Abstract
The risk of intrauterine transmission of cytomegalovirus (CMV) during pregnancy is much greater for women who contract primary CMV infection after conception than for women with evidence of infection (circulating CMV antibodies) before conception. Thus, laboratory tests that aid in the identification of recent primary CMV infection are important tools for managing the care of pregnant women suspected of having been exposed to CMV. CMV IgM detection is a sensitive marker of primary CMV infection, but its specificity is poor because CMV IgM is also produced during viral reactivation and persists following primary infection in some individuals. Studies conducted over the last 20 years convincingly demonstrate that measurement of CMV IgG avidity is both a sensitive and a specific method for identifying pregnant women with recent primary CMV infection and thus at increased risk for vertical CMV transmission. IgG avidity is defined as the strength with which IgG binds to antigenic epitopes expressed by a given protein; it matures gradually during the 6 months following primary infection. Low CMV IgG avidity is an accurate indicator of primary infection within the preceding 3 to 4 months, whereas high avidity excludes primary infection within the preceding 3 months. In this minireview, we summarize published data demonstrating the clinical utility of CMV IgG avidity results for estimating time since primary infection in pregnant women, describe commercially available CMV IgG avidity assays, and discuss some of the issues and controversies surrounding CMV IgG avidity testing during pregnancy.
INTRODUCTION
Congenital cytomegalovirus (CMV) infection is the most common intrauterine infection, occurring in approximately 40,000 newborns each year in the United States (1–4). The clinical manifestations include sensorineural hearing loss, visual impairment, mental retardation, and cognitive defects; 4% of infected infants do not survive (1, 2, 5, 6).
Several studies have demonstrated a strong link between primary CMV infection of the mother and in utero CMV transmission. The risk of congenital infection is approximately 40% in babies born to mothers who acquire a primary (initial) CMV infection after conception; in contrast, the risk is only about 1% in infants born to mothers who have evidence of CMV infection (i.e., circulating CMV antibodies) before conception (1, 3, 6–9). The few cases of CMV transmission in seropositive mothers reflect nonprimary CMV infections, defined as either viral reactivation or infection with a different strain of CMV during pregnancy (2, 3, 5). Preexisting maternal antibodies thus appear to offer substantial protection against congenital infection, most likely due to the ability of antibodies to control viremia (2, 9, 10).
The established link between primary CMV infection during pregnancy and congenital infection makes identification of primary CMV infection an important goal in maternal and neonatal health care. However, >95% of pregnant women with primary CMV infection are asymptomatic and thus cannot be diagnosed on clinical grounds (3, 11). The most straightforward indicator of primary CMV infection is documentation of seroconversion during pregnancy, but this approach is rarely effective due to the lack of preconception antibody screening programs allowing the identification of seronegative women (3, 9). Initial studies thus focused on detection of CMV IgM, due to its known utility as a transient marker of primary infection (3, 9, 12). These studies showed that CMV IgM detection is a sensitive marker for primary CMV infection, but its specificity is relatively poor; only about 50% of CMV IgM-positive individuals have primary infection (the reasons for this low specificity will be discussed later) (3, 5, 9, 13–15). These disappointing findings for CMV IgM led to a search for a different laboratory assay that could be used to identify primary CMV infection with high specificity, as well as sensitivity (2). Studies assessing CMV IgG avidity showed that low CMV IgG avidity is both a sensitive and a specific marker of primary CMV infection (1–3). Indeed, CMV IgG avidity is increasingly considered the “gold standard” for distinguishing primary from nonprimary CMV infection (1, 11, 12, 16) and is being used worldwide to identify primary CMV infection during pregnancy (14, 17–21).
This minireview focuses on 4 aspects of CMV IgG avidity testing: (a) the definition of avidity and the basic methodology used in initial studies linking CMV IgG avidity and primary infection; (b) a summary of results from the major studies demonstrating the utility of CMV IgG avidity assessment in pregnancy, including the advantages of avidity testing over CMV IgM testing alone; (c) a discussion of commercially available CMV IgG avidity assays, including newer automated assays; and (d) current issues and controversies in diagnosing primary CMV infections during pregnancy.
DEFINITION OF AVIDITY AND BASIC METHODOLOGY
Avidity is defined as the aggregate strength with which a mixture of polyclonal IgG molecules binds to multiple antigenic epitopes of proteins (12). It gradually matures over several months, reflective of antigen-driven selection of B cells producing IgG of increasing affinity (3, 11, 12). IgG antibodies produced during the first few months following primary infection exhibit low avidity (i.e., they bind weakly to the antigen), whereas antibodies produced by 6 months postinfection exhibit high avidity (i.e., they bind tightly to the antigen) (3, 11, 12).
The basic methodology used to measure avidity capitalizes on the weak binding of low-avidity IgG to a mixture of CMV antigens (typically viral lysate). Antigen-bound low-avidity IgG, but not high-avidity IgG, dissociates from the antigen in the presence of mild protein denaturants, such as urea, potassium thiocyanate, and guanidine chloride. The most common test format is an enzyme-linked immunosorbent assay (ELISA) utilizing urea as the dissociating agent (14, 21, 22). The patient's diluted serum is added to two microtiter wells coated with CMV antigen; after incubation, one well is washed with regular wash buffer, whereas the other well is washed with wash buffer containing urea. The ELISA is then finished per the routine procedure, and the optical density (OD) value of each well is measured. Results are expressed as an avidity index (AI), calculated using the formula AI = (OD of the urea-washed well/OD of the well washed with regular buffer) × 100, expressed as a percentage. The interpretive criteria for AI values must be established for each assay. The AI cutoff point for defining low avidity is typically 35% to 50%, whereas the AI cutoff point for defining high avidity is typically 50% to 65%. For example, in the assay used in our laboratory (22), low avidity is defined as an AI value of ≤50% and high avidity as an AI value of ≥60%; AI values of 51% to 59% are considered intermediate avidity.
STUDIES OF CMV IgG AVIDITY DURING PREGNANCY
The value of IgG avidity as a tool for discriminating recent from past viral infections was first recognized in the 1980s (23, 24). Its utilization in CMV infection was first reported in 1991 by Blackburn et al., who found low IgG avidity in CMV IgM-positive babies who were 7 to 12 months old (25). A connection between CMV IgG avidity and intrauterine infection was first reported in 1995, when Boppana and Britt used a radioimmunoassay to evaluate CMV IgG avidity in sera collected at delivery from mothers with primary CMV infection (defined as seroconversion or the presence of CMV IgG and IgM during the first trimester) (26). They found high avidity in 76% of nontransmitting mothers, compared to only 17% of transmitting mothers.
The core studies directly demonstrating the clinical utility of CMV IgG avidity for identifying pregnant women with primary infection were published between 1997 and 2002. These studies were conducted at 5 European medical centers, located in Brussels (Belgium), Paris (France), Stuttgart (Germany), Pavia (Italy), and Bologna (Italy). Although working independently and using different CMV IgG avidity assays, these 5 laboratories followed similar experimental protocols. CMV IgG avidity testing was performed on sera from 4 major patient groups: (i) a primary-infection group composed of patients with known primary CMV infection documented by seroconversion or virus isolation, (ii) a past-infection group composed of CMV IgG-positive and IgM-negative blood donors or pregnant women, (iii) a CMV reactivation group composed of women exhibiting a 4-fold increase in CMV IgG levels (with or without detectable CMV IgM) or CMV IgG-positive and IgM-positive patients known to be infected for more than a year, and (iv) an at-risk group composed of pregnant women whose first prenatal serum sample was CMV IgG positive and IgM positive, precluding determination of when primary infection occurred. When possible, pregnancy outcomes were monitored to identify congenital CMV transmission. The results generated at these 5 centers were remarkably consistent across sites; the major findings are discussed below.
CMV IgG avidity testing effectively distinguishes primary CMV infection from past CMV infection/reactivation.
All 5 laboratories found that the mean AI for the primary-infection group was significantly lower than the mean AIs for the past-infection and reactivation groups (9, 27–30). Those laboratories defining low or high avidity on the basis of the AI value found that >85% of patients with primary infection exhibited low IgG avidity and that >90% of patients with past infection or reactivation exhibited high avidity (27–29).
Maturation data indicate that CMV IgG avidity can be useful in determining time since infection.
Four laboratories, capitalizing on the availability of serial samples from patients with primary infection, tracked the maturation of CMV IgG avidity over time. All 4 laboratories reported a gradual maturation of avidity, with full maturation to high avidity accomplished by 5 to 6 months postinfection (3, 9, 28–31). Most pregnant women with primary infection showed low avidity for 3 to 4 months postinfection, with AI values then moving into the intermediate range for 1 to 2 months before reaching high AI levels. Thus, a high-avidity result during the first trimester is a strong indicator that infection occurred more than 5 months earlier, before conception. However, a low-avidity result during the third trimester strongly suggests that infection occurred within the prior 4 months, after conception.
CMV IgG avidity is an effective tool for assessing risk of transmission.
Three laboratories followed pregnancy outcomes to identify cases of congenital CMV infection; all found a strong association between low CMV IgG avidity during pregnancy and increased risk of in utero transmission (27, 31–34). The Paris and Bologna groups reported that all women who had CMV-infected offspring exhibited low IgG avidity (27, 28, 33). Similarly, one paper from the Brussels group reported that 89% of transmitting women exhibited low IgG avidity (34); in another report (31), however, they found that only 50% (7/14) of transmitting women had low IgG avidity (2 had intermediate avidity, 5 had high avidity). Further analysis revealed that the gestational age was >4 months in the 5 transmitting women with high IgG avidity. When evaluating the data from a different angle, namely, transmission rates in relation to avidity results, the combined data from Bologna and Brussels showed that 32% of women with a low AI value transmitted CMV to their offspring; in contrast, none of the women with high avidity during the first trimester transmitted CMV infection (31, 33, 34). As emphasized by the authors (31–35), these findings have significant implications for counseling pregnant women. Patients with high CMV IgG avidity during the first trimester can be assured that the risk of giving birth to an infected infant is low, and invasive procedures to identify fetal infection (e.g., collection of amniotic fluid for viral culture and CMV DNA detection [36]) are not needed. In contrast, women initially tested during the second or third trimester and exhibiting high avidity should be monitored for signs of intrauterine transmission, since primary infection after conception cannot be ruled out. Women with low avidity, regardless of trimester, should be considered candidates for further testing to assess fetal infection status (35).
CMV IgG avidity is better than CMV IgM for identifying primary CMV infection.
As mentioned earlier, CMV IgM detection has high sensitivity but low specificity for identifying primary CMV infection. One reason for poor specificity is that IgM can be produced during viral reactivation or reinfection with a different CMV strain (13, 27–29). Another reason is the long-term IgM persistence following primary infection in some individuals; about 25% of patients with primary CMV infection still have detectable IgM 4 months after infection, with IgM sometimes persisting for over a year (3, 37–39). This information, in conjunction with the CMV avidity maturation timeline discussed earlier, suggests that patients with IgM persistence will exhibit an IgM-positive but high-CMV-avidity result set if tested more than 5 to 6 months postinfection. This result pattern is common among patients being evaluated for possible primary CMV infection; core study groups found that about 50% of CMV IgM-positive at-risk patients had high CMV IgG avidity (27, 28, 33), a percentage confirmed by other investigators (13, 15, 22). Thus, some pregnant women with an IgM-positive/high-IgG-avidity result set may actually have nonrecent primary CMV infection in a setting of IgM persistence, rather than viral reactivation/reinfection. In any case, the high-IgG-avidity result indicates a low risk of vertical CMV transmission, provided that the testing occurred in the first trimester. IgM testing alone would incorrectly classify these women as having increased risk for intrauterine CMV transmission.
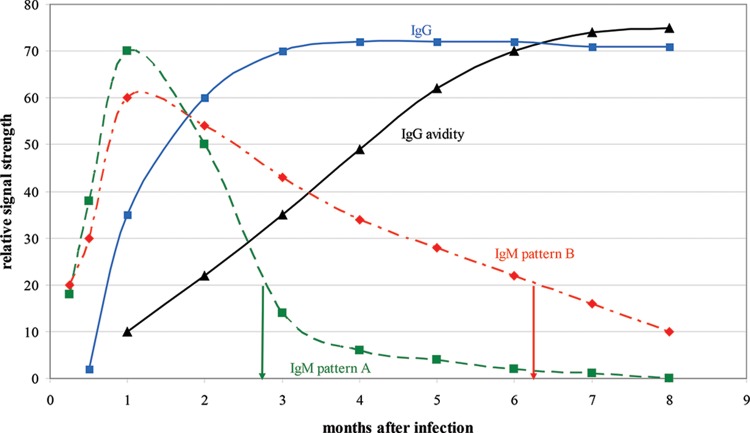
The following case illustrates the superiority of CMV IgG avidity testing for identifying primary CMV infection. The patient is a 32-year-old pregnant female whose first prenatal serum sample, collected at 12 weeks of gestation, was positive for CMV IgG and IgM. The IgG result was strongly positive, with a relative signal strength (RSS) of >60 (reference range, <10), and the IgM result was moderately positive, with an RSS of 20 (reference range, <5). As shown in Fig. 1, depending on whether the patient's IgM reversion pattern follows the common pattern (pattern A) or the persistence pattern (pattern B), an IgM-positive result of 20 may indicate two very different lengths of time since primary infection. If the pattern is A, the IgM result suggests primary infection within the last 3 months (i.e., postconception) and thus an increased risk for intrauterine CMV transmission. Alternatively, if the pattern is B (persistence pattern), the IgM result suggests that primary infection occurred about 6 months earlier (preconception) and thus the risk for intrauterine transmission is low. Of course, it is impossible to know which IgM seroreversion pattern the patient exhibits and thus impossible to determine the time since CMV infection using the IgM result. In contrast, CMV IgG avidity testing is an excellent tool for distinguishing between the two possible primary infection times. A low-avidity result (RSS, <50) supports infection within the prior 3 months and increased risk of transmission, whereas a high-avidity result (RSS, >60) indicates infection before conception and a very low risk of transmission. When the CMV IgG avidity assay was performed, an RSS of 70 (high avidity) was obtained, and the patient was assured that the CMV transmission risk was low.
FIG 1.
Relative changes in CMV IgM, IgG, and IgG avidity levels over time following primary CMV infection. IgM pattern A represents the typical IgM response pattern, whereas IgM pattern B represents long-term IgM persistence. In a CMV IgG-positive individual, an IgM-positive result of 20 indicates infection around 3 months previously if the individual exhibits IgM pattern A but around 6 months previously if the individual exhibits IgM pattern B. By employing CMV IgG avidity testing, the correct time since infection can be determined; a low-avidity result (expected to be about 30 based on this figure) indicates primary infection about 3 months previously, whereas a high-avidity result (expected to be about 70) indicates primary infection more than 6 months previously.
An exciting advancement in the clinical application of CMV IgG avidity results was reported recently by the Pavia group (40). They retrospectively identified pregnant women with primary CMV infection who had at least two archived sera available: one collected 0 to 3 months after infection (median, 1 month) and another collected >3 months after infection (median, 4.5 months). The two samples (referred to as T1 and T2, respectively) were retrieved and tested in the same CMV IgG avidity run. This avidity assay employed CMV nuclear antigen (rather than viral lysate); levels of IgG antibodies binding to nuclear antigen correlate with viral replication (40). When the OD values used to calculate the AI for T1 and T2 were compared, 77% of the women exhibited one of two distinctive avidity maturation patterns; exemplary OD and AI values (created by the authors of this minireview) for these two patterns are shown in Table 1. Characteristics of pattern 1 were that (i) the OD value following treatment with urea-containing buffer was markedly higher (>4-fold in this example) for T2 than for T1 and (ii) the OD values following treatment with control buffer (without urea) were quite similar for T2 and T1. In contrast, characteristics of pattern 2 were that (i) the urea buffer OD value was only slightly higher for T2 than for T1 and (ii) the control buffer OD value was markedly lower for T2 than for T1. IgG antibodies remaining on the plate after it was washed with urea buffer represent high-avidity antibodies; thus, pattern 1 exhibited a profound increase in high-avidity IgG between the two time points, whereas pattern 2 did not. Comparison of additional parameters in these two pattern groups revealed that the average load of CMV DNA in T1 was significantly higher in the pattern 1 group than in the pattern 2 group. The most significant finding, however, was a fetal infection rate of 63% (15 of 24 patients) in the pattern 1 group versus only 24% (7 of 29 patients) in the pattern 2 group. The authors hypothesized that the higher viral load in pattern 1 patients not only increases the odds of vertical transmission but also potently drives avidity maturation (40); stated a different way, pattern 1 appears to be a surrogate marker for a T1 viral load high enough to have a strong potential for vertical transmission. The lower control buffer OD in T2 than in T1 in pattern 2 apparently reflects lower levels of antibodies directed to CMV nuclear antigen, indicative of more-rapid viral clearance (40). Although further studies are required to determine if these distinctive avidity maturation patterns are detected using assays that include nonnuclear antigens, this analytical approach offers great promise for making CMV IgG avidity testing an even more powerful tool for identifying women with the highest risk of vertically transmitting CMV infection.
TABLE 1.
Examples for two distinctive CMV IgG avidity maturation patterns observed when serial samples from pregnant women with primary CMV infection were compareda
| Pattern | Sample time point | Urea buffer OD | Control buffer OD | Avidity index (%) | Rate of vertical transmission (%) |
|---|---|---|---|---|---|
| 1 | T1 | 0.25 | 1.70 | 15 | 63 |
| T2 | 1.10 | 1.85 | 59 | ||
| T2/T1 ratio | 4.40 | 1.09 | |||
| 2 | T1 | 0.45 | 1.75 | 26 | 24 |
| T2 | 0.55 | 1.00 | 55 | ||
| T2/T1 ratio | 1.22 | 0.57 |
See reference 40.
COMMERCIALLY AVAILABLE CMV IgG AVIDITY ASSAYS
All 5 European laboratories conducting the core studies described in the previous section utilized CMV IgG avidity ELISAs employing urea as the dissociating agent. The Bologna group used a commercially available CMV IgG avidity kit that employs 4.5 M urea, and the Pavia group used an in-house assay employing 6 M urea. The other 3 groups modified a CMV IgG kit to include a urea buffer wash step; the Brussels and Paris groups employed 8 M urea, whereas the Stuttgart group used 6 M urea. Other investigators have taken the latter approach of modifying CMV IgG kits from different manufacturers and typically employ 6 M urea (16, 17, 20, 22, 41, 42).
Table 2 lists the currently available CMV IgG avidity assays known to the authors. The list includes 7 ELISAs, an immunoblot assay, and 4 automated assays based on fluorescence or chemiluminescence; 11 of these 12 assays utilize a dissociating buffer, whereas 1 (Abbott Architect) uses a proprietary soluble CMV antigen reagent to block the binding of high-avidity IgG antibodies to CMV antigen covalently linked to the solid phase (43). Some of the ELISA kits and the immunoblot kit can be purchased in the United States; however, none are cleared by the U.S. Food and Drug Administration. Unfortunately, none of the automated assays can be purchased in the United States.
TABLE 2.
Commercially available CMV IgG avidity kitsa
| Manufacturer (test name) | Method | Dissociating agent | Low-avidity scoreb | High-avidity scoreb | Can be purchased in USA? |
|---|---|---|---|---|---|
| Bio-Rad | ELISA | Urea | <40 | >55 | No |
| Diesse | ELISA | Urea | <30 | >40 | Yes |
| Euroimmun | ELISA | Urea | <40 | >60 | Yes |
| Radim | ELISA | Urea | <35 | >45 | Yes |
| Technogenetics | ELISA | Potassium thiocyanate | <25 | >45 | No |
| Vidia | ELISA | Urea | <40 | >60 | Yes |
| Virion/Serion | ELISA | Urea | <45 | >55 | Yes |
| Mikrogen | IBL | Urea | NA | NA | Yes |
| bioMérieux (Vidas) | ELFA | Urea | <40 | >65 | No |
| DiaSorin (Liaison) | CIA | Urea | <20 | >30 | No |
| Roche (Elecsys) | V-CIA | Guanidine chloride | <45 | >55 | No |
| Abbott (Architect) | CMIA | Nonec | <50 | >60 | No |
Abbreviations: ELISA, enzyme-linked immunosorbent assay; IBL, immunoblotting; ELFA, enzyme-linked fluorescence assay; CIA, chemiluminescent immunoassay; V-CIA, voltage-induced chemiluminescent assay; CMIA, chemiluminescent microparticle immunoassay; NA, not applicable.
Avidity index values are formatted as percentages for consistency.
The assay utilizes CMV soluble antigen that blocks attachment of high-avidity IgG to CMV-coated microparticles.
The immunoblot assay, relatively new to the market, utilizes urea as the dissociating agent and requires a scanner to assess band intensity. Low avidity is defined as a more than 50% reduction in band intensity for 2 of 3 CMV antigens (IE1, p150, CM2) when urea-treated blot strips are compared to untreated strips (44). Two studies evaluating the immunoblot came to similar conclusions (44, 45); although the immunoblot assay correctly identified most primary and past CMV infections, it showed no significant benefit in performance or utility over conventional CMV IgG avidity assays.
The Vidas assay was the first automated assay to appear on the market, with a recommended AI diagnostic threshold of 80%. The Brussels and Paris groups evaluated this assay in 2001 and published essentially identical findings (46, 47). All patients with primary infection within the previous 3 months exhibited Vidas AI values of <80%, but only about 80% of patients with past infection exhibited AI values of >80%. Both groups reported that decreasing the AI diagnostic threshold from 80% to 70% had only a minor negative effective on the assay's ability to correctly identify primary infection (it decreased from 100% to >96%) but markedly improved its ability to correctly identify past infection (it increased from 80% to >95%) (46, 47). Other investigators came to this same conclusion (15, 48). In 2013, the Paris group conducted a systematic study of Vidas AI values using well-defined sera (49); they suggested that an AI of <40% be defined as low avidity and that an AI of >65% be defined as high avidity. These interpretive criteria have now been adopted by the manufacturer (bioMérieux).
The Liaison assay (DiaSorin) appeared soon after the Vidas assay, and evaluation data were published by the Pavia group in 2004 (50). At the manufacturer's suggested diagnostic AI threshold of 20%, the Liaison assay correctly identified 93% of primary infections and 85% of past infections. However, if the AI threshold was increased to 30%, the assay identified 100% of primary infections without affecting identifications of past infections.
The Elecsys assay (Roche Diagnostics) was recently the subject of a multicenter study conducted by the Paris, Pavia, and Bologna groups (51). The manufacturer's suggested interpretive criteria (an AI of <45% indicates low avidity, and an AI of ≥55% indicates high avidity) were used to evaluate the results. The combined findings from the 3 laboratories indicated that the assay correctly identified 95% of primary infections and 92% of past infections.
The Abbott Architect assay represents a departure from the conventional use of a dissociating agent to mediate the release of low-avidity IgG molecules from the solid phase. Rather, attachment of high-avidity antibodies to the solid phase is blocked by a proprietary soluble CMV antigen reagent. The manufacturer developed this alternative assay to avoid the potential detrimental effects of dissociating agents on the complex fluidic systems of the Architect analyzer (43). The calculation of AI was modified so that the numerical results are similar to those generated in conventional CMV IgG avidity assays. Two groups evaluated this assay in 2009, and both documented excellent performance (43, 52); 100% of samples from primary CMV infections exhibited low avidity, and 98% of samples from past CMV infections exhibited high avidity.
A recent report indicated that, in rare instances, avidity assays employing dissociating agents generate falsely low CMV IgG avidity results, as demonstrated by the lack of avidity maturation in a second sample collected a year later (53). When 4 such samples were tested in the Architect CMV IgG avidity assay, all exhibited high avidity (53), leading the authors to suggest that this alternative method offers an advantage over conventional assays. On the other hand, another recent report described exactly the opposite phenomenon (54); consecutive sera from 2 pregnant women exhibiting low, unchanging, CMV IgG avidity in the Architect assay exhibited high avidity in the Liaison assay. Luckily, problem samples such as these appear to be rare, but their existence supports the laboratorians' mantra, “remember that no test is perfect!”
Direct comparison of multiple CMV IgG avidity assays has been undertaken by 2 groups. A Spanish group compared 3 ELISAs (Bio-Rad, Euroimmun, Radim) and the Liaison assay (55) and concluded that all 4 assays were adequate laboratory tools for evaluating patients with suspected primary CMV infection. The Euroimmun and Liaison assays were a bit better for detecting primary infection, whereas the Bio-Rad and Radim assays were a bit better for excluding primary infection. A larger study was undertaken by the Pavia group, which compared 5 ELISAs and 3 automated assays (56). Their results revealed much less consistency than was observed in the Spanish study; none of the samples yielded identical scores by all 8 assays, and only 65% of sera exhibited the same qualitative IgG avidity result by at least 5 assays. However, receiver operator characteristic curve analysis of individual assay performance showed that all 8 assays performed reasonably well for diagnosing recent infection and that 7 of 8 assays performed reasonably well for diagnosing nonrecent infection. The authors concluded that, because CMV IgG avidity results influence decisions about performing invasive tests to detect fetal infection and possible termination of pregnancy, there is an immediate need for CMV IgG avidity kits to be improved and standardized.
ISSUES AND CONTROVERSIES
How should intermediate CMV IgG avidity results be used?
The major applications of CMV IgG avidity results as gleaned from the core publications are based on either low avidity or high avidity; low avidity indicates an increased risk of intrauterine transmission, whereas high avidity during the first trimester indicates a low risk of intrauterine transmission (27, 31, 34). Intermediate IgG avidity results were considered difficult to interpret for the purpose of risk assessment (3, 28, 30), yet a remarkably consistent finding in the literature is that 10% to 20% of patients within an at-risk group (CMV IgG positive and IgM positive but an unknown time of infection) exhibit intermediate avidity (13–15, 28, 31, 32, 34, 57–59). How should these women be counseled with respect to risk for intrauterine transmission and the need for invasive procedures to identify fetal infection? Are intermediate results comparable to low-avidity results or high-avidity results? The Bologna group analyzed the proportion of mothers transmitting the infection in relation to their CMV IgG avidity result and found transmission rates of 30% in the low-avidity group, 4% in the intermediate-avidity group, and 2% in the high-avidity group (58). Very similar transmission percentages were subsequently reported by another group (15). These findings thus suggested that, from a counseling standpoint, an intermediate-avidity result was more like a high-avidity result. A later study by the Bologna group (60) took the analysis a step further by also considering the trimester of pregnancy; 23% of CMV IgG-positive and IgM-positive women with intermediate CMV IgG avidity in the second trimester transmitted the infection to their offspring, compared to only 3% of IgG-positive and IgM-positive women with intermediate avidity in the first trimester. The authors concluded that intermediate CMV IgG avidity is a reliable marker of recent primary CMV infection in women initially tested in the second or third trimester. Stated a different way, from the standpoint of counseling, an intermediate-avidity result in the first trimester is comparable to a high-avidity result, whereas an intermediate-avidity result in the second or third trimester is comparable to a low-avidity result.
Should CMV IgG avidity be determined only for samples positive for CMV IgM?
Many laboratories using CMV IgG avidity to discriminate primary from nonprimary CMV infection follow a reflexive algorithm whereby only CMV IgG-positive samples that are also CMV IgM positive are tested for CMV IgG avidity (6, 17, 18, 31, 34, 48, 58); yet, investigators that have tested all CMV IgG-positive samples for avidity, regardless of the CMV IgM result, have consistently found that 1% to 3% of IgG-positive, IgM-negative samples exhibit low IgG avidity (16, 48, 57), suggesting an increased risk of intrauterine transmission. Indeed, transmission has been documented in patients who lacked CMV IgM but exhibited low IgG avidity (14, 61); it is likely that these women were in the narrow time window when IgM reversion has occurred but avidity is still low (3, 28–31). A reflexive algorithm requiring IgG avidity testing only on IgM-positive samples will thus miss a small number of primary infections. For this reason, some investigators recommend that all sera first be tested for CMV IgG and IgM and that all IgG-positive samples then be tested for CMV IgG avidity, regardless of the IgM result (5, 36, 50, 57). In addition to identifying IgM-negative patients with low IgG avidity, this approach also detects the small number of patients with an IgG-negative, IgM-positive result set, indicative of very recent CMV infection; seroconversion should be documented to ensure that the IgM result is a true positive. If budgetary and staffing issues require a laboratory to employ an algorithm whereby IgG avidity is performed only if the IgM result is equivocal or positive, the CMV IgM assay utilized should have high sensitivity in order to maximize the likelihood of detecting low CMV IgG avidity (21, 61–64).
Should all pregnant women be screened for evidence of primary CMV infection?
Screening of pregnant women for CMV antibodies is currently not recommended by any national government. The reasons cited include unjustified pregnancy termination due to patient anxiety, a lack of interventions proven to reduce or prevent transmission even if primary infection is identified, and the inability of screening programs to identify the small percentage of women who have CMV antibodies before conception but nevertheless transmit CMV to their offspring (1, 2, 65). However, in light of the convincing data demonstrating the clinical utility of CMV IgG avidity testing for assessing the risk of intrauterine CMV transmission, there has been renewed discussion of the benefits of screening pregnant women for CMV antibodies (3, 9, 60). If CMV IgG and IgM are measured during the first 2 months of gestation, the results enable specific counseling messages. (i) An IgG-positive, IgM-negative result at this early gestational age nearly always indicates past infection, with no need for further action; however, IgG avidity testing may be recommended if it is clinically warranted. (ii) An IgG-positive, IgM-positive result suggests recent primary infection and should trigger CMV IgG avidity testing. High or intermediate avidity indicates a low risk of congenital infection, whereas low avidity indicates an increased risk. If the result indicates low avidity, the patient should be counseled that vertical transmission is not a foregone conclusion, and additional procedures can be employed to identify fetal infection. (iii) An IgG-negative, IgM-positive result suggests very recent CMV infection and an increased risk of vertical transmission. The patient should be followed to document seroconversion and counseled the same as the patient with an IgG-positive, IgM-positive result. (iv) An IgG-negative, IgM-negative result indicates a risk for primary CMV infection, and the patient should be counseled to take measures to reduce the chance of infection, such as avoidance of direct contact with body fluids from other individuals (particularly preschool children) and frequent hand washing (1, 6, 66). These counseling messages are consistent with recommendations published by professional societies in Spain and Canada (67, 68).
CONCLUSIONS
CMV IgG avidity testing is now a proven, valuable laboratory tool for diagnosing primary CMV infection during pregnancy. Low avidity indicates primary infection within the preceding 3 to 4 months, with an increased risk of intrauterine transmission to the fetus/newborn. High avidity during the first trimester excludes postconception primary infection and indicates a low risk of intrauterine transmission. Although not quite as powerful as a high-avidity result, an intermediate-avidity result during the first trimester also indicates a low risk of intrauterine transmission. In contrast, an intermediate-avidity or high-avidity result during the second or third trimester does not rule out postconception primary infection and is associated with increased risk of transmission. The vast majority of patients with primary CMV infection exhibit both low CMV IgG avidity and detectable CMV IgM, but rarely is only one of these abnormal results present; thus, maximum detection of primary CMV infections, particularly when the first sample is collected after the first trimester, requires that both CMV IgG avidity and CMV IgM avidity testing be performed on CMV IgG-positive samples. Several diagnostic companies now offer CMV IgG avidity kits/assays, but many are not yet available for purchase worldwide. Manufacturers are encouraged to seek solutions to distribution and regulatory issues that currently block the widespread availability of their products, thus enabling the global application of CMV IgG avidity testing as a tool for assessing CMV transmission risk during pregnancy.
Footnotes
Published ahead of print 27 August 2014
REFERENCES
- 1.Coll O, Benoist G, Ville Y, Weisman LE, Botet F, the WAMP Infections Working Group. Anceschi MM, Greenough A, Gibbs RS, Carbonell-Estrany X. 2009. Guidelines on CMV congenital infection. J. Perinat. Med. 37:433–445. 10.1515/JPM.2009.127. [DOI] [PubMed] [Google Scholar]
- 2.Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK. 2013. The “silent” global burden of congenital cytomegalovirus. Clin. Microbiol. Rev. 26:86–102. 10.1128/CMR.00062-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Revello MG, Gerna G. 2002. Diagnosis and management of human cytomegalovirus infection in the mother, fetus, and newborn infant. Clin. Microbiol. Rev. 15:680–715. 10.1128/CMR.15.4.680-715.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baer HR, McBride HE, Caviness AC, Demmler-Harrison GJ. 2014. Survey of congenital cytomegalovirus (cCMV) knowledge among medical students. J. Clin. Virol. 60:222–242. 10.1016/j.jcv.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Revello MG, Fabbri E, Furione M, Zavattoni M, Lilleri D, Tassis B, Quarenghi A, Cena C, Arossa A, Montanari L, Rognoni V, Spinillo A, Gerna G. 2011. Role of prenatal diagnosis and counseling in the management of 735 pregnancies complicated by primary human cytomegalovirus infection: a 20-year experience. J. Clin. Virol. 50:303–307. 10.1016/j.jcv.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Guerra B, Simonazzi G, Banfi A, Lazzarotto T, Farina A, Lanari M, Rizzo N. 2007. Impact of diagnostic and confirmatory tests and prenatal counseling on the rate of pregnancy termination among women with seropositive cytomegalovirus immunoglobulin M antibody titers. Am. J. Obstet. Gynecol. 221:e1–e6. 10.1016/j.ajog.2006.08.039. [DOI] [PubMed] [Google Scholar]
- 7.Fowler KB, Stagno S, Pass RF, Britt WJ, Boll Alford TJCA. 1992. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N. Engl. J. Med. 326:663–667. 10.1056/NEJM199203053261003. [DOI] [PubMed] [Google Scholar]
- 8.Stagno S, Pass RF, Cloud G, Britt WJ, Henderson RE, Walton PD, Veren DA, Page F, Alford CA. 1986. Primary cytomegalovirus infection in pregnancy. Incidence, transmission to fetus, and clinical outcome. JAMA 256:1904–1908. [PubMed] [Google Scholar]
- 9.Revello MG, Gerna G. 1999. Diagnosis and implications of human cytomegalovirus infection in pregnancy. Fetal Matern. Med. Rev. 11:117–134. 10.1017/S0965539599000327. [DOI] [Google Scholar]
- 10.Revello MG, Gerna G. 2004. Pathogenesis and prenatal diagnosis of human cytomegalovirus infection. J. Clin. Virol. 29:71–83. 10.1016/j.jcv.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Lazzarotto T, Guerra B, Lanari M, Gabrielli L, Landini MP. 2008. New advances in the diagnosis of congenital cytomegalovirus infection. J. Clin. Virol. 41:192–197. 10.1016/j.jcv.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Hazell SL. 2007. Clinical utility of avidity assays. Expert Opin. Med. Diagn. 1:511–519. 10.1517/17530059.1.4.511. [DOI] [PubMed] [Google Scholar]
- 13.De Paschale M, Agrappi C, Manco MT, Clerici P. 2010. Positive predictive value of anti-HCMV IgM as an index of primary infection. J. Virol. Methods 168:121–125. 10.1016/j.jviromet.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Chakravarti A, Kashyap B, Wadhwa A. 2007. Relationship of IgG avidity index and IgM levels for the differential diagnosis of primary from recurrent cytomegalovirus infections. Iran. J. Allergy Asthma Immunol. 6:197–201. [PubMed] [Google Scholar]
- 15.Leruez-Ville M, Sellier Y, Salomon LJ, Stirnemann JJ, Jacquemard F, Ville Y. 2013. Prediction of fetal infection in cases with cytomegalovirus immunoglobulin M in the first trimester of pregnancy: a retrospective cohort. Clin. Infect. Dis. 56:1428–1435. 10.1093/cid/cit059. [DOI] [PubMed] [Google Scholar]
- 16.BaAlawi F, Robertson PW, Lahra M, Rawlinson WD. 2012. Comparison of five CMV IgM immunoassays with CMV IgG avidity for diagnosis of primary CMV infection. Pathology 44:381–383. 10.1097/PAT.0b013e328353bec0. [DOI] [PubMed] [Google Scholar]
- 17.Kouri V, Correa CB, Verdasquera D, Martinez PA, Alvarez A, Aleman Y, Perez L, Golpe MA, Someilan T, Chong Y, Fresno C, Navarro MA, Perez E, Moro I, Sanchez R, Llanusa C, Melin P. 2010. Diagnosis and screening for cytomegalovirus infection in pregnant women in Cuba as prognostic markers of congenital infection in newborns 2007–2008. Pediatr. Infect. Dis. J. 29:1105–1110. 10.1097/INF.0b013e3181eb7388. [DOI] [PubMed] [Google Scholar]
- 18.Kamel N, Metwally L, Sayed Ahmed WA, Lotfi M, Younis S. 2014. Primary cytomegalovirus infection in pregnant Egyptian women confirmed by cytomegalovirus IgG avidity testing. Med. Princ. Pract. 23:29–33. 10.1159/000354758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanengisser-Pines B, Hazan Y, Pines G, Appelman Z. 2009. High cytomegalovirus IgG avidity is a reliable indicator of past infection in patients with positive IgM detected during the first trimester of pregnancy. J. Perinat. Med. 37:15–18. 10.1515/JPM.2009.012. [DOI] [PubMed] [Google Scholar]
- 20.Sonoyama A, Ebina Y, Morioko I, Tanimura K, Morizane M, Tairaku S, Minematsu T, Inoue N, Yamada H. 2012. Low IgG avidity and ultrasound fetal abnormality predict congenital cytomegalovirus infection. J. Med. Virol. 84:1928–1933. 10.1002/jmv.23387. [DOI] [PubMed] [Google Scholar]
- 21.Munro SC, Hall B, Whybin LR, Leader L, Robertson P, Maine GT, Rawlinson WD. 2005. Diagnosis of and screening for cytomegalovirus infection in pregnant women. J. Clin. Microbiol. 43:4713–4718. 10.1128/JCM.43.9.4713-4718.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Prince HE, Lapé-Nixon M, Novak-Weekley SM. 2014. Performance of a cytomegalovirus IgG enzyme immunoassay kit modified to measure avidity. Clin. Vaccine Immunol. 21:808–812. 10.1128/CVI.00105-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inouye S, Hasagawa A, Matsuno S, Katow S. 1984. Changes in antibody avidity after virus infections: detection by an immunosorbent assay in which a mild protein-denaturing agent is employed. J. Clin. Microbiol. 20:525–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hedman K, Seppala I. 1988. Recent rubella virus infection indicated by a low avidity of specific IgG. J. Clin. Immunol. 8:214–221. 10.1007/BF00917569. [DOI] [PubMed] [Google Scholar]
- 25.Blackburn NK, Besselaar TG, Schoub BD, O'Connell KF. 1991. Differentiation of primary cytomegalovirus infection from reactivation using the urea denaturation test for measuring antibody avidity. J. Med. Virol. 33:6–9. 10.1002/jmv.1890330103. [DOI] [PubMed] [Google Scholar]
- 26.Boppana SB, Britt WJ. 1995. Antiviral antibody responses and intrauterine transmission after primary maternal cytomegalovirus infection. J. Infect. Dis. 171:1115–1121. 10.1093/infdis/171.5.1115. [DOI] [PubMed] [Google Scholar]
- 27.Grangeot-Keros L, Mayaux MJ, Lebon P, Freymuth F, Eugene G, Stricker R, Dussaix E. 1997. Value of cytomegalovirus (CMV) IgG avidity index for the diagnosis of primary CMV infection in pregnant women. J. Infect. Dis. 175:944–996. 10.1086/513996. [DOI] [PubMed] [Google Scholar]
- 28.Bodeus M, Feyder S, Goubau P. 1998. Avidity of IgG antibodies distinguishes primary from non-primary cytomegalovirus infection in pregnant women. Clin. Diagn. Virol. 9:9–16. 10.1016/S0928-0197(97)10016-2. [DOI] [PubMed] [Google Scholar]
- 29.Lazzarotto T, Spezzacatena P, Pradelli P, Abate DA, Varani S, Landini MP. 1997. Avidity of immunoglobulin G directed against human cytomegalovirus during primary and secondary infections in immunocompetent and immunocompromised subjects. Clin. Diagn. Lab. Immunol. 4:469–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eggers M, Bader U, Enders G. 2000. Combination of microneutralization and avidity assays: improved diagnosis of recent primary human cytomegalovirus infection in single serum sample of second trimester pregnancy. J. Med. Virol. 60:324–330. . [DOI] [PubMed] [Google Scholar]
- 31.Bodeus M, Van Ranst M, Bernard P, Hubinont C, Goubau P. 2002. Anticytomegalovirus IgG avidity in pregnancy: a 2-year prospective study. Fetal Diagn. Ther. 17:362–366. 10.1159/000065386. [DOI] [PubMed] [Google Scholar]
- 32.Lazzarotto T, Spezzacatena P, Varani S, Gabrielli L, Pradelli P, Guerra B, Landini MP. 1999. Anticytomegalovirus (anti-CMV) immunoglobulin G avidity in identification of pregnant women at risk of transmitting congenital CMV infection. Clin. Diagn. Lab. Immunol. 6:127–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lazzarotto T, Varani S, Spezzacatena P, Gabrielli L, Pradelli P, Guerra B, Landini MP. 2000. Maternal IgG avidity and IgM detected by blot as diagnostic tools to identify pregnant women at risk of transmitting cytomegalovirus. Viral Immunol. 13:137–141. 10.1089/vim.2000.13.137. [DOI] [PubMed] [Google Scholar]
- 34.Bodeus M, Goubau P. 1999. Predictive value of maternal-IgG avidity for congenital human cytomegalovirus infection. J. Clin. Virol. 12:3–8. [DOI] [PubMed] [Google Scholar]
- 35.Lazzarotto T, Varani S, Guerra B, Nicolosi A, Lanari M, Landini MP. 2000. Prenatal indicators of congenital cytomegalovirus infection. J. Pediatr. 137:90–95. 10.1067/mpd.2000.107110. [DOI] [PubMed] [Google Scholar]
- 36.Lazzarotto T, Varani S, Gabrielli L, Spezzacatena P, Landini MP. 1999. New advances in the diagnosis of congenital cytomegalovirus infection. Intervirology 42:390–397. 10.1159/000053976. [DOI] [PubMed] [Google Scholar]
- 37.Stagno S, Tinker MK, Elrod C, Fuccillo DA, Cloud G, O'Beirne AJ. 1985. Immunoglobulin M antibodies detected by enzyme-linked immunosorbent assay and radioimmunoassay in the diagnosis of cytomegalovirus infections in pregnant women and newborn infants. J. Clin. Microbiol. 21:930–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grazia Revello M, Percivalle E, Zannino M, Rossi V, Gerna G. 1991. Development and evaluation of a capture ELISA for IgM antibody to the human cytomegalovirus major DNA binding proteins. J. Virol. Methods 35:315–329. 10.1016/0166-0934(91)90073-9. [DOI] [PubMed] [Google Scholar]
- 39.Daiminger A, Bader U, Eggers M, Lazzarotto T, Enders G. 1999. Evaluation of two novel enzyme immunoassays using recombinant antigens to detect cytomegalovirus-specific immunoglobulin M in sera from pregnant women. J. Clin. Virol. 13:161–171. 10.1016/S1386-6532(99)00028-1. [DOI] [PubMed] [Google Scholar]
- 40.Furione M, Rognoni V, Sarasini A, Zavattoni M, Lilleri D, Gerna G, Revello MG. 2013. Slow increase in IgG avidity correlates with prevention of human cytomegalovirus transmission to the fetus. J. Med. Virol. 85:1960–1967. 10.1002/jmv.23691. [DOI] [PubMed] [Google Scholar]
- 41.de Souza S, Bonon SHA, Costa SCB, Rossi CL. 2003. Evaluation of an in-house specific immunoglobulin G (IgG) avidity ELISA for distinguishing recent primary from long-term human cytomegalovirus (HCMV) infection. Rev. Inst. Med. Trop. Sao Paulo 45:323–326. 10.1590/S0036-46652003000600005. [DOI] [PubMed] [Google Scholar]
- 42.Tagawa M, Minematsu T, Masuzaki H, Ishimaru T, Moriuchi H. 2010. Seroepidemiological survey of cytomegalovirus infection among pregnant women in Nagasaki, Japan. Pediatr. Intl. 52:459–462. 10.1111/j.1442-200X.2009.03005.x. [DOI] [PubMed] [Google Scholar]
- 43.Curdt I, Praast G, Sickinger E, Schultess J, Herold I, Braun HB, Bernhardt S, Maine GT, Smith DD, Hsu S, Christ HM, Pucci D, Hausmann M, Herzogenrath J. 2009. Development of fully automated determination of marker-specific immunoglobulin G (IgG) avidity based on the avidity competition assay format: application for Abbott Architect cytomegalovirus and toxo IgG avidity assays. J. Clin. Microbiol. 47:603–613. 10.1128/JCM.01076-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Enders G, Daiminger A, Bader U, Exler S, Schimpf Y, Enders M. 2013. The value of CMV IgG avidity and immunoblot for timing the onset of primary CMV infection in pregnancy. J. Clin. Virol. 56:102–107. 10.1016/j.jcv.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 45.Rajasekariah H, Scott G, Robertson PW, Rawlinson WD. 2013. Improving diagnosis of primary cytomegalovirus infection in pregnant women using immunoblot. J. Med. Virol. 85:315–319. 10.1002/jmv.23471. [DOI] [PubMed] [Google Scholar]
- 46.Bodeus M, Beulne D, Goubau P. 2001. Ability of three IgG-avidity assays to exclude recent cytomegalovirus infection. Eur. J. Clin. Microbiol. Infect. Dis. 20:248–252. 10.1007/s100960100484. [DOI] [PubMed] [Google Scholar]
- 47.Baccard-Longere M, Freymuth F, Cointe D, Seigneurin JM, Grangeot-Keros L. 2001. Multicenter evaluation of a rapid and convenient method for determination of cytomegalovirus immunoglobulin G avidity. Clin. Diagn. Lab. Immunol. 8:429–431. 10.1128/CDLI.8.2.429-431.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dollard SC, Staras SAS, Amin MM, Schmid DS, Cannon MJ. 2011. National prevalence estimates for cytomegalovirus IgM and IgG avidity and association between high IgM antibody titer and low IgG avidity. Clin. Vaccine Immunol. 18:1895–1899. 10.1128/CVI.05228-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vauloup-Fellous C, Berth M, Heskia F, Dugua J-M, Grangeot-Keros L. 2013. Re-evaluation of the VIDAS® cytomegalovirus (CMV) IgG avidity assay: determination of new cut-off values based on the study of kinetics of CMV-IgG maturation. J. Clin. Virol. 56:118–123. 10.1016/j.jcv.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 50.Revello MG, Gorini G, Gerna G. 2004. Clinical evaluation of a chemiluminescence immunoassay for determination of immunoglobulin G avidity to human cytomegalovirus. Clin. Diagn. Lab. Immunol. 11:801–805. 10.1128/CDLI.11.4.801-805.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vauloup-Fellous C, Lazzarotto T, Revello MG, Grangeot-Keros L. 2 March 2014. Clinical evaluation of the Roche Elecsys® CMV IgG avidity assay. Eur. J. Clin. Microbiol. Infect. Dis. 10.1007/s10096-014-2080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lagrou K, Bodeus M, Van Ranst M, Goubau P. 2009. Evaluation of the new architect cytomegalovirus immunoglobulin M (IgM), IgG, and IgG avidity assays. J. Clin. Microbiol. 47:1695–1699. 10.1128/JCM.02172-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Berth M, Grangeot-Keros L, Heskia F, Dugua J-M, Vauloup-Fellous C. 30 April 2014. Analytical issues possibly affecting the performance of commercial human cytomegalovirus IgG avidity assays. Eur. J. Clin. Microbiol. Infect. Dis. 10.1007/s10096-014-2109-8. [DOI] [PubMed] [Google Scholar]
- 54.Lumley S, Patel M, Griffiths PD. 2014. The combination of specific IgM antibodies and IgG antibodies of low avidity does not always indicate primary infection with cytomegalovirus. J. Med. Virol. 86:834–837. 10.1002/jmv.23863. [DOI] [PubMed] [Google Scholar]
- 55.Guisasola ME, Ramos B, Sanz JC, Garcia-Bermejo I, de Ory Manchon F. 2010. Comparison of IgG avidity assays in the confirmation of the diagnosis of cytomegalovirus primary infection. APMIS 118:991–993. 10.1111/j.1600-0463.2010.02682.x. [DOI] [PubMed] [Google Scholar]
- 56.Revello MG, Genini E, Gorini G, Klersy C, Piralla A, Gerna G. 2010. Comparative evaluation of eight commercial human cytomegalovirus IgG avidity assays. J. Clin. Virol. 48:255–259. 10.1016/j.jcv.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 57.Prince HE, Lapé-Nixon M, Brenner A, Pitstick N, Couturier MR. 2014. Potential impact of different cytomegalovirus (CMV) IgM assays on an algorithm requiring IgM reactivity as a criterion for measuring CMV IgG avidity. Clin. Vaccine Immunol. 21:813–816. 10.1128/CVI.00106-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lazzarotto T, Gabrielli L, Lanari M, Guerra B, Bellucci T, Sassi M, Landini MP. 2004. Congenital cytomegalovirus infection: recent advances in the diagnosis of maternal infection. Hum. Immunol. 65:410–415. 10.1016/j.humimm.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 59.Prince HE, Leber AL. 2002. Validation of an in-house assay for cytomegalovirus immunoglobulin G (CMV IgG) avidity and relationship of avidity to CMV IgM levels. Clin. Diagn. Lab. Immunol. 9:824–827. 10.1128/CDLI.9.4.824-827.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lazzarotto T, Guerra B, Gabrielli L, Lanari M, Landini MP. 2011. Update on the prevention, diagnosis and management of cytomegalovirus infection during pregnancy. Clin. Microbiol. Infect. 17:1285–1293. 10.1111/j.1469-0691.2011.03564.x. [DOI] [PubMed] [Google Scholar]
- 61.Lazzarotto T, Galli C, Pulvirenti R, Rescaldani R, Vezzo R, La Gioia A, Martinelli C, La Rocca S, Agresti G, Grillner L, Nordin M, Van Ranst M, Combs B, Maine GT, Landini MP. 2001. Evaluation of the Abbott AxSYM cytomegalovirus (CMV) immunoglobulin M (IgM) assay in conjunction with other CMV IgM tests and a CMV IgG avidity assay. Clin. Diagn. Lab. Immunol. 8:196–198. 10.1128/CDLI.8.1.196-198.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Maine GT, Stricker R, Stricker R. 2012. Kinetics of CMV seroconversion in a Swiss pregnant women population. Diagn. Microbiol. Infect. Dis. 73:275–277. 10.1016/j.diagmicrobio.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 63.Gentile M, Galli C, Pagnotti P, Di Marco P, Tzantzoglou S, Bellomi F, Ferreri ML, Selvaggi C, Antonelli G. 2009. Measurement of the sensitivity of different commercial assays in the diagnosis of CMV infection in pregnancy. Eur. J. Clin. Microbiol. Infect. Dis. 28:977–981. 10.1007/s10096-009-0738-0. [DOI] [PubMed] [Google Scholar]
- 64.Macé M, Sisoeff L, Rudent A, Grangeot-Keros L. 2004. A serological testing algorithm for the diagnosis of primary CMV infection in pregnant women. Prenat. Diagn. 24:861–863. 10.1002/pd.1001. [DOI] [PubMed] [Google Scholar]
- 65.Johnson JM, Anderson BL. 2013. Cytomegalovirus: should we screen pregnant women for primary infection? Am. J. Perinatol. 3:121–124. 10.1055/s-0032-1333133. [DOI] [PubMed] [Google Scholar]
- 66.Adler SP, Finney JW, Manganello AM, Best AM. 2004. Prevention of child-to-mother transmission of cytomegalovirus among pregnant women. J. Pediatr. 145:485–491. 10.1016/j.jpeds.2004.05.041. [DOI] [PubMed] [Google Scholar]
- 67.Baquero-Artigao F, Grupo de Trabajo de Tuberculosis de la Sociedad Española de Infectolog ía Pediátrica 2009. Consensus document from the Spanish Society of Paediatric Infectious Diseases (SEIP) on the diagnosis and treatment of congenital cytomegalovirus infection. An. Pediatr. (Barc.) 71:535–547 (In Spanish.) 10.1016/j.anpedi.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 68.Yinon Y, Farine D, Yudin MH, Gagnon R, Hudon L, Basso M, Bos H, Delisle MF, Menticoglou S, Mundle W, Ouellet A, Pressey T, Roggensack A, Boucher M, Castillo E, Gruslin A, Money DM, Murphy K, Ogilvie G, Paquet C, Van Eyk N, van Schalkwyk J, Fetal Medicine Committee Society of Obstetricians and Gynaecologists of Canada 2010. Cytomegalovirus infection in pregnancy. J. Obstet. Gynaecol. Can. 32:348–354. [DOI] [PubMed] [Google Scholar]