Abstract
Infants born preterm are at a higher risk of complications and hospitalization in cases of rotavirus diarrhea than children born at term. We evaluated the impact of a rotavirus vaccination campaign (May 2007 to May 2010) on hospitalizations for rotavirus gastroenteritis in a population of children under 3 years old born prematurely (before 37 weeks of gestation) in the Brest University Hospital birth zone. Active surveillance from 2002 to 2006 and a prospective collection of hospitalizations for rotavirus diarrhea were initiated in the pediatric units of Brest University Hospital until May 2010. Numbers of hospitalizations for rotavirus diarrhea among the population of children born prematurely, before and after the start of the vaccination program, were compared using a Poisson regression model controlling for epidemic-to-epidemic variation. A total of 217 premature infants were vaccinated from 2007 to 2010. Vaccine coverage for a complete course of three doses was 41.9%. The vaccine safety in premature infants was similar to that in term infants. The vaccination program led to a division by a factor of 2.6 (95% confidence interval [CI], 1.3 to 5.2) in the number of hospitalizations for rotavirus diarrhea during the first two epidemic seasons following vaccine introduction and by a factor of 11 (95% CI, 3.5 to 34.8) during the third season. We observed significant effectiveness of the pentavalent rotavirus vaccine on the number of hospitalizations in a population of prematurely born infants younger than 3 years of age. A multicenter national study would provide better assessment of this impact. (This study [Impact of Systematic Infants Vaccination Against Rotavirus on Gastroenteritis Hospitalization: a Prospective Study in Brest District, France (IVANHOE)] has been registered at ClinicalTrials.gov under registration no. NCT00740935.)
INTRODUCTION
Rotavirus infection is the leading cause of severe acute diarrhea in infants worldwide. The vast majority of cases occur before the age of 5 years (1, 2). Preterm infants are at higher risk for complications and hospitalization in cases of rotavirus diarrhea than children born at term (3–6). Among these children, those who have low birth weight (<2,500 g) or very low birth weight (<1,500 g) present the highest risk of complications (odds ratio [OR] of 2.6 and 95% confidence interval [CI] of 1.6 to 4.1 or OR of 1.6 and 95% CI of 1.3 to 2.1, respectively) (3, 4). Complications present as gastrointestinal hemorrhage and necrotizing enterocolitis in particular (4, 7). The greater severity of these infections in premature infants might be explained by the relative immaturity of their immune systems and the lower levels of maternal antibodies transferred transplacentally before birth than term infants (3, 4). Rotarix and RotaTeq are the two rotavirus vaccines currently available. Their efficacies against severe rotavirus diarrhea, including that in preterm infants, have been demonstrated (3, 8, 9).
Observational studies after routine vaccination have been conducted from sentinel networks in developed countries (10–13). These have shown a marked decrease in the numbers of cases of rotavirus diarrhea in the year following the vaccine introduction. Despite the presence of inherent biases in the observational studies, the results were consistent. Taking into account the natural secular variability in rotavirus epidemics, our French population-based Impact of Systematic Infants Vaccination Against Rotavirus on Gastroenteritis Hospitalization: a Prospective Study in Brest District, France (IVANHOE) study (registered at ClinicalTrials.gov under registration no. NCT00740935) (14) confirmed these observations by showing a 2-fold decrease (95% CI, 1.6 to 2.7) in hospitalization rates for rotavirus diarrhea in infants under 2 years old with vaccine coverage of 47.1%. Since then, the effectiveness of RotaTeq has been observed in several developed countries (15–17). However, data on the impact of this vaccine in premature infants are limited.
The main objective of our analysis was to evaluate the impact of the pentavalent rotavirus vaccine on the number of hospitalizations for rotavirus diarrhea in preterm infants enrolled in the IVANHOE study. The secondary objectives were to analyze the vaccine coverage and safety in this population. The vaccine chosen for the study was the pentavalent rotavirus vaccine (RotaTeq).
MATERIALS AND METHODS
Active hospital-based surveillance system.
The study methodology of IVANHOE has been previously published (14). Briefly, it consisted of an active hospital-based surveillance system initiated 5 years before vaccine introduction (May 2007), providing baseline hospitalization rates for rotavirus disease. Brest University Hospital has the only Pediatric Department that serves the population of northwestern Brittany. The subgroup for our analysis was composed of children born prematurely, between 25 and 36 weeks and 6 days of gestational age, at Brest University Hospital from December 2000 to the end of May 2010. Based on the postal code of the parents' place of residence, children were categorized as belonging to the catchment area of Brest University Hospital as defined by the Agence Régionale de Santé (ARS) (Regional Agency for Health) of Brittany (18). To estimate the vaccine impact on hospitalization, we restricted the study population to infants born prematurely at Brest University Hospital and residing in this catchment area. A population-based study with follow-up of all children born prematurely in this catchment area and with the confirmed cases of rotavirus diarrhea in this population was thus possible. A case of rotavirus diarrhea was defined by a combination of symptoms (either a frequent flow of watery and abnormally loose stools with or without vomiting or diarrhea associated with two additional symptoms [vomiting, abdominal pain, or fever]) and confirmation through the detection of rotavirus in the stool specimen using the Rida Quick rotavirus/adenovirus Combi test (R-Biopharm AG, Darmstadt, Germany). Its sensitivity and specificity for rotavirus are 75% and 95% (19). Information, including demographics, clinical data, laboratory data, and vaccination history, was entered into standardized case report forms for all confirmed rotavirus diarrhea cases. By conducting 5 years of baseline surveillance, the secular variation of the rotavirus seasons (December to May included) was captured, providing confidence in the role of the vaccination on the observed declines. Data were entered into a database using the Epi-Info 6.1 software program. The International Classification of Diseases (ICD-10) codes for gastroenteritis were also used to ensure that all children admitted to Brest University Hospital for rotavirus diarrhea had been identified.
Vaccination program implementation.
From March 2007 onward, an information letter has been given to all mothers who gave birth in Brest. The vaccine used for the study was RotaTeq, a live pentavalent rotavirus vaccine. It was freely available, directly from pediatricians, from the Protection Maternelle et Infantile (PMI) (free public outpatient clinics dedicated to the care of mothers and their children) and from the Brest University Hospital pharmacy when prescribed by a general practitioner or neonatologist. The vaccination schedule was three 2-ml oral doses of vaccine, dose 1 between 6 and 12 weeks of age and doses 2 and 3 at 4-week intervals, with all three doses being given before 26 weeks of age. Children born prematurely, between 33 and 36 weeks and 6 days of gestational age, were vaccinated according to the same modalities as children born at term as for all other vaccinations at the real age of the child. For children born prematurely between 25 and 32 weeks and 6 days of gestational age, whether still hospitalized or not, vaccination was performed under cardiorespiratory monitoring in the hospital during 48 h. A case report form, including vaccination dates, was completed by the caregivers.
Evaluation of vaccine coverage.
The ratio of vaccinated premature infants to the total number of premature infants, born at the Brest University Hospital and eligible for the complete vaccination schedule, was calculated. The infants concerned were those born after 20 February 2007 and before 1 December 2009, i.e., eligible for vaccination between May 2007 and May 2010. Vaccine coverage was calculated for the catchment area of Brest University Hospital for a complete vaccine schedule. The vaccination rate was also calculated for at least one dose of vaccine.
Vaccine impact on hospitalization for rotavirus diarrhea.
Hospitalizations (hospital admission) for rotavirus-specific diarrhea during the 2009-2010 epidemic in premature infants younger than 3 years of age, who were born at Brest University Hospital and whose parents lived within the catchment area of this hospital, were chosen as the primary outcome. In fact, only infants younger than 3 years of age could have been vaccinated at the end of the third year of the program. Trends in hospitalizations for rotavirus-specific diarrhea before and after vaccine introduction were monitored in our primary analysis. To control for epidemic-to-epidemic variation in disease burden, we used a population not targeted by the vaccination program, that is, a population among whom the number of hospitalizations in previous epidemics was strongly correlated with the number of hospitalizations in our population of interest. Infants aged 3 to 5 years fulfilled these criteria, as shown in Fig. 1.
FIG 1.
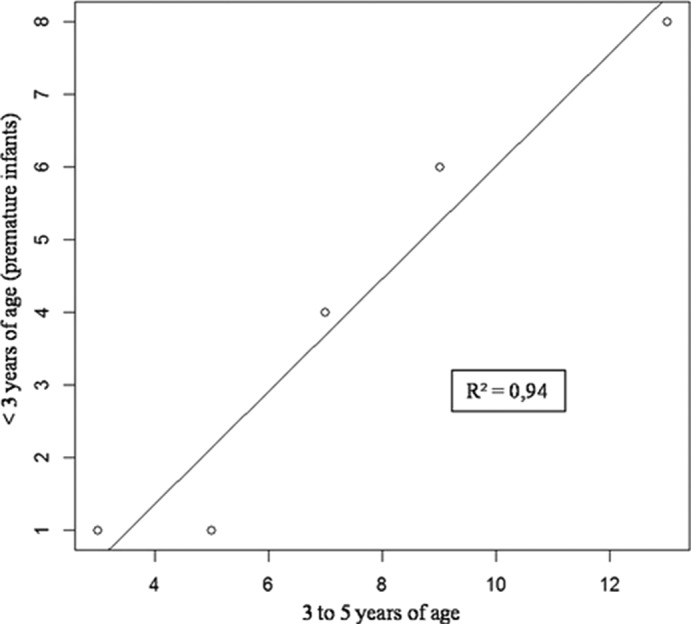
Numbers of hospitalized infants living within the catchment area of Brest University Hospital and hospitalized for rotavirus-specific diarrhea according to age groups during five epidemics (2002-2003 to 2006-2007) before vaccine introduction.
As in the IVANHOE study, we used a Poisson model. The number of hospitalizations for rotavirus diarrhea in infants younger than 3 years of age during the epidemic season (December to May) was modeled as a function of (i) the number of hospitalizations in infants 3 to 5 years of age and (ii) vaccine introduction. As the vaccination program covered infants younger than 1 year in the first year and infants younger than 2 years in the second year, we expected a potential difference in the impact of vaccination between each of the three seasons. To estimate the vaccination impact for each season, while counting the number of hospitalizations, we introduced three dummy variables into a Poisson model, log[E(Y) = α + α1X + β1X + β1Z1 + β2Z2 + β3Z3, where Y is the number of hospitalizations for rotavirus-specific diarrhea within the catchment area of Brest University Hospital in prematurely born infants younger than 3 years of age for each epidemic, X is the number of hospitalizations for rotavirus-specific diarrhea within the catchment area of Brest University Hospital in infants 3 to 5 years of age for each epidemic, Z1 is equal to 1 for the 2007-2008 epidemic and 0 otherwise, Z2 is equal to 1 for the 2008-2009 epidemic and 0 otherwise, and Z3 is equal to 1 for the 2008-2009 epidemic and 0 otherwise. This modeling did not use an offset because it concerned the number of hospitalizations and not the incidence rates.
Safety analysis.
All vaccinated children born after 20 February 2007 and before 1 December 2009, i.e., those eligible for vaccination between May 2007 and May 2010, were followed with respect to all-cause hospitalizations for up to 42 days after the last dose. To ensure that all vaccinated children admitted to Brest University Hospital for intussusceptions and Kawasaki disease had been identified, we also used ICD-10 codes. The safety analysis included all subjects receiving at least one dose. The incidence of serious adverse events (SAEs) in preterm infants born between 25 and 36 weeks and 6 days of gestational age was therefore compared to that of full-term infants. For this analysis, we have deliberately ignored the place of residence of the parents to analyze all the adverse events.
Ethical aspects.
The institutional research ethics board and French national agency for drug security (Agence Nationale de Sécurité du Médicament [ANSM]) approved the IVANHOE study, which included the secondary analysis of subgroups. In addition, the computer file used for the study was the subject of a license from the French National Commission for Informatics and Liberties (CNIL). All patients' names were anonymized. Written informed consent was obtained from parents of patients before vaccination.
RESULTS
Vaccination program results and vaccine coverage.
A total of 217 premature infants born at Brest University Hospital whose parents lived within its catchment area were enrolled in the IVANHOE study (Fig. 2). They all received at least one dose of vaccine from May 2007 to May 2010. Among these infants, 201 received a complete vaccination schedule. The number of children, according to the vaccine doses they actually received, is presented in Table 1. Vaccine coverage for a complete vaccination schedule, calculated from the 201 preterm infants, was equal to 41.9%. This coverage was higher in the last 2 years of the vaccination program (from 23% in the first year to >50% in subsequent years) (Table 1).
FIG 2.
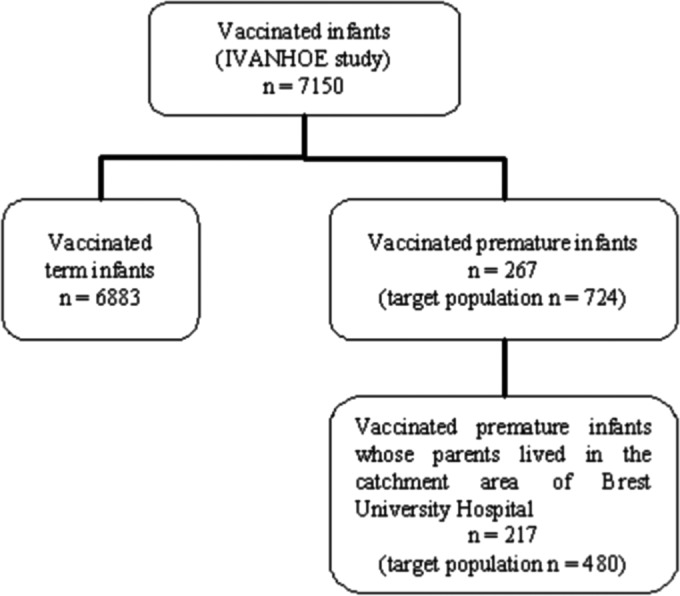
Number of infants vaccinated between May 2007 and May 2010, including those born at Brest University Hospital.
TABLE 1.
Number of infants born prematurely at Brest University Hospital whose parents lived in the catchment area of this hospital according to vaccine doses actually received from May 2007 to May 2010
| Infants | Total no. (%) of infants during: |
|||
|---|---|---|---|---|
| 15 May 2007 to 14 May 2008 | 15 May 2008 to 14 May 2009 | 15 May 2009 to 14 May 2010 | 15 May 2007 to 14 May 2010 | |
| Target population | 152 | 182 | 146 | 480 |
| Infants who received: | ||||
| 1 dose | 0 | 6 | 3 | 9 |
| 2 doses | 1 | 4 | 2 | 7 |
| 3 doses | 35 (23.0) | 86 (47.2) | 80 (54.8) | 201 (41.9) |
| ≥1 dose | 36 (23.7) | 96 (52.7) | 85 (58.2) | 217 (45.2) |
Vaccine impact on hospitalization for rotavirus diarrhea.
During the 2002-2003 to 2009-2010 epidemic seasons, 27 premature infants born at Brest University Hospital whose parents lived within its catchment area were hospitalized for rotavirus diarrhea (Fig. 3). Regarding the control population of children aged 3 to 5 years whose parents lived in the same geographic area, 76 of them were hospitalized for rotavirus diarrhea in the same period (Fig. 3). The characteristics of these two populations are described in Table 2 for the last three epidemics. The numbers of hospitalizations in these two populations, before and after the introduction of the vaccination program, are plotted in Fig. 3. The number of hospitalizations for rotavirus diarrhea in children aged 3 to 5 years clearly tended to increase after the start of the vaccination program, while that of premature infants tended to be static or even decrease. Modeling allowed us to quantify the relative reduction in the number of hospitalizations in prematurely born infants younger than 3 years of age compared to the expected number of hospitalizations. This modeling was performed for the three epidemic seasons following the start of the vaccination program. The modeling results are presented in Table 3. The number of hospitalizations in premature infants younger than 3 years of age was divided by a factor of 2.6 (95% CI, 1.3 to 5.2) in the first epidemic season following the start of the vaccination, by a factor of 2.6 (95% CI, 1.3 to 5.2) in the second season, and by a factor of 11 (95% CI, 3.5 to 34.8) in the third season. The numbers of observed and expected hospitalizations in prematurely born infants younger than 3 years of age for each epidemic are plotted in Fig. 3.
FIG 3.
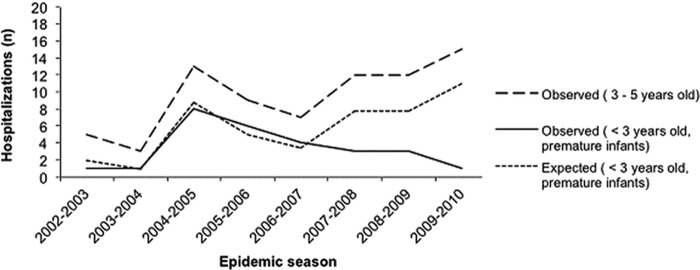
Observed and expected hospitalizations for rotavirus diarrhea during epidemic seasons.
TABLE 2.
Characteristics of premature infants aged <3 years and 3 to 5 years hospitalized for rotavirus diarrhea during the 2007-2008, 2008-2009, and 2009-2010 epidemics whose parents lived in the Brest University Hospital catchment area
| Variable | 2007-2008 epidemic |
2008-2009 epidemic |
2009-2010 epidemic |
|||
|---|---|---|---|---|---|---|
| Premature infants <3 yr (n = 3) | Infants aged 3–5 yr (n = 12) | Premature infants <3 yr (n = 3) | Infants aged 3–5 yr (n = 12) | Premature infants <3 yr (n = 3) | Infants aged 3–5 yr (n = 12) | |
| Age at entry (median [range]) (mo) | 19 (15–19) | 43 (36–59) | 14 (13–28) | 42 (38–50) | 26 | 48 (36–59) |
| Gestational age (median [range]) (wk) | 35 (26–35) | 29 (25–35) | 28 | |||
| Birth wt (median [range]) (g) | 2215 (1030–2260) | 1210 (880–2250) | 1330 | |||
| Temp of >38°C (no. [%]) | 3 (100) | 6 (50) | 0 (0) | 5 (42) | 1 (100) | 7 (46) |
| Duration of diarrhea (median [range]) (days) | 1 (1–2) | 2 (1–4) | 1 (1–4) | 2 (0–5) | 2 | 2 (0–5) |
| >7 diarrhea episodes in previous 24 h (no. [%]) | 0 (0) | 2 (16) | 1 (33) | 2 (16) | 1 (100) | 1 (6) |
| No. of episodes of vomiting in previous 24 h (median [range]) | 6 (1–9); NKa = 1 | 5 (0–20); NK = 1 | 5 (1–7) | 4 (1–20) | 9 | 10 (0–19) |
| No. of symptoms of dehydration (median [range]) | 1 (1–2) | 3 (0–5) | 2 (0–2) | 2 (1–7) | 1 | 2 (0–5) |
| Estimated wt loss of infants (no. [%]) | ||||||
| <5% | 3 | 3 (26) | 3 | 5 (42) | 1 | 6 (46) |
| 5–10% | 0 | 7 (58) | 0 | 5 (42) | 0 | 5 (38) |
| >10% | 0 | 2 (16) | 0 | 2 (16) | 0 | 2 (16); NK = 2 |
| Infants requiring treatment with intravenous fluids, (no. [%]) | 2 (66) | 12 (100) | 2 (66) | 15 (100) | 1 (100) | 15 (100) |
NK, not known.
TABLE 3.
Statistical modeling results
| Variable | Relative reduction | Parameter estimate | SD | P |
|---|---|---|---|---|
| Intercept | β0 = −1.83 | 0.70 | <0.01 | |
| Log (control population, 3–5 yr old) | β1 = 1.56 | 0.31 | <0.01 | |
| Scale | 0.55 | |||
| Impact of the vaccination campaigns on the no. of hospitalizations (median [range]) | ||||
| 2007 | 0.38 (0.27–0.55) | β2 = −0.95 | 0.35 | <0.01 |
| 2008 | 0.38 (0.27–0.55) | β3 = −0.95 | 0.35 | <0.01 |
| 2009 | 0.09 (0.05–0.16) | β4 = −2.40 | 0.59 | <0.01 |
Safety analysis.
The numbers of adverse events reported in term and preterm infants are presented in Fig. 4. The frequency of SAEs was higher in term infants (8.1%) than in preterm infants (5.2%). This difference was not statistically significant (P = 0.09, chi-square test). Among the SAEs reported as possibly related with the vaccine, the frequencies were similar in the two populations (1.8% for term infants and 1.9% for preterm infants [P = 0.96, chi-square test]). Two cases of bronchiolitis, one case of bronchopneumonia, and two cases of rhinitis were identified as SAEs in the premature infants. No cases of intussusception nor of Kawasaki disease was reported in the premature infants. No diarrhea, fever, or other reactogenic symptoms were reported within the 6 weeks following the last dose among the prematurely born infants.
FIG 4.
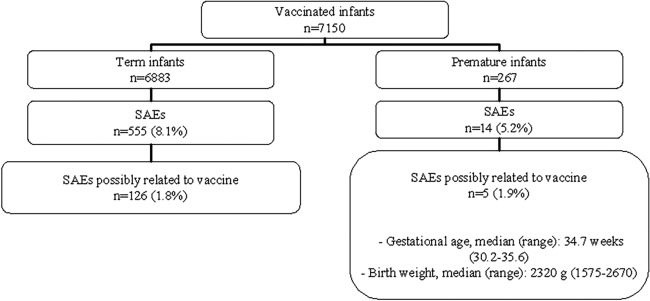
Serious adverse events (SAEs) reported from May 2007 to May 2010. The analysis was not restricted to the catchment area of Brest University Hospital.
DISCUSSION
This study highlighted the positive impact of a vaccination program using the pentavalent rotavirus vaccine in a population of prematurely born infants younger than 3 years of age. After introduction of the vaccination program, we report a 2.6-fold (95% CI, 1.3 to 5.2) and an 11-fold (95% CI, 3.5 to 34.8) decrease in the number of hospitalizations for rotavirus in this population in the two epidemic seasons following the vaccine introduction and in the third season. This impact was greater than the impact shown in the IVANHOE study for infants younger than 2 years of age, whose number of hospitalizations was divided by a factor of 2 (95% CI, 1.6 to 2.7) during the second epidemic season following vaccine introduction (14). The results of the IVANHOE study were in line with those obtained from observational studies implemented in developed countries after the introduction of routine immunization (10–13, 20, 21), where a decrease was found in cases of rotavirus diarrhea in the year following the introduction of routine vaccination.
The use of pentavalent rotavirus vaccine was well tolerated by premature infants. Indeed, among infants who received at least one dose of vaccine, analysis of SAEs did not show any significant difference between premature infants and term infants (5.2% versus 8.1%, P = 0.09, chi-square test). No cases of intussusception nor of Kawasaki disease were reported in our population. A similar frequency of SAEs (5.5%) was observed in a population of 1,005 preterm infants with no difference compared with those receiving a placebo (6.2%) (3). No reactogenic symptoms were reported among our population. However, the small sample size caused us to moderate our conclusions.
Vaccine coverage was lower in premature infants than in term infants (41.9% versus 47.1%) for a complete course of three doses throughout the study period. However, an improvement was noted between the first and the last 2 years of the study with an increase from 23% in the first year to 47.2% and 54.8% thereafter. We expected greater vaccine coverage in the premature infant population since this group contains infants at higher risk for hospitalization in cases of rotavirus diarrhea. The reason for this lower rate may be the initial fear of doctors and parents in regard to the safety of the vaccine in premature infants. The greater relative reduction in hospitalizations during the third season raised the possibility of herd immunity induced by rotavirus vaccination. This herd immunity after a vaccination program against rotavirus has now been observed in several studies (21–25). However, this indirect herd protection was not deduced from our small sample, and the improvement in vaccine coverage rates in the second and third years of the study could also have had an impact on reducing the number of hospitalizations.
The IVANHOE study, being the first population-based study on the impact of rotavirus vaccination to consider the natural secular variability in rotavirus epidemics, confirmed the results of observational studies showing a decrease in the number of hospitalizations for rotavirus diarrhea in infants under 2 years old with a vaccine coverage estimated to be 47.1% (10–14, 20, 21). Such secular variability was demonstrated in a study of three US hospitals over the same periods (2006-2007 and 2007-2008) (26). In addition, Sato et al. showed that the effectiveness of the vaccine against rotavirus may be masked by not including this concept in the analysis (27). This effectiveness has now been observed in other studies that have taken into account the variability of the rotavirus epidemics (15–17). Furthermore, the robustness of our study design was highlighted by the experts from the Division of Viral Diseases of the US Centers for Disease Control and Prevention (28). The importance of assessing the effectiveness and real-world impact of a vaccine was underlined. This implied evaluation of the vaccine under conditions of routine use, controlling for secular variations in the disease burden. The 5-year baseline surveillance confirmed the secular variation of the rotavirus seasons and ensured the benefit of vaccination on the observed declines.
The design of our analysis was similar to that of the IVANHOE study. The impact of the vaccination program was evaluated by using a Poisson model that made a significant adjustment for the observed data before the vaccine introduction. This demonstrated the robustness of our modeling to predict hospitalizations of premature infants without a vaccine program. The Poisson model is actually designed to analyze rare events (29). Moreover, the fact that the control population was different from the population of interest, in terms of gestational age at birth and age at the time of hospitalization, did not affect our analysis. In fact, it acted as an exogenous variable in the modeling adjustment uninfluenced by the vaccine introduction. Our modeling showed a decline in the number of hospitalizations for rotavirus diarrhea within the three epidemic seasons following vaccine introduction. The consistency of these results enhanced the validity of our modeling and its ability to predict the impact of vaccination in this population of premature infants.
Finally, it would have been interesting to study the impact of the vaccine program in relation to the severity of prematurity, the birth weight, or the type of feeding of these children (breastfeeding versus infant formula). Unfortunately, our population of premature infants was too small to identify a significant difference or to set up modeling for each subgroup.
Despite low vaccine coverage, our study showed a significant impact of rotavirus vaccine on the number of hospitalizations of premature infants within 3 years following the immunization. This was the first population-based study taking into account the natural secular variability in rotavirus epidemics with a specific analysis of children born prematurely. Our results can be used for further assessment of rotavirus vaccine and development of guidelines for a subgroup of vulnerable infants. A national multicenter study would allow a better assessment of the potential impact of rotavirus vaccine in the subgroup of premature infants.
ACKNOWLEDGMENTS
The Brest University Hospital sponsored this study. Sanofi-Pasteur MSD provided vaccine and funded the study. Sanofi-Pasteur did not play any role in the study design or in the collection, analysis, or interpretation of the data.
We thank Zarrin Alavi, INSERM CIC 0502, CHRU de Brest, for her advice in revising the manuscript.
Footnotes
Published ahead of print 30 July 2014
REFERENCES
- 1.Parashar UD, Hummelman EG, Bresee JS, Miller MA, Glass RI. 2003. Global illness and deaths caused by rotavirus disease in children. Emerg. Infect. Dis. 9:565–572. 10.3201/eid0905.020562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parashar UD, Burton A, Lanata C, Boschi-Pinto C, Shibuya K, Steele D, Birmingham M, Glass RI. 2009. Global mortality associated with rotavirus disease among children in 2004. J. Infect. Dis. 200(Suppl 1):S9–S15. 10.1086/605025. [DOI] [PubMed] [Google Scholar]
- 3.Goveia MG, Rodriguez ZM, Dallas MJ, Itzler RF, Boslego JW, Heaton PM, DiNubile MJ. 2007. Safety and efficacy of the pentavalent human-bovine (WC3) reassortant rotavirus vaccine in healthy premature infants. Pediatr. Infect. Dis. J. 26:1099–1104. 10.1097/INF.0b013e31814521cb. [DOI] [PubMed] [Google Scholar]
- 4.Newman RD, Grupp-Phelan J, Shay DK, Davis RL. 1999. Perinatal risk factors for infant hospitalization with viral gastroenteritis. Pediatrics 103:E3. 10.1542/peds.103.1.e3. [DOI] [PubMed] [Google Scholar]
- 5.Bresee JS, Glass RI, Ivanoff B, Gentsch JR. 1999. Current status and future priorities for rotavirus vaccine development, evaluation and implementation in developing countries. Vaccine 17:2207–2222. 10.1016/S0264-410X(98)00376-4. [DOI] [PubMed] [Google Scholar]
- 6.Ford-Jones EL, Wang E, Petric M, Corey P, Moineddin R, Fearon M. 2000. Hospitalization for community-acquired, rotavirus-associated diarrhea: a prospective, longitudinal, population-based study during the seasonal outbreak. The Greater Toronto Area/Peel Region PRESI Study Group. Pediatric Rotavirus Epidemiology Study for Immunization. Arch. Pediatr. Adolesc. Med. 154:578–585. 10.1001/archpedi.154.6.578. [DOI] [PubMed] [Google Scholar]
- 7.Sharma R, Hudak ML, Premachandra BR, Stevens G, Monteiro CB, Bradshaw JA, Kaunitz AM, Hollister RA. 2002. Clinical manifestations of rotavirus infection in the neonatal intensive care unit. Pediatr. Infect. Dis. J. 21:1099–1105. 10.1097/00006454-200212000-00003. [DOI] [PubMed] [Google Scholar]
- 8.Vesikari T, Clark HF, Offit PA, Dallas MJ, DiStefano DJ, Goveia MG, Ward RL, Schodel F, Karvonen A, Drummond JE, DiNubile MJ, Heaton PM. 2006. Effects of the potency and composition of the multivalent human-bovine (WC3) reassortant rotavirus vaccine on efficacy, safety and immunogenicity in healthy infants. Vaccine 24:4821–4829. 10.1016/j.vaccine.2006.03.025. [DOI] [PubMed] [Google Scholar]
- 9.Van der Wielen M, Van Damme P. 2008. Pentavalent human-bovine (WC3) reassortant rotavirus vaccine in special populations: a review of data from the Rotavirus Efficacy and Safety Trial. Eur. J. Clin. Microbiol. Infect. Dis. 27:495–501. 10.1007/s10096-008-0479-5. [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention. 2009. Reduction in rotavirus after vaccine introduction—United States, 2000-2009. MMWR Morb. Mortal. Wkly. Rep. 58:1146–1149. [PubMed] [Google Scholar]
- 11.Lambert SB, Faux CE, Hall L, Birrell FA, Peterson KV, Selvey CE, Sloots TP, Nissen MD, Grimwood K. 2009. Early evidence for direct and indirect effects of the infant rotavirus vaccine program in Queensland. Med. J. Aust. 191:157–160. [DOI] [PubMed] [Google Scholar]
- 12.Paulke-Korinek M, Rendi-Wagner P, Kundi M, Kronik R, Kollaritsch H. 2010. Universal mass vaccination against rotavirus gastroenteritis: impact on hospitalization rates in Austrian children. Pediatr. Infect. Dis. J. 29:319–323. 10.1097/INF.0b013e3181c18434. [DOI] [PubMed] [Google Scholar]
- 13.Hanquet G, Ducoffre G, Vergison A, Neels P, Sabbe M, Van Damme P, Van Herck K. 2011. Impact of rotavirus vaccination on laboratory confirmed cases in Belgium. Vaccine 29:4698–4703. 10.1016/j.vaccine.2011.04.098. [DOI] [PubMed] [Google Scholar]
- 14.Gagneur A, Nowak E, Lemaitre T, Segura JF, Delaperriere N, Abalea L, Poulhazan E, Jossens A, Auzanneau L, Tran A, Payan C, Jay N, de Parscau L, Oger E. 2011. Impact of rotavirus vaccination on hospitalizations for rotavirus diarrhea: the IVANHOE study. Vaccine 29:3753–3759. 10.1016/j.vaccine.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 15.Pendleton A, Galic M, Clarke C, Ng SP, Ledesma E, Ramakrishnan G, Liu Y. 2013. Impact of rotavirus vaccination in Australian children below 5 years of age: a database study. Hum. Vaccin. Immunother. 9:1617–1625. 10.4161/hv.24831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vesikari T, Uhari M, Renko M, Hemming M, Salminen M, Torcel-Pagnon L, Bricout H, Simondon F. 2013. Impact and effectiveness of RotaTeq vaccine based on 3 years of surveillance following introduction of a rotavirus immunization program in Finland. Pediatr. Infect. Dis. J. 32:1365–1373. 10.1097/INF.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 17.Rha B, Tate JE, Payne DC, Cortese MM, Lopman BA, Curns AT, Parashar UD. 2014. Effectiveness and impact of rotavirus vaccines in the United States—2006-2012. Expert Rev. Vaccines 13:365–376. 10.1586/14760584.2014.877846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tardif L. 2002. Hospitalization and territories in Brittany. Octant 90. (In French.) [Google Scholar]
- 19.Weitzel T, Reither K, Mockenhaupt FP, Stark K, Ignatius R, Saad E, Seidu-Korkor A, Bienzle U, Schreier E. 2007. Field evaluation of a rota- and adenovirus immunochromatographic assay using stool samples from children with acute diarrhea in Ghana. J. Clin. Microbiol. 45:2695–2697. 10.1128/JCM.00562-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buttery JP, Lambert SB, Grimwood K, Nissen MD, Field EJ, Macartney KK, Akikusa JD, Kelly JJ, Kirkwood CD. 2011. Reduction in rotavirus-associated acute gastroenteritis following introduction of rotavirus vaccine into Australia's National Childhood vaccine schedule. Pediatr. Infect. Dis. J. 30:S25–S29. 10.1097/INF.0b013e3181fefdee. [DOI] [PubMed] [Google Scholar]
- 21.Clark HF, Lawley D, Mallette LA, DiNubile MJ, Hodinka RL. 2009. Decline in cases of rotavirus gastroenteritis presenting to The Children's Hospital of Philadelphia after introduction of a pentavalent rotavirus vaccine. Clin. Vaccine Immunol. 16:382–386. 10.1128/CVI.00382-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Paulke-Korinek M, Kundi M, Rendi-Wagner P, de Martin A, Eder G, Schmidle-Loss B, Vecsei A, Kollaritsch H. 2011. Herd immunity after two years of the universal mass vaccination program against rotavirus gastroenteritis in Austria. Vaccine 29:2791–2796. 10.1016/j.vaccine.2011.01.104. [DOI] [PubMed] [Google Scholar]
- 23.Jit M, Bilcke J, Mangen MJ, Salo H, Melliez H, Edmunds WJ, Yazdan Y, Beutels P. 2009. The cost-effectiveness of rotavirus vaccination: comparative analyses for five European countries and transferability in Europe. Vaccine 27:6121–6128. 10.1016/j.vaccine.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 24.Panozzo CA, Becker-Dreps S, Pate V, Weber DJ, Jonsson Funk M, Sturmer T, Brookhart MA. 2014. Direct, indirect, total, and overall effectiveness of the rotavirus vaccines for the prevention of gastroenteritis hospitalizations in privately insured US children, 2007-2010. Am. J. Epidemiol. 179:895–909. 10.1093/aje/kwu001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Effelterre T, Soriano-Gabarro M, Debrus S, Claire Newbern E, Gray J. 2010. A mathematical model of the indirect effects of rotavirus vaccination. Epidemiol. Infect. 138:884–897. 10.1017/S0950268809991245. [DOI] [PubMed] [Google Scholar]
- 26.Payne DC, Szilagyi PG, Staat MA, Edwards KM, Gentsch JR, Weinberg GA, Hall CB, Curns AT, Clayton H, Griffin MR, Fairbrother G, Parashar UD. 2009. Secular variation in United States rotavirus disease rates and serotypes: implications for assessing the rotavirus vaccination program. Pediatr. Infect. Dis. J. 28:948–953. 10.1097/INF.0b013e3181a6ad6e. [DOI] [PubMed] [Google Scholar]
- 27.Sato T, Nakagomi T, Naghipour M, Nakagomi O. 2010. Modeling seasonal variation in rotavirus hospitalizations for use in evaluating the effect of rotavirus vaccine. J. Med. Virol. 82:1468–1474. 10.1002/jmv.21806. [DOI] [PubMed] [Google Scholar]
- 28.Tate JE, Parashar UD. 2011. Monitoring impact and effectiveness of rotavirus vaccination. Expert Rev. Vaccines 10:1123–1125. 10.1586/erv.11.94. [DOI] [PubMed] [Google Scholar]
- 29.Falissard B. 2005. Understanding and use of statistics in the life sciences, 3rd ed. Masson, Paris, France: (In French.) [Google Scholar]


