Abstract
Vaccination with live attenuated classical swine fever virus (CSFV) vaccines can rapidly confer protection in the absence of neutralizing antibodies. With an aim of providing information on the cellular mechanisms that may mediate this protection, we explored the interaction of porcine natural killer (NK) cells and γδ T cells with CSFV. Both NK and γδ T cells were refractory to infection with attenuated or virulent CSFV, and no stimulatory effects, as assessed by the expression of major histocompatibility complex (MHC) class II (MHC-II), perforin, and gamma interferon (IFN-γ), were observed when the cells were cultured in the presence of CSFV. Coculture with CSFV and myeloid dendritic cells (mDCs) or plasmacytoid dendritic cells (pDCs) showed that pDCs led to a partial activation of both NK and γδ T cells, with upregulation of MHC-II being observed. An analysis of cytokine expression by infected DC subsets suggested that this effect was due to IFN-α secreted by infected pDCs. These results were supported by ex vivo analyses of NK and γδ T cells in the tonsils and retropharyngeal lymph nodes from pigs that had been vaccinated with live attenuated CSFV and/or virulent CSFV. At 5 days postchallenge, there was evidence of significant upregulation of MHC-II but not perforin on NK and γδ T cells, which was observed only following a challenge of the unvaccinated pigs and correlated with increased CSFV replication and IFN-α expression in both the tonsils and serum. Together, these data suggest that it is unlikely that NK or γδ T cells contribute to the cellular effector mechanisms induced by live attenuated CSFV.
INTRODUCTION
Classical swine fever (CSF) is a highly contagious and often fatal disease of domestic pigs and wild boar. The etiological agent is the classical swine fever virus (CSFV), a small, enveloped, positive-sense, and single-stranded RNA virus belonging to the family Flaviviridae (1). Due to the ethical and economic consequences of controlling CSF outbreaks in the European Union (EU) through a stamping-out policy, there is an urgent need for the development of alternative control strategies, such as marker vaccines (2, 3). Vaccination with live attenuated C-strain vaccines can protect against CSF before the appearance of a neutralizing antibody response but not before virus-specific gamma interferon (IFN-γ)-secreting cells appear in the peripheral blood (4). Studies have suggested that C-strain CSFV is a potent inducer of type I T-cell responses, which may play a role in the protection afforded in the absence of antibody responses (5–7). An improved understanding of the cellular immune mechanisms triggered by the C-strain vaccine would therefore aid in the development of the next generation of CSFV marker vaccines.
Little is known about the contribution of porcine NK and γδ T cells in the cellular immune response against CSFV. Their activation/inhibition might be crucial, given that swine possess only a small number of cytotoxic T cells but large numbers of lymphocytes with innate cytotoxic activity, especially γδ T cells (8). In young pigs, γδ T cells and NK cells represent 50% and 10% of the total peripheral blood lymphocyte population, respectively, although their frequencies decrease with age (8, 9). It is well known that NK cells possess the ability to attack pathogen-infected and malignant cells and to produce immunostimulatory cytokines, such as IFN-γ and tumor necrosis factor alpha (TNF-α) (9). Specifically, NK cells are triggered to kill or ignore transformed or pathogen-infected cells, depending on a balance of inhibitory and activating signals received through ligands on potential target cells (10). Although some pathogens can directly activate NK cells, such as influenza virus activation of human NK cells through hemagglutinin-NKp46 receptor binding (11) or murine cytomegalovirus-activating NK cells via the m157 glycoprotein-Ly49 receptor interaction (12), the activation of these cells by most pathogens seems to be initiated by antigen-presenting cells (APCs), which provide both indirect (cytokines) and direct (contact-dependent) signals (13). The cross talk between NK cells and dendritic cells (DCs) may also be bidirectional, and IL-2-activated human NK cells can directly induce the maturation of DCs, thereby enhancing their ability to stimulate naive T cells (14). Porcine NK cells were originally defined by a CD3− CD8α+ perforin+ CD2+ CD16+ phenotype (8, 9). Similar to the NK cells from other species, porcine NK cells may be activated with IL-2 or IL-15 or synergistically with interleukin-12 (IL-12) and IL-18, which in addition to inducing cytokine and cytotoxic responses, increase the expression of major histocompatibility complex (MHC) class II, which is normally found at low levels in resting NK cells (15, 16), suggesting that MHC class II may serve as a marker of activated NK cells, as has been proposed for porcine T cells (9). Cytokine-induced activation of porcine NK cells has been shown to enhance the killing of virus-infected cells (16). The recent development of monoclonal antibodies (MAbs) against porcine NKp46 unexpectedly revealed both NKp46+ and NKp46− NK cells in the blood, with NKp46+ cells showing capacity for enhanced IFN-γ expression (17). While absent from the blood, a third NK cell population with a CD8α−/low NKp46high phenotype has been described in porcine spleens, with high cytolytic and cytokine expression capacity (18). Studies of the role of porcine NK cells in the response to viral infection are limited, but there are data showing that porcine NK cell responses are impaired following infection with foot-and-mouth disease virus (FMDV) (19) and porcine reproductive and respiratory syndrome virus (PRRSV) (20, 21). One study was conducted in the context of virulent CSFV infection and similarly showed diminished NK cell cytolytic activity following the onset stage of disease (22). Several studies have reported the effects of a related flavivirus, hepatitis C virus (HCV), on human NK cells. Human NK cell activity can be directly inhibited by the recombinant HCV core protein (23) and by the structural protein E2 (24). The HCV nonstructural protein 5A (NS5A) is able to impair human NK cell activity indirectly by inducing monocytes to secrete cytokines that result in the downregulation of NKG2D expression on NK cells (25). This inhibition of NK cells, which occurs early in infection, would allow HCV to establish a replicative advantage prior to the induction of the specific immune response and would also destroy NK-DC cross talk, impairing the DC-mediated priming of CD8+ cytotoxic T lymphocyte (CTL) responses (26).
γδ T cells represent another significant lymphocyte population of the innate immune system, which is regulated by the balance between activating and inhibitory signals (27). γδ T cells have been shown to exert several different functions, suggesting that they are involved in a multitude of immunological processes. They are able to release perforin and granzymes to kill infected or malignant cells (28). They can produce cytokines involved in the protection against viruses and other intracellular pathogens (IFN-γ and TNF-α), extracellular parasites (IL-4, IL-5, and IL-13), extracellular bacteria (IL-17), or immunoregulation (transforming growth factor beta [TGF-β] and IL-10) (27). The activation of γδ T cells can be direct or indirect, mediated by the interaction with APCs, such as DCs (29). Moreover, in humans and pigs, it has been shown that γδ T cells not only interact with DCs but also display characteristics of professional APCs themselves (30, 31). In swine, γδ T cells can express both CD8α and MHC-II molecules, which have been proposed to be markers of porcine T-cell activation (9), and it has been reported that a subpopulation of circulating porcine γδ T cells is able to present antigens to memory helper T cells through MHC-II (31). γδ T-cell responses to CSFV have not been described; however, in HCV-infected patients, γδ T cells from the liver, but not blood, are able to be expanded in vitro after exposure to cytokines, and it is thought that γδ T cells are activated through the cross-linking of CD81, which binds the HCV structural protein E2 with high affinity (32).
In a previous study, we did not detect CSFV-specific IFN-γ release by NK and γδ T cells in the blood of C-strain-vaccinated pigs (33). However, we hypothesize that these cells play an important role in the lymphoid tissues by contributing to protection at the sites of primary infection and by shaping the T-cell response against the virus. Similar to HCV infection, we might find evidence of cellular activation at the local tissue level, which in the case of CSFV would be the tonsils, as well as in the lymph nodes that drain the oronasal cavity. To explore both the direct and indirect effects, we investigated the interaction of NK and γδ T cells with CSFV virions and CSFV-infected DC populations in vitro, and we extended these findings to an ex vivo analysis of NK and γδ T cells in the tonsils and retropharyngeal lymph nodes from pigs vaccinated with C-strain and/or challenged with virulent CSFV.
MATERIALS AND METHODS
Viruses.
A live attenuated C-strain CSFV (AC Riemser Schweinepestvakzine, Riemser Arzneimittel AG, Riems, Germany) and the virulent CSFV Brescia strain were propagated in PK15 cell monolayers. Both mock virus and virus stocks were prepared, and titers were determined, as described previously (33).
Ethics statement.
All animal work was approved by the Animal Health and Veterinary Laboratories Agency ethics committee, and all procedures were conducted in accordance with the Animals (Scientific Procedures) Act 1986 (United Kingdom) under project license permits PPL 70/6559 and 70/7057. To minimize animal suffering, careful completion of the clinical score sheets and regular observation were conducted, which informed euthanasia decisions based on a predefined humane endpoint (clinical score, >15 or temperature, >41°C and >2 individual scores [other than the temperature score] with a value of 3). However, none of the animals experienced clinical signs necessitating euthanasia. Each animal was euthanized on predetermined days by stunning and exsanguination.
Animals.
For an assessment of the interaction of NK and γδ T cells with CSFV in vitro, blood samples were collected from CSFV-naive Large White/Landrace crossbreed pigs, 6 to 24 months of age, by puncture of the external jugular vein. To analyze the responses of NK and γδ T cells to CSFV ex vivo, a C-strain vaccination and challenge study was performed as previously described (33). In brief, 42 Large White/Landrace crossbreed pigs of 8 to 10 weeks of age were randomly assigned to one of four groups. Five days prior to challenge (day −5), the animals in groups 1 (n = 6) and 3 (n = 12) were vaccinated intranasally with 105 the 50% tissue culture infective dose (TCID50) of C-strain CSFV (1 ml divided equally between each nostril and administered using a mucosal atomization device [MAD-300; Wolfe Tory Medical, USA]), group 2 (n = 12) was vaccinated with 2 ml of C-strain vaccine into the brachiocephalous muscle (as recommended by the manufacturer [Riemser Arzneimittel AG]), and group 4 (n = 12) was intranasally inoculated with 1 ml of mock virus supernatant, as described for groups 1 and 2. On day 0, groups 2 to 4 were inoculated intranasally with 105 TCID50 of CSFV Brescia strain, as described above, and group 1 received a similar inoculation of mock virus supernatant. On days −2 and 0, three animals were euthanized from groups 2 to 4, and on days 3 and 5, three animals were euthanized from all four groups, and the tonsils and retropharyngeal lymph nodes were collected.
Clinical, hematological, and virological methods.
The animals were inspected by the Animal Health and Veterinary Laboratories Agency (AHVLA) Animal Service Unit staff twice daily (am and pm), and 9 parameters relevant to an indication of CSF (affecting liveliness, body tension, body shape, breathing, walking, skin, eye/conjunctiva, appetite/left over food at feedings, and defecation) were examined and scored as 0 (normal), 1 (slightly altered), 2 (distinct clinical sign), or 3 (known CSF symptom) (34). A total clinical score for each animal was assigned twice daily, and their temperatures were monitored by rectal thermometer readings and recorded once daily. Temperature and clinical score monitoring commenced on day −11 postchallenge and continued until the termination of the experiment (day 5 postchallenge). Peripheral blood leukocytes and CSFV RNA were monitored in EDTA blood samples collected every 3 days using volumetric flow cytometry and reverse transcription-quantitative RT-PCR (qRT-PCR), respectively (34).
Isolation and cryopreservation of tonsil and lymph node cells and PBMC.
At days −2, 0, 3, and 5 postchallenge, each animal was stunned and euthanized by exsanguination. The tonsils and medial and lateral retropharyngeal lymph nodes were removed. The lymphoid tissues, first separated from the connective and epithelial tissues, were placed in a small volume of Hanks' balanced salt solution (HBSS) (Life Technologies) supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin and chopped into fine pieces with sterile pointed scissors. The tissues were transferred to a 100-μm cell strainer (BD Biosciences) inserted in a 50-ml collection tube. A plunger from a 5-ml syringe was used to disrupt the tissues, HBSS was passed regularly through the cell strainer, and the process was continued until only connective tissue remained in the cell strainer. The cells were washed twice in HBSS and resuspended in RPMI 1640 medium (Life Technologies) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin, and 100 μg/ml streptomycin (cRPMI). Peripheral blood mononuclear cells (PBMC) were prepared as described earlier (33). For both the tonsil/lymph node cells and PBMC, cell numbers were determined using a MACSQuant Analyzer flow cytometer (Miltenyi Biotec, Bisley, United Kingdom) by gating on events with typical forward scatter (FSC) and side scatter (SSC) properties for lymphocytes. The cells were adjusted to a density of 1 × 107 to 2 × 107 cells/ml in cold 10% dimethyl sulfoxide (DMSO) (Sigma, Poole, United Kingdom) in FBS and cryopreserved as described previously (33).
Quantification of CSFV RNA in tonsils and retropharyngeal lymph nodes.
To quantify CSFV RNA in the tonsils and retropharyngeal lymph nodes, RNA was extracted from the tissue homogenates. Briefly, 200 mg of tissue was homogenized in 4 ml RLT buffer (Qiagen, Crawley, United Kingdom) using the Precellys 24-Dual tissue homogenizer (Precellys, Stretton Scientific Ltd., Stretton, United Kingdom), with 2 30-s cycles at 6,000 rpm. The tissue homogenates were clarified by centrifugation for 10 min at 1,000 × g and RNA extracted using the RNeasy maxi kit with the additional DNase treatment, as per the manufacturer's instructions (Qiagen). CSFV RNA was quantified by qRT-PCR as described previously (34) and normalized to 18S RNA using the Eukaryotic 18S rRNA endogenous control (Life Technologies).
Detection of IFN-α-secreting cells in tonsils.
Samples from the tonsils were collected, fixed in Bouin's solution, and embedded in paraffin wax. Four-micrometer sections were dewaxed, rehydrated, and then treated in hydrogen peroxide 3% in methanol for 15 min to eliminate endogenous peroxidase activity. The tissue sections were then pretreated for antigen retrieval by immersing in 0.01% Tween 20 in phosphate-buffered saline (PBS) for 10 min at room temperature and then washing in running tap water for 5 min. The sections were mounted in a Sequenza immunostaining center (Shandon Scientific, Runcorn, United Kingdom) and rinsed with 5 mM Tris-buffered saline (pH 7.6) with 0.05% Tween 20 (TBST). Primary antibody cross-reactivity with the tissue constituents was blocked with 10% FBS, 0.3% (wt/vol) glycine, and 0.001% (wt/vol) bovine serum albumin (BSA) in PBS. A mouse MAb against porcine IFN-α (clone F17; R&D Systems) was added at 1.82 μg/ml and sections incubated at 4°C for 18 to 20 h. The sections were washed in TBST and incubated for 30 min with a 1:100 dilution of biotinylated secondary antibody (biotin-SP-conjugated AffiniPure Fab fragment goat anti-mouse IgG [H+L]; Jackson ImmunoResearch, Stratech, Newmarket, United Kingdom), and normal goat serum (1:66 dilution) and normal pig serum (1:33 dilution) were added before being washed twice in TBST. The sections were incubated for 30 min at room temperature with avidin-biotin complex (ABC Vector Elite; Vector Laboratories, Peterborough, United Kingdom), and the signal was detected using 3,3′-diaminobenzidine tetrahydrochloride (DAB). Finally, the sections were lightly counterstained with Mayer's hematoxylin (Surgipath, Peterborough, United Kingdom) for 5 min, dehydrated in absolute alcohol, and cleared in xylene before being coverslipped. Negative-control sections were included in each run, substituting primary antibody with an isotype control (mouse IgG1; Dako, Ely, United Kingdom) at the same concentration. Immunolabeled cells were counted in 25 nonoverlapping consecutive fields of 0.2 mm2 for interfollicular tonsil lymphoid tissue or 25 tonsillar lymphoid follicles per animal. The slides were identified with a unique code, which did not include references to the animal or experimental group and were thus examined in a blind manner.
Detection of type I IFN activity in serum.
At days −2, 0, 3, and 5 postchallenge, blood was collected in serum separator tubes (BD Biosciences), and serum was obtained by centrifugation at 1,500 × g for 10 min and stored at −80°C until analyzed. Type I IFN bioactivity was determined using an Mx protein chloramphenicol acetyltransferase (Mx/CAT) reporter gene assay originally developed for to quantify bovine IFN-α/β (35). The serum samples were diluted to 20% (vol/vol) in Dulbecco's modified Eagle's medium (DMEM) (Life Technologies) supplemented with 10 μg/ml blasticidin (Life Technologies) and added to reporter bovine kidney cells (MDBK-t2) for 24 h. A titration of recombinant porcine IFN-α (R&D Systems) was added as a standard. The lysates were prepared from the cultures and CAT enzyme measured by ELISA (Roche, Welwyn Garden City, United Kingdom). Absorbance was read using a FLUOstar Optima microplate reader (BMG Labtech, Aylesbury, United Kingdom).
Enrichment of DCs, γδ T cells, and NK cells from porcine blood.
PBMC were prepared as described above. The blood DC populations were enriched by two rounds of magnetic cell separation (CD14 depletion and CD172a enrichment), and the subsets were isolated by subsequent flow cytometric sorting. Specifically, PBMC were resuspended in CD14 microbeads (10 μl/107 cells; Miltenyi Biotec) and incubated at room temperature (RT) for 10 min. After two washes with 50 ml of PBS supplemented with 2% FBS, the cells were resuspended in PBS supplemented with 2% FBS and 5 mM EDTA (MACS buffer, 1 ml/2.5 × 108 cells), passed through a 100-μm cell strainer (BD Biosciences) to eliminate cell aggregates, and applied to a MACS LD column on a QuadroMACS unit (Miltenyi Biotec). The column flowthrough and two washes of 3 ml MACS buffer were collected and washed in PBS with 2% FBS. The cells were stained with an MAb against CD172a (clone 74-22-15A, 1 μg MAb/107 cells; Washington State University Monoclonal Antibody Center [WSUMAC], Pullman, WA, USA) and incubated at RT for 10 min. The cells were then washed and incubated with mouse IgG microbeads (10 μl/107 cells; Miltenyi Biotec) for a further 10 min. After two washes with 50 ml of PBS with 2% FBS, the CD172a+ cells were enriched using LS columns, as described by the manufacturer (Miltenyi Biotec). To assess the purity of the enriched DCs and/or to isolate myeloid DCs (mDCs) and plasmacytoid DCs (pDCs), the cells were stained with anti-CD4-PerCP-Cy5.5 MAb (clone 74-12-4, 0.4 μg/107 cells; BD Biosciences) and a lineage cocktail of IgG1 MAbs against CD3 (clone 8E6, 2 μg/107 cells; WSUMAC), CD8α (clone PT36B, 2 μg/107 cells; WSUMAC), and CD21 (clone B-Ly4, 1 μg/107 cells; BD Biosciences), followed by staining with APC-conjugated rat anti-mouse IgG1 antibody (clone X56, 1 μg/107 cells; BD Biosciences). For sorting, the cells were washed, resuspended at a density of 107 cells/ml in cRPMI, and lineage− CD4− mDCs and lineage− CD4+ pDCs were sorted using a MoFlo Astrios cell sorter (Beckman Coulter, High Wycombe, United Kingdom). The purity of the enriched and sorted DC populations was assessed by flow cytometry. The DCs were typically enriched by magnetic sorting to ∼60%, with comparable numbers of mDCs and pDCs, and following flow cytometric sorting, the mDCs and pDCs were shown in all instances to be >90% pure. After sorting, the cell densities were adjusted to 2 × 106 cells/ml in cRPMI, and 50 μl/well was added to round-bottom 96-well plates.
Porcine γδ T cells were enriched by positive magnetic sorting of PBMC stained with an MAb against 1 μg/107 cells γδ-TcR1-N4 (clone PGBL22A; WSUMAC), followed by mouse IgG microbeads and a collection of the positive fraction following separation on an LS column (Miltenyi Biotec). To assess purity, an aliquot of cells was stained with the MAb TcR1-N4 labeled with Zenon Alexa Fluor 405, as described by the manufacturer (Life Technologies), and analyzed by flow cytometry. The purity of the γδ T cells ranged from 85 to 95%. The porcine NK cells were isolated by two rounds of magnetic sorting. PBMC were first depleted of T cells by indirect staining with an MAb against CD3 (clone 8E6, 1 μg/107 cells; WSUMAC), followed by staining with mouse IgG microbeads, collection of the flowthrough, and washes on an LD column, as described above. The CD3− fraction was stained with an MAb against CD8α (clone PT36B, 1 μg/107 cells; WSUMAC), and after staining with mouse IgG, the microbeads were enriched using an LS column. Purity was assessed after staining an aliquot with an MAb against CD8α-phycoerythrin (PE) (clone 76-2-11; BD Biosciences) and CD3-APC (clone BB238E68C8; BD Biosciences) and showed a purity of the NK cells that ranged from 70 to 80%. After sorting, the cell densities of the NK and γδ T cells were adjusted to 2 × 106 or 4 × 106 cells/ml with cRPMI, and 100 or 50 μl was added to the wells of a round-bottom 96-well plate. All the washes used centrifugation at 930 × g for 5 min.
Coculture of NK and γδ T cells with CSFV, DCs, and recombinant porcine cytokines.
Enriched NK cells or γδ T cells (2 × 105 cells/well), alone or cocultured with enriched or sorted DC populations (1 × 105 cells/well), were cultured with CSFV C-strain or Brescia (100 μl/well). To maintain pDC viability in vitro, the DC-containing cultures were supplemented with 40 ng/ml recombinant porcine IL-3 (kindly provided by Kirsten Morris, CSIRO Animal, Food, & Health Science, Australian Animal Health Laboratory, Geelong, Australia). For experiments involving only enriched NK or γδ T cells, a multiplicity of infection (MOI) of 1 was used, whereas an MOI of 0.66 was used for the DC coculture experiments. In defined experiments, the cells were cultured in the presence of recombinant porcine cytokines IL-12 (100 ng/ml), IL-18 (100 ng/ml), IFN-α (4,000, 2,000, or 1,000 U/ml), and/or TNF-α (800, 400, or 200 pg/ml) (all R&D Systems). In all experiments, mock virus supernatant or cRPMI medium was used as a negative control. The cultures were incubated for 24 to 48 h at 37°C in a 5% CO2 humidified atmosphere.
Assessment of cytokine production from DC, NK, and γδ T-cell cultures.
After 24 h of culture, the cells were resuspended, centrifuged at 930 × g for 2 min, and the supernatants were collected and stored at −80°C until analyzed. The quantitative simultaneous measurement of porcine IFN-α, IFN-γ, IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, and TNF-α in culture supernatants from mock- and CSFV-infected sorted mDC and pDC populations was performed using a multiplex ELISA (Cira porcine cytokine 1 array; Aushon, Billerica, MA, USA), according to the manufacturer's instructions. IL-12 was quantified from the culture supernatants from mock- and CSFV-infected sorted mDC and pDC populations using a commercial ELISA kit (Porcine IL-12 DuoSet; R&D Systems). Type I IFN activity was quantified from the culture supernatants from mock- and CSFV-infected sorted mDC and pDC populations, as described above, following the addition of dilutions of culture supernatants to reporter bovine kidney cells (MDBK-t2) for 24 h and an assessment of CAT expression by ELISA. IFN-γ was quantified in the supernatants from the NK and γδ T-cell cultures using a commercial ELISA kit (Porcine IFN-gamma Quantikine ELISA kit; R&D Systems).
Multiparameter cytofluorometric analysis of NK and γδ T cells.
To analyze NK and γδ T-cell populations from the in vitro experiments, cytometric staining was performed directly. For the analysis of NK and γδ T cells from CSFV vaccinated/challenged animals, cryopreserved retropharyngeal lymph node or tonsil cells were rapidly thawed in a 37°C water bath and washed in prewarmed cRPMI. The cell densities were determined as described above, adjusted to 1 × 107 cells/ml, and 100 μl was transferred to the wells of a 96-well round-bottom plate.
In both the in vitro and ex vivo experiments, the cells were harvested and washed in Dulbecco's PBS without Mg2+ and Ca2+ (Life Technologies). To assess viability, the cells were stained with LIVE/DEAD fixable near infrared viability dye (Life Technologies) for 30 min at 4°C and then washed twice with PBS supplemented with 2% FBS and 0.09% sodium azide (fluorescence-activated cell sorter [FACS] buffer). The cells were stained with the MAbs specific for surface markers for 10 min at RT and then washed twice with FACS buffer. The MAbs used were: MHC class II DR-fluorescein isothiocyanate (FITC) (clone 2E9/13; AbD Serotec), CD8α-PE (clone 76-2-11; BD Biosciences), CD4-PerCP-Cy5.5 (clone 74-12-4; BD Biosciences), CD3-APC (clone BB238E68C8; BD Biosciences), CD3 (clone BB238E6; Southern Biotech, Cambridge Bioscience, Cambridge, United Kingdom), and TcR1-N4 delta chain (clone PGBL22A; WSUMAC). Unconjugated CD3 and TcR1-N4 MAbs were labeled using the Zenon Alexa Fluor 647 and Alexa Fluor 405 mouse IgG1 labeling kits, respectively, according to the manufacturer's instructions (Life Technologies). Surface-stained cells were fixed using CellFIX (BD Biosciences) for 10 min at RT and then analyzed or were fixed and permeabilized using CytoFix/CytoPerm solution (BD Biosciences) for 20 min at 4°C. After two washes in BD Perm/Wash buffer (BD Biosciences), the cells were incubated with MAbs at RT for 10 min in the dark. The MAbs used for intracellular staining were anti-human perforin-FITC (clone δG9; BD Biosciences) and anti-CSFV E2 MAb (clone WH303; AHVLA Cell and Tissue Culture Unit). Staining with anti-CSFV E2 MAb was visualized by subsequent staining with rat anti-mouse IgG1-APC (clone X56; BD Biosciences). The cells were given two final washes in BD Perm/Wash buffer and resuspended in FACS buffer prior to flow cytometric analysis. The cells were analyzed by gating on viable cells (LIVE/DEAD fixable dead cell stain negative) in the lymphocyte population, and the defined lymphocyte subpopulations were then gated upon and their expression of MHC-II, perforin, and CSFV-E2 assessed. The irrelevant isotype control MAbs used to control staining with MHC-II, perforin, and CSFV E2 were FITC-IgG2b isotype control (AbD Serotec), FITC-IgG2b isotype control (27-35; BD Biosciences), and unconjugated IgG1 isotype control (AbD Serotec), respectively. All the washes used centrifugation at 930 × g for 2 min, and the cells were analyzed on MACSQuant Analyzer (Miltenyi Biotec) or CyAn ADP (Beckman Coulter) flow cytometers. To assess the potential effects of cryopreservation on the expression of MHC-II and perforin by NK and γδ T cells, PBMC were cultured in the presence or absence of IL-12/IL-18 prior to their cryopreservation. Flow cytometric analysis of the resuscitated cells demonstrated that previously activated NK and γδ T cells retained increased MHC-II and perforin expression compared to that of unstimulated cells (data not shown).
Data analysis and statistics.
Graphical and statistical analyses were performed using GraphPad Prism 5.04 (GraphPad Software, Inc., La Jolla, CA, USA). The data were represented as the mean with standard error of the mean (SEM). A two-tailed unpaired t test or a one-way analysis of variance (ANOVA), followed by a Bonferroni's posttest or Dunnett's test, was used, and a P value of <0.05 was considered statistically significant.
RESULTS
In vitro analysis of the interaction of NK and γδ T cells with CSFV.
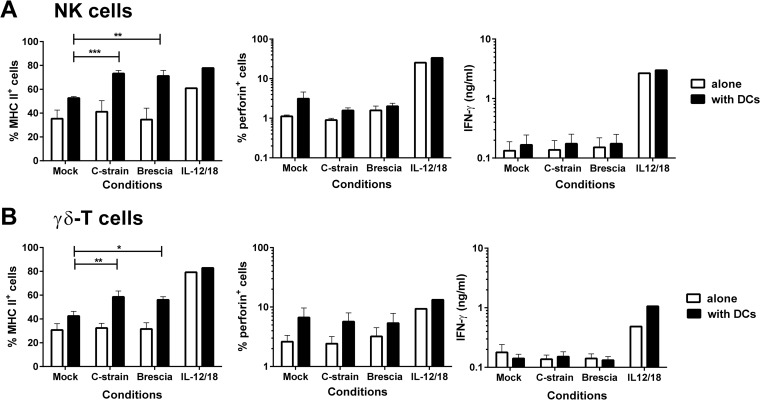
The direct effects of a live attenuated C-strain and the virulent Brescia strain of CSFV were assessed on NK and γδ T cells in vitro (Fig. 1). In contrast to stimulation with a combination of recombinant porcine IL-12 and IL-18, neither virus inoculum induced the activation of either cell type, as assessed by an increase in the proportion of cells expressing MHC-II or intracellular perforin or by the secretion of IFN-γ. Furthermore, it was found that both cell populations were resistant to in vitro infection by attenuated or virulent CSFV, as assessed by intracellular staining for the CSFV E2 protein (data not shown). To assess the indirect effects of CSFV on NK and γδ T cells, these cells were cocultured with CSFV and enriched blood DCs, which contained both pDCs and mDCs as a source of CSFV-susceptible antigen-presenting cells. Coculture with DCs and both C-strain and Brescia CSFV led to a significant increase in the proportions of both NK and γδ T cells expressing MHC-II but did not induce upregulation in perforin or stimulate an IFN-γ response.
FIG 1.
Analysis of the direct and indirect effects of CSFV on NK and γδ T cells. Enriched NK and γδ T cells were cocultured with an attenuated C-strain or virulent Brescia strain of CSFV, either alone or with enriched blood DCs, at a ratio of 2:1. A mock virus-infected cryolysate supernatant and a cocktail of recombinant porcine IL-12 and IL-18 were used as negative and positive controls, respectively. Twenty-four hours postculture, the percentages of MHC-II- and perforin-expressing cells were assessed on the NK cells (A) and γδ T cells (B) by flow cytometry, and the IFN-γ content of the culture supernatants was assessed by ELISA. The mean data ± SEM from three independent experiments utilizing different animals are shown. The values for each virus-stimulated condition were compared to the corresponding mock-stimulated control using a one-way ANOVA, followed by Dunnett's multiple comparison test: ***, P < 0.001; **, P < 0.01; *, P < 0.05.
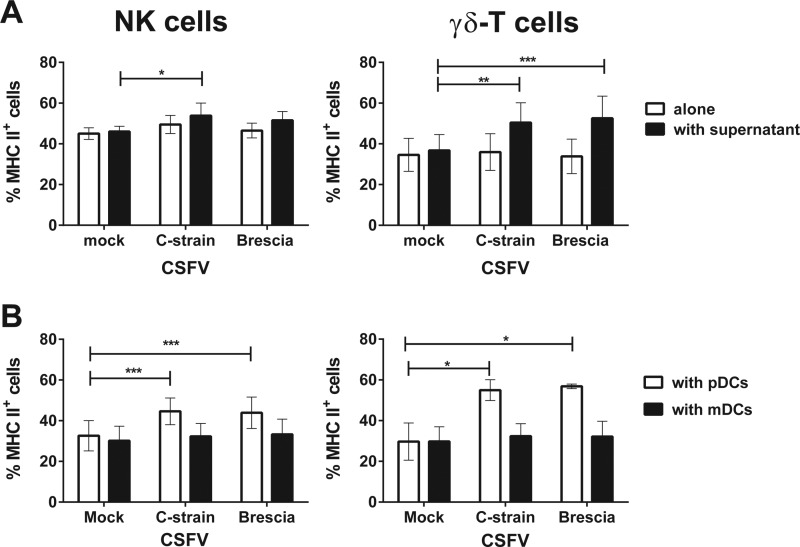
To determine whether MHC-II upregulation on NK and γδ T cells was due to soluble factors released by the DCs in response to CSFV infection, experiments were conducted using culture supernatants collected 24 h following infection of enriched blood DCs with Brescia or C-strain CSFV. The culture of NK and γδ T cells with the supernatants from DCs infected with either strain, but not the uninfected DC cultures, led to a significant increase in the proportion of cells expressing MHC-II (Fig. 2A). In order to establish whether mDCs or pDCs were responsible for this partial activation, NK and γδ T cells were cocultured with viruses in the presence of mDCs or pDCs (Fig. 2B). A significant increase in the cells expressing MHC-II was observed on the surface of both NK and γδ T cells when cultured with virus and pDCs, whereas culture with mDCs had no stimulatory effect.
FIG 2.
Effect of soluble factors released by CSFV-infected DCs on MHC class II expression by NK and γδ T cells, and an assessment of the cellular source. Enriched NK and γδ T cells were cocultured with attenuated C-strain or virulent Brescia strain CSFV or with culture supernatants from enriched blood DCs previously infected with the two strains (A). To determine whether pDCs or mDCs were responsible for this effect, enriched NK and γδ T cells were cocultured with attenuated C-strain or virulent Brescia strain CSFV, alone or together with sorted pDCs or mDCs, at a ratio 2:1 (B). A mock virus-infected cryolysate supernatant was used as a negative control. Twenty-four hours postculture, the expression of MHC-II was assessed on NK and γδ T cells by flow cytometry. The mean ± SEM data from three independent experiments utilizing different animals are shown. The values for each virus-stimulated condition were compared to the corresponding mock-stimulated control using a one-way ANOVA, followed by Dunnett's multiple comparison test: ***, P < 0.001; **, P < 0.01; *, P < 0.05.
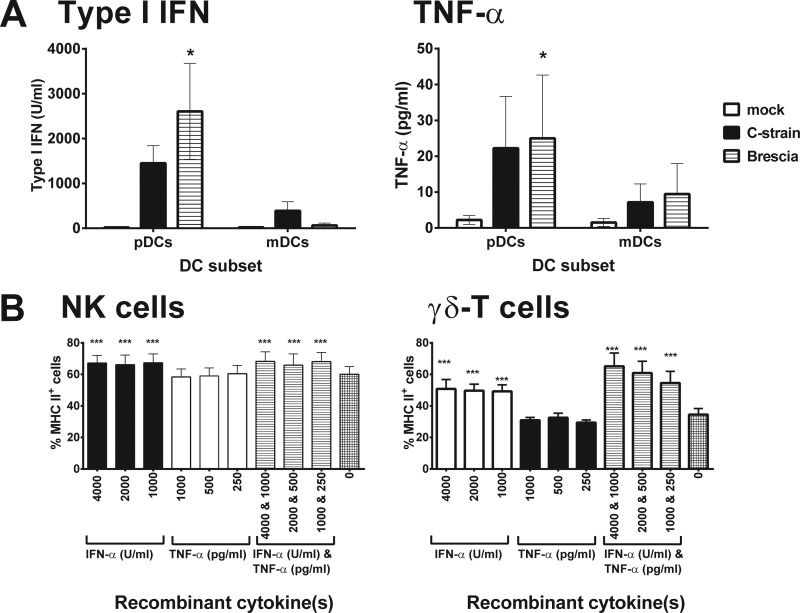
We next assessed the cytokine profiles of the supernatants from the cultures of CSFV-infected mDCs and pDCs in an attempt to identify differentially expressed cytokines that may be responsible for the upregulation of MHC-II. In response to both CSFV strains, no significant differences were observed in the levels of IL-1β, IL-2, IL-4, IL-6, IL-8, IL-10, IL-12, or IFN-γ detected in the supernatants of the mDC and pDC cultures (data not shown), while significantly higher levels of type I IFN and TNF-α were detected in the supernatants of virus-infected pDC cultures compared to those of mDCs (Fig. 3A). Enriched NK and γδ T cells were therefore cultured with recombinant porcine IFN-α and TNF-α at concentrations comparable to those detected in the infected pDC culture supernatants, either individually or in combination, and the effects on MHC-II expression were assessed (Fig. 3B). Stimulation with IFN-α but not TNF-α induced significant upregulation of MHC-II on the NK and γδ T cells, and there was evidence for further enhancement of MHC-II expression by γδ T cells when the two cytokines were combined. The lack of dose dependency in the observed responses may reflect that all doses of cytokines tested were at a saturating concentration.
FIG 3.
Investigation of CSFV-infected pDC-derived cytokines on the MHC class II expression of NK and γδ T cells. (A) pDCs or mDCs were exposed to CSFV C-strain, Brescia strain, or a mock virus-infected cryolysate supernatant, and after 24 h in culture, the levels of TNF-α and type I IFN in the supernatants were analyzed using a porcine cytokine multiplex ELISA and Mx/CAT reporter gene assay, respectively. (B) Enriched NK and γδ T cells were then cultured in cRPMI medium alone or in the presence of porcine recombinant IFN-α and/or TNF-α, at concentrations comparable to those detected in infected pDC cocultures, and the expression of MHC-II was assessed by flow cytometry after 24 h. The mean ± SEM data from three independent experiments utilizing different animals are shown. The values for each group were compared to the corresponding unstimulated control (mock stimulation in panel A and no cytokine treatment in panel B) using a one-way ANOVA, followed by Dunnett's multiple comparison test: ***, P < 0.001; **, P < 0.01; *, P < 0.05.
Ex vivo analysis of NK and γδ T cells isolated from the tonsils and retropharyngeal lymph nodes of pigs vaccinated and/or challenged with CSFV.
Ex vivo experiments were performed in the context of CSFV vaccination and/or challenge in order to complement the in vitro results. An experiment was conducted in which pigs were vaccinated with the C-strain virus and challenged 5 days later with the virulent strain Brescia. A challenge control group was included in the study. The vaccinated animals were solidly protected from challenge, while the unvaccinated pigs developed clinical signs of CSF, viremia, and leukopenia (33). We focused the analysis of the phenotypes of NK and γδ T cells in the tonsils and retropharyngeal lymph nodes, which are the primary sites of CSFV infection and thus where innate immunity might play an important role. The surface expression of MHC-II and the intracellular levels of perforin were assessed in the cells isolated from tissues collected on days −2, 0, 3, and 5 postchallenge. While no differences were observed in the proportions of NK and γδ T cells expressing perforin (data not shown), we observed differences between the groups in terms of upregulation of MHC-II (Fig. 4). Specifically, on day 0 postchallenge (day 5 postvaccination), there was some evidence of an upregulation of MHC-II on γδ T cells in the draining lymph nodes from intranasally vaccinated animals (Fig. 4B), although greater differences were observed 5 days postchallenge (Fig. 4B). At this time point, there was a significantly greater proportion of MHC-II expressing NK and γδ T cells in both the tonsils and retropharyngeal lymph nodes of the challenge control pigs compared to that of both the vaccinated and challenged pigs and those that were vaccinated but unchallenged. A comparison of the viral loads in these tissues confirmed that the challenge control pigs had significantly larger amounts of viral RNA present on day 5 postchallenge (Fig. 5). Concomitant with the increased viral loads, we also found that by day 5 postchallenge, the unvaccinated and challenged pigs showed increased numbers of IFN-α-expressing cells, primarily in the interfollicular areas of the tonsils (Fig. 6A), as well as high levels of type I IFN circulating in serum (Fig. 6B).
FIG 4.
Ex vivo analysis of the MHC class II expression on lymphoid tissue-derived NK and γδ T cells 5 days following C-strain vaccination and 5 days post-virulent CSFV challenge. The cells were isolated from tonsils and retropharyngeal lymph nodes (RPLN) 5 days after vaccination (day 0) and 5 days after Brescia challenge (day 5), and MHC-II expression on NK and γδ T cells was assessed using flow cytometry. (A) Gating strategy used to interrogate responses in live γδ T cells (CD3+ γδ-TCR+) and NK cells (CD3− CD8αlow). (B) Mean percentage of MHC-II-expressing NK and γδ T cells for each experimental group (group 1, C-strain vaccination [intranasal {i.n.}] and mock challenge; group 2, C-strain vaccination [intramuscular {i.m.}] and CSFV Brescia challenge; group 3, C-strain vaccination [i.n.] and CSFV Brescia challenge; group 4, mock vaccination and CSFV Brescia challenge), tissue, and time point. The data represent the mean of 3 pigs/group/time point ± SEM. Statistical analyses were performed using a one-way ANOVA, followed by Bonferroni's multiple comparison test: ***, P < 0.001; **, P < 0.01; *, P < 0.05.
FIG 5.
Quantification of CSFV loads in tonsils and retropharyngeal lymph nodes following C-strain vaccination and/or virulent Brescia challenge. The tonsils and retropharyngeal lymph nodes were collected longitudinally following C-strain vaccination and challenge 5 days later with CSFV Brescia strain. CSFV RNA loads were quantified in the tissues by qRT-PCR. The data represent the mean of 3 pigs/group ± SEM. Statistical analyses were performed using a one-way ANOVA, followed by Bonferroni's multiple comparison test: **, P < 0.01.
FIG 6.
Quantification of local and systemic type I IFN following C-strain vaccination and/or virulent CSFV Brescia challenge. Serum and tonsils were collected longitudinally following C-strain vaccination and challenge 5 days later with CSFV Brescia strain. Cells expressing IFN-α were enumerated in the interfollicular areas (closed symbols) and follicles (open symbols) of the tonsils by immunohistochemistry (A), and the levels of type I IFN in serum were determined using an Mx/CAT reporter gene assay (B). The data represent the mean of 3 pigs/group ± SEM. Statistical analyses were performed using a one-way ANOVA, followed by Bonferroni's multiple comparison test: ***, P < 0.001; *, P < 0.05.
DISCUSSION
This study aimed to provide insight into the potential roles of NK and γδ T cells in the immune response against CSFV, which may explain in part the capacity of live attenuated CSFV vaccines to provide protection within days of administration (7). Through a combination of in vitro experimentation and ex vivo analyses of the key lymphoid tissues, our data suggest that neither cell type appears to be appropriately activated for contribution to antiviral effector functions, but they are at least partially activated following stimulation with type I IFN produced by pDCs infected with CSFV.
Coculture of NK and γδ T cells with CSFV-infected pDCs, but not mDCs, led to an upregulation of MHC-II on the surface of these cells, which was associated with high levels of IFN-α release. In response to CSFV infection, the mDCs released significantly smaller amounts of IFN-α compared to that of pDCs, which is most likely due to the inhibition of the type I IFN induction cascade by CSFV Npro-induced proteasomal degradation of interferon regulatory factor 3 (IRF3) (36–38). By virtue of their utilization of IRF7 instead of IRF3, pDCs possess a functional type I IFN response to CSFV infection, although it was recently shown that Npro also interacts with IRF7 and reduces the capacity of pDCs to produce IFN-α (39). While IFN-α is the prototypic antiviral cytokine, it also plays an important immunoregulatory role, enhancing both innate and adaptive immune responses (40). Moreover, it is well known that IFN-α can upregulate MHC-II on professional APCs, although there are few reports demonstrating that it can induce expression on nonconventional APCs (41). For the first time, we report this effect of IFN-α on two nonconventional APCs, NK and γδ T cells, in pigs. Future work should address whether this effect is mediated specifically by particular porcine IFN-α subtypes or whether other type I IFNs may also play a role. Seventeen different porcine IFN-α subtypes have been defined (42). While only IFN-α1 is commercially available and is currently used as the basis for IFN-α ELISAs, commercial antibodies recognize ≥16 porcine IFN-α subtypes (Olubukola Soule, AHVLA, personal communication).
The results of the ex vivo analysis showed that the upregulation of MHC-II on tonsillar and lymph node resident NK and γδ T cells was associated with virulent Brescia infection and appeared to be inversely correlated to C-strain-induced protection. Indeed, the activated phenotype was observed in tissues with both the highest levels of CSFV infection and IFN-α-secreting cells, which was reflected in the elevated levels of circulating type I IFN. It is likely that virulent CSFV infection of pDCs is responsible for the type I IFN response, since it has been described that pDCs rapidly increase in numbers in the tonsils following CSFV infection accompanied with high levels of IFN-α (43). While there was little evidence for MHC-II upregulation associated with C-strain infection in vivo, our results did show a C-strain-mediated response in vitro. This may be explained by the more limited replication of C-strain in vivo and/or a failure of pDCs to traffic to the tonsils, resulting in the reduced type I IFN levels observed. Alternatively, it is possible that some form of immune regulation is induced by C-strain infection, such as IL-10 induction, which may inhibit the pDC-mediated type I IFN response (44). Moreover, it has recently been shown that the interaction of pDCs with CSFV-infected cells induces greater IFN-α production than that from direct infection of pDCs (45); therefore, the ability of virulent CSFV strains to rapidly replicate in macrophages and mDCs may potentiate the IFN-α response by pDCs in the lymphoid tissues. This exacerbated type I IFN response to CSFV mediated by pDCs has been hypothesized as a key driver of the pathogenesis of CSF, including the hallmark trait of leukopenia (46, 47). A novel subset of porcine NK cells expressing high levels of NKp46 and low levels of CD8α has recently been described in the spleen, with a highly activated status (18); future analyses should assess whether this population resides in other secondary lymphoid tissues, such as the tonsils, and determine if they show evidence of activation following infection with C-strain and/or virulent CSFV.
The expression of MHC-II on activated NK and γδ T cells raises the question of whether these cells acquire an antigen-presenting cell function in response to CSFV infection, but this is beyond the scope of the present study. A previous study reported that porcine γδ T cells were able to acquire a phenotype similar to that of professional APCs after vaccination against foot-and-mouth disease (FMD), and these cells were able to process and present soluble antigen to CD4+ T cells, likely modulating the outcome of the adaptive immune response (48). Human γδ T cells have also been described as gaining APC function following contact with microbes, enabling them to induce conventional CD4+ and CD8+ T-cell responses (30). It has been suggested that γδ T cells act as APCs only in the initiation of proinflammatory immune responses, and they are not able to substitute DCs in controlling self-tolerance and immune regulation (30). The ability of NK cells to express MHC-II after interaction with DCs has been described in mice. Nakayama et al. (49) observed that NK cell acquisition of MHC-II was mediated by intercellular membrane transfer from interacting DCs and that NK cells also acquired costimulatory molecules, such as CD80 and CD86, from DCs through a process of trogocytosis. However, in the present study, we observed that both the recombinant cytokines and supernatants derived from CSFV-infected pDC cultures induced the upregulation of MHC-II on NK cells, suggesting that this was a consequence of de novo expression of MHC-II by the stimulated cells rather than via “cross-dressing.” An assessment of MHC class II transcription levels following NK cell stimulation may help elucidate whether the observed upregulation of surface expression is due to trogocytosis. Interestingly, MHC-II-dressed NK cells have been shown to inhibit DC-induced CD4+ T-cell responses via competitive antigen presentation (49), and it remains to be determined whether the MHC-II porcine NK cells observed here inhibit or promote CSFV-specific T-cell activation.
Despite significant upregulation of MHC-II, the apparent lack of activation of NK and γδ T cells enabling the release of antiviral cytokines, like IFN-γ, or perforin-mediated cytotoxic function raises the possibility that CSFV actively inhibits this process. CSFV infection of blood-derived pDCs and mDCs induces the maturation of these cells, as assessed by an increase in costimulatory molecule expression (J. C. Edwards and S. P. Graham, unpublished data), and in addition to type I IFN, DCs secrete a range of other cytokines, such as IL-2 and IL-12, with the capacity to activate NK and γδ T cells. Studies of HCV, another member of the Flaviviridae family, have shown that several viral proteins, core, E2, and NS5A, can either directly or indirectly, in the case of NS5A, inhibit human NK cell activation (23–25). Since NK cells may also play an important role in the induction of T-cell responses via a reciprocal activating cross talk with DCs, these inhibitory mechanisms may offer the virus a significant advantage in evading immune responses at several levels. However, since we saw a lack of complete activation of NK cells with both C-strain and virulent CSFV, the upregulation of perforin, or the secretion of IFN-γ, it is unlikely that this has a negative impact on T-cell induction in vivo, since C-strain induces a virus-specific T-cell response that is undetectable in virulent-CSFV-infected animals (6, 7, 33, 50). In summary, CSFV induced a partial activation of NK and γδ T cells, as characterized by MHC-II upregulation, which appeared to be mediated by type I IFN produced by infected pDCs in vitro and was most clearly observed in the tissues with high viral loads and accompanying circulating type I IFN. The current data failed to show that these cells gained antiviral effector functions, and it therefore appears unlikely that either population contributes directly to the rapid protection conferred by C-strain CSFV. Further studies should assess cytokine and cytotoxic functions at later time points following interaction with infected DCs and determine whether MHC-II-bearing NK and γδ T cells can function as APCs or rather might inhibit DC-induced T-cell responses against CSFV.
ACKNOWLEDGMENTS
We thank Bentley Crudgington, Helen Mokhtar, and Olobukola Soule, AHVLA Virology Department, for technical assistance during the study; Derek Clifford and colleagues, AHVLA Animal Services Unit, for the care of animals and provision of samples; Phil Hogarth and colleagues, AHVLA Flow Cytometry Sorting Facility, for assistance with dendritic cell sorting; Sophie Morgan, AHVLA Virology Department, for assistance with the cytokine array; Julie Gough, AHVLA Specialist Scientific Support, for performing the IFN-α immunohistochemistry; Kirsten Morris, CSIRO Animal, Food & Health Sciences, Australian Animal Health Laboratory, Geelong, Australia, for the kind gift of recombinant porcine IL-3; and Falko Steinbach, AHVLA Virology Department, for critical review of the manuscript.
This work was supported by Project SE0796 from the Department for the Environment, Food, and Rural Affairs of the United Kingdom. N.V.K. was supported by a DBT CREST Award from the Ministry of Science & Technology, Government of India. P.J.S.-C. was supported by grants of the Ministry of Education, Spain (José Castillejo Program 2010–2012) and University of Córdoba, Spain (Research Promotion Program 2011–2013).
We declare no commercial or other associations that may pose a conflict of interest.
Footnotes
Published ahead of print 30 July 2014
REFERENCES
- 1.Moennig V. 2000. Introduction to classical swine fever: virus, disease and control policy. Vet. Microbiol. 73:93–102. 10.1016/S0378-1135(00)00137-1. [DOI] [PubMed] [Google Scholar]
- 2.van Oirschot JT. 2003. Emergency vaccination against classical swine fever. Dev. Biol. (Basel) 114:259–267. [PubMed] [Google Scholar]
- 3.Beer M, Reimann I, Hoffmann B, Depner K. 2007. Novel marker vaccines against classical swine fever. Vaccine 25:5665–5670. 10.1016/j.vaccine.2006.12.036. [DOI] [PubMed] [Google Scholar]
- 4.Dewulf J, Laevens H, Koenen F, Mintiens K, de Kruif A. 2004. Efficacy of E2-sub-unit marker and C-strain vaccines in reducing horizontal transmission of classical swine fever virus in weaner pigs. Prev. Vet. Med. 65:121–133. 10.1016/j.prevetmed.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Suradhat S, Sada W, Buranapradiktun S, Damrongwatanapokin S. 2005. The kinetics of cytokine production and CD25 expression by porcine lymphocyte subpopulations following exposure to classical swine fever virus (CSFV). Vet. Immunol. Immunopathol. 106:197–208. 10.1016/j.vetimm.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 6.Graham SP, Haines F, Johns H, Sosan O, La Rocca SA, Lamp B, Rümenapf T, Everett HE, Crooke HR. 2012. Characterisation of vaccine-induced, broadly cross-reactive IFN-γ secreting T cell responses that correlate with rapid protection against classical swine fever virus. Vaccine 30:2742–2748. 10.1016/j.vaccine.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 7.Graham SP, Everett HE, Haines FJ, Johns HL, Sosan OA, Salguero FJ, Clifford DJ, Steinbach F, Drew TW, Crooke HR. 2012. Challenge of pigs with classical swine fever viruses after C-strain vaccination reveals remarkably rapid protection and insights into early immunity. PLoS One 7:e29310. 10.1371/journal.pone.0029310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denyer MS, Wileman TE, Stirling CM, Zuber B, Takamatsu HH. 2006. Perforin expression can define CD8 positive lymphocyte subsets in pigs allowing phenotypic and functional analysis of natural killer, cytotoxic T, natural killer T and MHC un-restricted cytotoxic T-cells. Vet. Immunol. Immunopathol. 110:279–292. 10.1016/j.vetimm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Gerner W, Käser T, Saalmüller A. 2009. Porcine T lymphocytes and NK cells–an update. Dev. Comp. Immunol. 33:310–320. 10.1016/j.dci.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 10.Lanier L. 2005. NK cell recognition. Annu. Rev. Immunol. 23:225–274. 10.1146/annurev.immunol.23.021704.115526. [DOI] [PubMed] [Google Scholar]
- 11.Mandelboim O, Lieberman N, Lev M, Paul L, Arnon TI, Bushkin Y, Davis DM, Strominger JL, Yewdell JW, Porgador A. 2001. Recognition of haemagglutinins on virus-infected cells by NKp46 activates lysis by human NK cells. Nature 409:1055–1060. 10.1038/35059110. [DOI] [PubMed] [Google Scholar]
- 12.Smith HRC, Heusel JW, Mehta IK, Kim S, Dorner BG, Naidenko OV, Iizuka K, Furukawa H, Beckman DL, Pingel JT, Scalzo AA, Fremont DH, Yokoyama WM. 2002. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc. Natl. Acad. Sci. U. S. A. 99:8826–8831. 10.1073/pnas.092258599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Newman KC, Riley EM. 2007. Whatever turns you on: accessory-cell-dependent activation of NK cells by pathogens. Nat. Rev. Immunol. 7:279–291. 10.1038/nri2057. [DOI] [PubMed] [Google Scholar]
- 14.Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. 2002. Reciprocal activating interaction between natural killer cells and dendritic cells. J. Exp. Med. 195:327–333. 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pintaric M, Gerner W, Saalmüller A. 2008. Synergistic effects of IL-2, IL-12 and IL-18 on cytolytic activity, perforin expression and IFN-γ production of porcine natural killer cells. Vet. Immunol. Immunopathol. 121:68–82. 10.1016/j.vetimm.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 16.Toka FN, Nfon CK, Dawson H, Estes DM, Golde WT. 2009. Activation of porcine natural killer cells and lysis of foot-and-mouth disease virus infected cells. J. Interferon Cytokine Res. 29:179–192. 10.1089/jir.2008.0058. [DOI] [PubMed] [Google Scholar]
- 17.Mair KH, Essler SE, Patzl M, Storset AK, Saalmüller A, Gerner W. 2012. NKp46 expression discriminates porcine NK cells with different functional properties. Eur. J. Immunol. 42:1261–1271. 10.1002/eji.201141989. [DOI] [PubMed] [Google Scholar]
- 18.Mair KH, Müllebner A, Essler SE, Duvigneau JC, Storset AK, Saalmüller A, Gerner W. 2013. Porcine CD8αdim/−NKp46high NK cells are in a highly activated state. Vet. Res. 44:13. 10.1186/1297-9716-44-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Toka FN, Nfon C, Dawson H, Golde WT. 2009. Natural killer cell dysfunction during acute infection with foot-and-mouth disease virus. Clin. Vaccine Immunol. 6:1738–1749. 10.1128/CVI.00280-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Renukaradhya GJ, Alekseev K, Jung K, Fang Y, Saif LJ. 2010. Porcine reproductive and respiratory syndrome virus-induced immunosuppression exacerbates the inflammatory response to porcine respiratory coronavirus in pigs. Viral Immunol. 23:457–466. 10.1089/vim.2010.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manickam C, Dwivedi V, Patterson R, Papenfuss T, Renukaradhya GJ. 2013. Porcine reproductive and respiratory syndrome virus induces pronounced immune modulatory responses at mucosal tissues in the parental vaccine strain VR2332 infected pigs. Vet. Microbiol. 162:68–77. 10.1016/j.vetmic.2012.08.021. [DOI] [PubMed] [Google Scholar]
- 22.Pauly T, König M, Thiel HJ, Saalmüller A. 1998. Infection with classical swine fever virus: effects on phenotype and immune responsiveness of porcine T lymphocytes. J. Gen. Virol. 79:31–40. [DOI] [PubMed] [Google Scholar]
- 23.Herzer K, Falk C, Encke J, Eichhorst S, Ulsenheimer A, Seliger B, Krammer P. 2003. Upregulation of major histocompatibility complex class I on liver cells by hepatitis C virus core protein via p53 and TAP1 impairs natural killer cells cytotoxicity. J. Virol. 77:8299–8309. 10.1128/JVI.77.15.8299-8309.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crotta S, Stilla A, Wack A, D'Andrea A, Nuti S, D'Oro U, Mosca M, Filliponi F, Brunetto R, Bonino F, Abrignani S, Valiante N. 2002. Inhibition of natural killer cells through engagement of CD81 by the major hepatitis C virus envelope protein. J. Exp. Med. 195:35–41. 10.1084/jem.20011124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sène D, Levasseur F, Abel M, Lambert M, Camous X, Hernandez C, Pène V, Rosenberg AR, Jouvin-Marche E, Marche PN, Cacoub P, Caillat-Zucman S. 2010. Hepatitis C virus (HCV) evades NKG2D-dependent NK cell responses through NS5A-mediated imbalance of inflammatory cytokines. PLoS Pathog. 6:e1001184. 10.1371/journal.ppat.1001184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golden-Mason L, Rosen H. 2006. Natural killer cells: primary target for hepatitis C virus immune evasion strategies? Liver Transpl. 12:363–372. 10.1002/lt.20708. [DOI] [PubMed] [Google Scholar]
- 27.Bonneville M, O'Brien R, Born W. 2010. γδ T cell effector functions: a blend of innate programming and acquired plasticity. Nat. Rev. Immunol. 10:467–478. 10.1038/nri2781. [DOI] [PubMed] [Google Scholar]
- 28.Dieli F, Troye-Blomberg M, Farouk SE, Sireci G, Salerno A. 2001. Biology of gammadelta T cells in tuberculosis and malaria. Curr. Mol. Med. 1:437–446. 10.2174/1566524013363627. [DOI] [PubMed] [Google Scholar]
- 29.Devilder MC, Allain S, Dousset C, Bonneville M, Scotet E. 2009. Early triggering of exclusive IFN-γ responses of human V γ9Vδ2 T cells by TLR-activated myeloid and plasmacytoid dendritic cells. J. Immunol. 183:3625–3633. 10.4049/jimmunol.0901571. [DOI] [PubMed] [Google Scholar]
- 30.Brandes M, Willimann K, Moser B. 2005. Professional antigen-presentation function by human γδ-T cells. Science 309:264–268. 10.1126/science.1110267. [DOI] [PubMed] [Google Scholar]
- 31.Takamatsu HH, Denyer MS, Wileman TE. 2002. A sub-population of circulating porcine γδ T cells can act as professional antigen presenting cells. Vet. Immunol. Immunopathol. 87:223–224. 10.1016/S0165-2427(02)00083-1. [DOI] [PubMed] [Google Scholar]
- 32.Tseng CT, Miskovly E, Houghton M, Kimpel GR. 2001. Characterization of liver T-cell receptor γδ+ T cells obtained from individuals chronically infected with hepatitis C virus (HCV): evidence for these T cells playing a role in the liver pathology associated with HCV infections. Hepatology 33:1312–1320. 10.1053/jhep.2001.24269. [DOI] [PubMed] [Google Scholar]
- 33.Franzoni G, Kurkure NK, Edgar DS, Everett HE, Gerner W, Bodman-Smith KB, Crooke HR, Graham SP. 2013. Assessment of the phenotype and functionality of porcine CD8 T cell responses following vaccination with live attenuated classical swine fever virus (CSFV) and virulent CSFV challenge. Clin. Vaccine Immunol. 20:1604–1616. 10.1128/CVI.00415-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Everett H, Salguero FJ, Graham SP, Haines F, Johns H, Clifford D, Nunez A, La Rocca SA, Parchariyanon S, Steinbach F, Drew T, Crooke H. 2010. Characterisation of experimental infection of pigs with genotype 2.1 and 3.3 isolates of classical swine fever virus. Vet. Microbiol. 142:26–33. 10.1016/j.vetmic.2009.09.039. [DOI] [PubMed] [Google Scholar]
- 35.Fray MD, Mann GE, Charleston B. 2001. Validation of an Mx/CAT reporter gene assay for the quantification of bovine type-I interferon. J. Immunol. Methods 249:235–244. 10.1016/S0022-1759(00)00359-8. [DOI] [PubMed] [Google Scholar]
- 36.Bauhofer O, Summerfield A, Sakoda Y, Tratschin JD, Hofmann MA, Ruggli N. 2007. Classical swine fever virus Npro interacts with interferon regulatory factor 3 and induces its proteasomal degradation. J. Virol. 81:3087–3096. 10.1128/JVI.02032-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ruggli N, Bird BH, Liu L, Bauhofer O, Tratschin JD, Hofmann MA. 2005. N(pro) of classical swine fever virus is an antagonist of double-stranded RNA-mediated apoptosis and IFN-alpha/beta induction. Virology 340:265–276. 10.1016/j.virol.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 38.La Rocca SA, Herbert RJ, Crooke H, Drew TW, Wileman TE, Powell PP. 2005. Loss of interferon regulatory factor 3 in cells infected with classical swine fever virus involves the N-terminal protease, Npro. J. Virol. 79:7239–7247. 10.1128/JVI.79.11.7239-7247.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fiebach AR, Guzylack-Piriou L, Python S, Summerfield A, Ruggli N. 2008. Classical swine fever virus N(pro) limits type I interferon induction in plasmacytoid dendritic cells by interacting with interferon regulatory factor 7. J. Virol. 85:8002–8011. 10.1128/JVI.00330-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Colonna M, Trichieri G, Liu YJ. 2004. Plasmacytoid dendritic cells in immunity. Nat. Immunol. 5:1219–1226. 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 41.Bukowski JF, Welsh RM. 1986. Enhanced susceptibility to cytotoxic T lymphocytes of target cells isolated from virus-infected or interferon-treated mice. J. Virol. 59:735–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sang Y, Rowland RR, Hesse RA, Blecha F. 2010. Differential expression and activity of the porcine type I interferon family. Physiol. Genomics 42:248–258. 10.1152/physiolgenomics.00198.2009. [DOI] [PubMed] [Google Scholar]
- 43.Jamin A, Gorin S, Cariolet R, Le Potier MF, Kuntz-Simon G. 2008. Classical swine fever virus induces activation of plasmacytoid and conventional dendritic cells in tonsil, blood, and spleen of infected pigs. Vet. Res. 39:7. 10.1051/vetres:2007045. [DOI] [PubMed] [Google Scholar]
- 44.Ito S, Ansari P, Sakatsume M, Dickensheets H, Vazquez N, Donnelly RP, Larner AC, Finbloom DS. 1999. Interleukin-10 inhibits expression of both interferon alpha- and interferon gamma-induced genes by suppressing tyrosine phosphorylation of STAT1. Blood 93:1456–1463. [PubMed] [Google Scholar]
- 45.Python S, Gerber M, Suter R, Ruggli N, Summerfield A. 2013. Efficient sensing of infected cells in absence of virus particles by plasmacytoid dendritic cells is blocked by the viral ribonuclease E(rns). PLoS Pathog. 9:e1003412. 10.1371/journal.ppat.1003412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Summerfield A, Alves M, Ruggli N, de Bruin MG, McCullough KC. 2006. High IFN-alpha responses associated with depletion of lymphocytes and natural IFN-producing cells during classical swine fever. J. Interferon Cytokine Res. 26:248–255. 10.1089/jir.2006.26.248. [DOI] [PubMed] [Google Scholar]
- 47.Renson P, Blanchard Y, Le Dimna M, Felix H, Cariolet R, Jestin A, Le Potier MF. 2010. Acute induction of cell death-related IFN stimulated genes (ISG) differentiates highly from moderately virulent CSFV strains. Vet. Res. 41:7. 10.1051/vetres/2009055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takamatsu HH, Denyer MS, Stirling C, Cox S, Aggarwal N, Dash P, Wileman TE, Barnett PV. 2006. Porcine gammadelta T cells: possible roles on the innate and adaptive immune responses following virus infection. Vet. Immunol. Immunopathol. 112:49–61. 10.1016/j.vetimm.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 49.Nakayama M, Takeda K, Kawano M, Takal T, Ishil N, Ogasawara K. 2011. Natural killer (NK)-dendritic cell interactions generate MHC class II-dressed NK cells that regulate CD4+ T cells. Proc. Natl. Acad. Sci. U. S. A. 108:18360–18365. 10.1073/pnas.1110584108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Graham SP, Everett HE, Johns HL, Haines FJ, La Rocca SA, Khatri M, Wright IK, Drew T, Crooke HR. 2010. Characterisation of virus-specific peripheral blood cell cytokine responses following vaccination or infection with classical swine fever viruses. Vet. Microbiol. 142:34–40. 10.1016/j.vetmic.2009.09.040. [DOI] [PubMed] [Google Scholar]