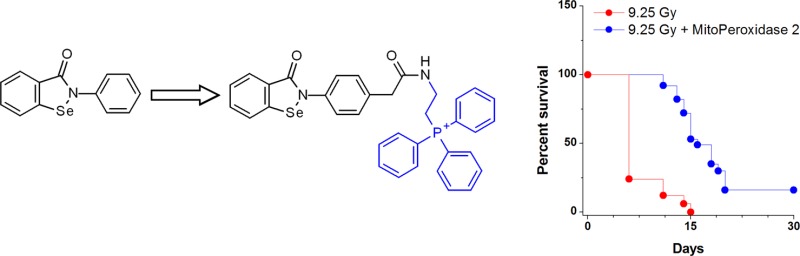
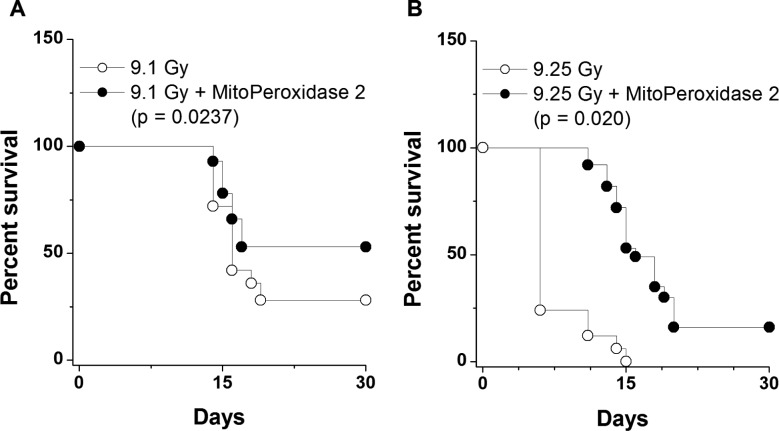
We further assessed whether the in vitro radiomitigative effect of MitoPeroxidase 2 was translated into an in vivo effect. Groups of 15 mice were treated with MitoPeroxidase 2 i.v. at 24 h after WBI. As shown in Figure 4, at two different radiation doses, administration of MitoPeroxidase 2 afforded an increase in survival.
Figure 4.
Radiomitigative properties of MitoPeroxidase 2 administered 24 h after exposure to γ-rays in mice (n = 15 mice per group).
In conclusion, the data presented herein indicate that MitoPeroxidase 2 acts as a potent radiation mitigator. Our data complement previous studies on the medicinal chemistry of ebselen within the context of radiomitigation upon late, 24 h postirradiation treatment. This is an important pharmacological advantage, as IR damage to organisms is often the result of accidents, whereby immediate post-IR treatment may be impractical.
Glossary
Abbreviations
- GPx
glutathione peroxidase
- ER
endoplasmic reticulum
- IR
ionizing radiation
- RMs
radiation mitigators
- RPs
radiation protectors
- WBI
whole-body irradiation
- MEC
mouse embryonic cells
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/ml5003635.
Details of biological assays, chemical synthesis, and structural analysis (PDF)
This work was supported by NIH Grant U19AI068021, HL114453 and NS061817, and Human Frontier Science Program RGP0013.
The authors declare no competing financial interest.
A systematic error was introduced in the page numbers of the References list in the version published November 18, 2014. The page number ranges have been corrected as of June 1, 2016.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Weiss J. Radiochemistry of Aqueous Solutions. Nature 1944, 153, 748–750 10.1038/153748a0. [DOI] [Google Scholar]
- Patt H. M.; Tyree E. B.; Straube R. L.; Smith D. E. Cysteine Protection against X Irradiation. Science 1949, 110, 213–214 10.1126/science.110.2852.213. [DOI] [PubMed] [Google Scholar]
- Bacq Z. M.; Herve A.; Fischer P.; Lecomte J.; Pirotte M.; Deschamps G.; Le Bihan H.; Rayet P. Preventive and therapeutic effects of mercapto-ethylamin (becaptan) on radiation sickness. Rev. Med. Liege 1953, 8, 104–109. [PubMed] [Google Scholar]
- Radivojevitch D.; Bacq Z. M.; Beaumariage M. L. Radio-protective action of cysteamine, cystamine and histamine on the dipilation of the young mouse exposed to x-radiation. J. Physiol. (Paris) 1960, 52, 205–206. [PubMed] [Google Scholar]
- Sweeney T. R.A Survey of Compounds from the Antiradiation Drug Development Program of the US Army Medical Research and Development Command; Walter Reed Army Institute of Research: Washington DC, 1979. [Google Scholar]
- Kouvaris J. R.; Kouloulias V. E.; Vlahos L. J. Amifostine: the first selective-target and broad-spectrum radioprotector. Oncologist 2007, 12, 738–747 10.1634/theoncologist.12-6-738. [DOI] [PubMed] [Google Scholar]
- Kam W. W.; Banati R. B. Effects of ionizing radiation on mitochondria. Free Radical Biol. Med. 2013, 65, 607–619 10.1016/j.freeradbiomed.2013.07.024. [DOI] [PubMed] [Google Scholar]
- Coelho D.; Holl V.; Weltin D.; Lacornerie T.; Magnenet P.; Dufour P.; Bischoff P. Caspase-3-like activity determines the type of cell death following ionizing radiation in MOLT-4 human leukaemia cells. Br. J. Cancer 2000, 83, 642–649 10.1054/bjoc.2000.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayir H.; Kagan V. E.; Borisenko G. G.; Tyurina Y. Y.; Janesko K. L.; Vagni V. A.; Billiar T. R.; Williams D. L.; Kochanek P. M. Enhanced oxidative stress in iNOS-deficient mice after traumatic brain injury: support for a neuroprotective role of iNOS. J. Cereb. Blood Flow Metab. 2005, 25, 673–684 10.1038/sj.jcbfm.9600068. [DOI] [PubMed] [Google Scholar]
- Kagan V. E.; Bayir H.; Shvedova A. A. Nanomedicine and nanotoxicology: two sides of the same coin. Nanomedicine 2005, 1, 313–316 10.1016/j.nano.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Fabisiak J. P.; Tyurina Y. Y.; Tyurin V. A.; Kagan V. E. Quantification of selective phosphatidylserine oxidation during apoptosis. Methods Mol. Biol. 2005, 291, 449–456 10.1385/1-59259-840-4:449. [DOI] [PubMed] [Google Scholar]
- Imai H.; Nakagawa Y. Biological significance of phospholipid hydroperoxide glutathione peroxidase (PHGPx, GPx4) in mammalian cells. Free Radical Biol. Med. 2003, 34, 145–169 10.1016/S0891-5849(02)01197-8. [DOI] [PubMed] [Google Scholar]
- Nomura K.; Imai H.; Koumura T.; Kobayashi T.; Nakagawa Y. Mitochondrial phospholipid hydroperoxide glutathione peroxidase inhibits the release of cytochrome c from mitochondria by suppressing the peroxidation of cardiolipin in hypoglycaemia-induced apoptosis. Biochem. J. 2000, 351, 183–193 10.1042/0264-6021:3510183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura K.; Imai H.; Koumura T.; Nakagawa Y. Involvement of mitochondrial phospholipid hydroperoxide glutathione peroxidase as an antiapoptotic factor. Neurosignals 2001, 10, 81–92 10.1159/000046877. [DOI] [PubMed] [Google Scholar]
- Epperly M. W.; Melendez J. A.; Zhang X.; Nie S.; Pearce L.; Peterson J.; Franicola D.; Dixon T.; Greenberger B. A.; Komanduri P.; Wang H.; Greenberger J. S. Mitochondrial targeting of a catalase transgene product by plasmid liposomes increases radioresistance in vitro and in vivo. Radiat. Res. 2009, 171, 588–595 10.1667/RR1424.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tak J. K.; Park J. W. The use of ebselen for radioprotection in cultured cells and mice. Free Radical Biol. Med. 2009, 46, 1177–1185 10.1016/j.freeradbiomed.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Aitken J. B.; Lay P. A.; Duong T. T.; Aran R.; Witting P. K.; Harris H. H.; Lai B.; Vogt S.; Giles G. I. Synchrotron radiation induced X-ray emission studies of the antioxidant mechanism of the organoselenium drug ebselen. JBIC, J. Biol. Inorg. Chem. 2012, 17, 589–598 10.1007/s00775-012-0879-y. [DOI] [PubMed] [Google Scholar]
- Azad G. K.; Tomar R. S. Ebselen, a promising antioxidant drug: mechanisms of action and targets of biological pathways. Mol. Biol. Rep. 2014, 41, 4865–4879 10.1007/s11033-014-3417-x. [DOI] [PubMed] [Google Scholar]
- Singh N.; Halliday A. C.; Thomas J. M.; Kuznetsova O. V.; Baldwin R.; Woon E. C.; Aley P. K.; Antoniadou I.; Sharp T.; Vasudevan S. R.; Churchill G. C. A safe lithium mimetic for bipolar disorder. Nat. Commun. 2013, 4, 1332. 10.1038/ncomms2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipovska A.; Kelso G. F.; Brown S. E.; Beer S. M.; Smith R. A.; Murphy M. P. Synthesis and characterization of a triphenylphosphonium-conjugated peroxidase mimetic. Insights into the interaction of ebselen with mitochondria. J. Biol. Chem. 2005, 280, 24113–24126 10.1074/jbc.M501148200. [DOI] [PubMed] [Google Scholar]
- Smith R. A.; Hartley R. C.; Murphy M. P. Mitochondria-targeted small molecule therapeutics and probes. Antioxid. Redox Signaling 2011, 15, 3021–3038 10.1089/ars.2011.3969. [DOI] [PubMed] [Google Scholar]
- Kabe Y.; Ohmori M.; Shinouchi K.; Tsuboi Y.; Hirao S.; Azuma M.; Watanabe H.; Okura I.; Handa H. Porphyrin accumulation in mitochondria is mediated by 2-oxoglutarate carrier. J. Biol. Chem. 2006, 281, 31729–31735 10.1074/jbc.M604729200. [DOI] [PubMed] [Google Scholar]
- Stoyanovsky D. A.; Huang Z.; Jiang J.; Belikova N. A.; Tyurin V.; Epperly M. W.; Greenberger J. S.; Bayir H.; Kagan V. E. A manganese-porphyrin complex decomposes H(2)O(2), inhibits apoptosis, and acts as a radiation mitigator in vivo. ACS Med. Chem. Lett. 2011, 2, 814–817 10.1021/ml200142x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puntel R. L.; Roos D. H.; Folmer V.; Nogueira C. W.; Galina A.; Aschner M.; Rocha J. B. Mitochondrial dysfunction induced by different organochalchogens is mediated by thiol oxidation and is not dependent of the classical mitochondrial permeability transition pore opening. Toxicol. Sci. 2010, 117, 133–143 10.1093/toxsci/kfq185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press D. J.; Mercier E. A.; Kuzma D.; Back T. G. Substituent effects upon the catalytic activity of aromatic cyclic seleninate esters and spirodioxyselenuranes that act as glutathione peroxidase mimetics. J. Org. Chem. 2008, 73, 4252–4255 10.1021/jo800381s. [DOI] [PubMed] [Google Scholar]
- Bhabak K. P.; Mugesh G. Functional mimics of glutathione peroxidase: bioinspired synthetic antioxidants. Acc. Chem. Res. 2010, 43, 1408–1419 10.1021/ar100059g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.