Abstract
Despite the widespread use of Mycobacterium bovis BCG, the only licensed vaccine against tuberculosis (TB), TB remains a global epidemic. To assess whether more direct targeting of the lung mucosa by respiratory immunization would enhance the potency and longevity of BCG-induced anti-TB protective immunity, the long-term impact of intranasal (i.n.) BCG vaccination was compared to conventional subcutaneous (s.c.) immunization by using a mouse model of pulmonary tuberculosis. Although significantly improved protection in the lung was seen at early time points (2 and 4 months postvaccination) in i.n. BCG-immunized mice, no differences in pulmonary protection were seen 8 and 10 months postvaccination. In contrast, in all of the study periods, i.n. BCG vaccination induced significantly elevated protective splenic responses relative to s.c. immunization. At five of nine time points, we observed a splenic protective response exceeding 1.9 log10 protection relative to the s.c. route. Furthermore, higher frequencies of CD4 T cells expressing gamma interferon (IFN-γ) and IFN-γ/tumor necrosis factor alpha, as well as CD8 T cells expressing IFN-γ, were detected in the spleens of i.n. vaccinated mice. Using PCR arrays, significantly elevated levels of IFN-γ, interleukin-9 (IL-9), IL-11, and IL-21 expression were also seen in the spleen at 8 months after respiratory BCG immunization. Overall, while i.n. BCG vaccination provided short-term enhancement of protection in the lung relative to s.c. immunization, potent and extremely persistent splenic protective responses were seen for at least 10 months following respiratory immunization.
INTRODUCTION
More than a century after Robert Koch first described how to culture tubercle bacilli, Mycobacterium tuberculosis remains one of the most successful human pathogens and a major global public health threat. According to the recent WHO global tuberculosis (TB) report, 1.3 million people died from TB in 2012 and 8.6 million new cases of the disease were detected (1). Next to HIV, TB is the most important cause of death from an infectious agent in low-income countries. Recommended by the WHO, live, attenuated Mycobacterium bovis BCG is the only TB vaccine approved for human use, being administered intradermally (i.d.) to more than 70% of children worldwide (2). Although i.d. BCG vaccination has been shown to be effective in preventing severe forms of extrapulmonary TB, its ability to protect against pulmonary TB in controlled clinical trials has been highly variable (0 to 80%) (3–5). Importantly, it has been estimated that i.d. BCG immunization prevents only about 5% of vaccine-preventable deaths (6). Clearly, novel, more effective TB immunization strategies are needed.
Despite 9 decades of BCG vaccine use, the optimal vaccine dose and route of administration have not been fully defined. Since TB is primarily a pulmonary infection, respiratory BCG immunization has long been considered an attractive alternative to i.d. vaccination. With immune lymphoid mucosal and bronchial tissues being targeted by respiratory immunization, it is possible that direct vaccination at pulmonary sites is superior to vaccination by other routes for inducing protective responses against infectious diseases of the lung. However, preclinical studies in animal models of TB that have compared the intranasal (i.n.) BCG vaccination route with injections of BCG through the skin (i.d. or subcutaneously [s.c.]) have yielded contradictory protection results. Several primarily short-term studies have shown that i.n. BCG vaccination is more effective than s.c. immunization, while other experiments have failed to demonstrate the superiority of the i.n. BCG vaccination route (7–16). Overall differences in study protocols, including various vaccination-challenge time periods and postchallenge evaluation time frames, have confounded the interpretation of comparative i.n. versus s.c. immunization protection experiments. Interestingly, these earlier studies have shown that BCG can be administered safely by the respiratory route. Aerosolized BCG given to guinea pigs and monkeys elicited minimal harmful side effects (7, 8, 12). More importantly in humans, BCG administered by the respiratory route to healthy adults and children, including patients with metastatic lung cancer, did not yield severe side effects, obvious BCG infections, or significant changes in pulmonary function (17–19). However, in these small studies, no clinical benefit of using aerosolized BCG has been shown thus far.
Given the extensive global coverage of BCG immunization and the protection i.d. BCG vaccination confers against severe disseminated forms of childhood TB, prime-boost strategies are being developed to boost BCG-induced anti-TB responses (20). However, the recent disappointing results of an MVA85 BCG boosting trial have suggested that other novel vaccination protocols must be tested, including alternative routes of administration (21). In this study, we reevaluated the preclinical effectiveness of i.n. BCG immunization in long-term murine vaccination-challenge studies. Here we show that while i.n. BCG immunization confers improved short-term pulmonary protection against an aerosol M. tuberculosis challenge relative to that afforded by s.c. BCG vaccination, the i.n. route confers considerable long-term protection in reducing M. tuberculosis dissemination from the lungs to distal sites such as the spleen.
MATERIALS AND METHODS
Animals.
C57BL/6 female mice (6 to 8 weeks of age) were obtained from the Jackson Laboratories (Bar Harbor, ME). All of the mice used in this study were maintained under appropriate conditions at the Center for Biologics Evaluation and Research, Bethesda, MD. This study was done in accordance with the guidelines for the care and use of laboratory animals specified by the National Institutes of Health. This protocol was approved by the Institutional Animal Care and Use Committee of the Center for Biologics Evaluation and Research.
Vaccinations.
Mice were immunized s.c. or i.n. with 5 × 105 CFU of BCG Pasteur strain originally obtained from the Trudeau Institute (Saranac Lake, NY). For i.n. immunizations, mice were anesthetized with ketamine and xylazine; this was followed by delivery of the bacterial solution into the nares in a volume of 40 μl. Subcutaneous immunizations were done by delivering 200 μl of the bacterial suspension into the back.
Evaluation of vaccine-induced protective immunity.
At various time points postimmunization, five mice per group were infected with M. tuberculosis Erdman by aerosol at a concentration known to deliver about 200 CFU into the lungs over a 30-min exposure in a Middlebrook chamber (Glas Col, Terre Haute, IN). At each time point, the lungs and spleens were homogenized separately in phosphate-buffered saline (PBS) with 0.05% Tween 80 with a Seward Stomacher 80 blender (Tekmar, Cincinnati, OH). The homogenates were serially diluted in PBS plus 0.05% Tween 80 and plated on Middlebrook 7H11 agar (Difco) plates containing 10% oleic acid-albumin-dextrose-catalase enrichment medium (Becton Dickinson, Sparks, MD), 10 μg/ml ampicillin, 50 μg/ml cycloheximide, and 2 μg/ml 2-thiophenecarboxylic acid hydride (TCH) (Sigma, St. Louis, MO). The addition of this concentration of TCH to the agar plates inhibits BCG growth but has no effect on M. tuberculosis growth. Plates were incubated at 37°C for 14 to 17 days before counting to determine the number of mycobacterial CFU per organ.
Flow cytometry.
By multiparameter flow cytometry, four or five BCG-vaccinated mice were used to determine the frequency of CD4+ or CD8+ monofunctional gamma interferon-positive (IFN-γ+) T cells or multifunctional T (MFT) cells induced at 2 months after s.c. or i.n. BCG immunization. Unvaccinated (naive) mice served as the negative control. The splenic T cell responses of naive and vaccinated mice were measured as previously described (22). Following 36 to 40 h of incubation with BCG (multiplicity of infection [MOI] = 0.02), the spleen cells were incubated with GolgiPlug (1.0 μl/ml/well of a 24-well plate; BD Biosciences, San Jose, CA) for 4 h. Unbound cells were then removed from the wells and transferred to tubes (12 by 75 mm), washed with PBS, and resuspended in 50 μl of PBS. Violet live-dead stain (10 μl of a 1:100 dilution; Invitrogen, Carlsbad, CA) was added to each tube, which was then incubated for 30 min at 4°C to allow for gating on viable cells. After the cells were washed with PBS plus 2% fetal bovine serum (FBS), an antibody (Ab) against CD16/CD32 (FcγIII/II receptor, clone 2.4G2) (Fc block) was added in a volume of 50 μl and the cells were incubated at 4°C for 15 min. The cells were then stained for 30 min at 4°C by adding Abs against the CD4 (Alexa Fluor 700-conjugated rat anti-mouse CD4 Ab, clone RM4-5), and CD8 (peridinin chlorophyll protein complex-conjugated rat anti-mouse CD8 Ab, clone 53 to 6.7) proteins at 0.1 and 0.4 μg per tube, respectively. Following incubation, the cells were washed twice with PBS-FBS and then fixed for 30 min at 4°C with 2% paraformaldehyde in PBS. Fixed cells were washed twice with perm-wash buffer (1% FBS, 0.01 M HEPES, and 0.1% saponin in PBS); this was followed by intracellular staining with the following Abs at 0.2 μg per tube in a 50-μl volume: phycoerythrin-conjugated rat anti-mouse IFN-γ Ab, clone XMG1.2; fluorescein isothiocyanate-conjugated rat anti-mouse tumor necrosis factor alpha (TNF-α) Ab, clone MP6-XT22; and allophycocyanin-conjugated rat anti-mouse interleukin-2 (IL-2) Ab, clone JES6-5H4. The cells were then incubated at 4°C for 30 min and washed twice with perm-wash buffer and twice with PBS-FBS. All Abs were obtained from BD Bioscience.
Lung T cell responses were determined by disrupting the lung tissue from naive or immunized mice with razor blades and incubating it with type I collagenase (0.7 mg/ml; Invitrogen, Carlsbad, CA) for 1 h. The lung homogenates were then passed through a 70-μm cell strainer to removed tissue clumps. The cells were pelleted and resuspended in 5 ml ACK lysing buffer (Lanza, Walkersville, MD) for 1 min to deplete the cell suspension of erythrocytes. After washing, the cells were counted and 2 million viable lung cells per well were added to a 96-well plate with 10 μg ampicillin and BCG (MOI = 0.5) in a 0.2-ml volume of Dulbecco's modified Eagle's medium with 10 mM HEPES, 2.0 mM l-glutamine, 0.1 mM minimum essential medium nonessential amino acids, and 10% FBS. After overnight incubation, GolgiPlug was added to the wells and the plate was incubated for an additional 4 h. The nonadherent cells were then harvested and stained with live-dead stain and fluorescent Abs as described above for surface and intracellular staining.
Cytokine responses from stimulated spleen and lung cells were determined with a LSRII flow cytometer (Becton Dickinson) and FlowJo software (Tree Star Inc., Ashland, OR). We acquired 250,000 events per sample and then, by using FlowJo, gated on live, single lymphocytes. To determine the frequencies of different populations of MFT cells, we gated on CD4+ or CD8+ T cells staining positive for TNF-α and IFN-γ, TNF-α and IL-2, IFN-γ and IL-2, or all three cytokines (triple positive [TP]).
PCR array.
In order to obtain a more comprehensive evaluation of overall cytokine responses following s.c. or i.n. immunization with BCG, a PCR array was performed with separate stimulated spleen or lung single-cell suspensions described above from the same four or five BCG-vaccinated mice. Total RNA was isolated from the cells with an RNeasy Plus minikit (Qiagen, Valencia, CA). Equal amounts of RNA from each sample were then reverse transcribed to cDNA with a High Capacity RNA to cDNA kit (Applied Biosystems, Foster City, CA). The quality of the cDNA conversion was confirmed by performing PCR assays of individual samples with glyceraldehyde 3-phosphate dehydrogenase (GAPDH) primers. To quantitate cytokine responses, the expression levels of 84 cytokines were evaluated with the RT2 profiler PCR array mouse common cytokine and cytokine/chemokine 96-well PCR plates (SABiosciences) and an Applied Biosciences ViiA sequence detection system. The mRNA expression level obtained for each gene was normalized to the expression of the GAPDH housekeeping gene by using the following equation: relative mRNA expression = 2−(CT of cytokine − CT of GAPDH) (where CT is the threshold cycle). The normalized gene expression levels in experimental samples (relative to the levels for naive controls) were determined by dividing the relative gene expression values in vaccinated animals by the expression levels in naive controls. Each reported cytokine or chemokine value represents the mean increase (or decrease) in mRNA expression relative to the levels for naive controls in four experiments.
Lung inflammation assessment.
In order to measure the level of inflammation in the lungs after s.c. or i.n. BCG vaccination, hematoxylin-and-eosin (H&E)-stained lung sections were evaluated 1 month after the M. tuberculosis challenge as previously described (23). Using the Image Pro Plus computer program (Media Cybernetics, Silver Spring, MD), we assessed the level of inflammation present in images obtained from a Nikon Optiphot 2 microscope fitted with a camera. In these images, the inflamed areas stained darker than the noninflamed areas. On the basis of these differences, we assigned the inflamed areas the color red, the noninflamed areas the color green, and the background the color yellow. The percentage of the lung sections colored red, green, or yellow was then determined by the computer software. To quantitate the percentage of the area inflamed, the mean percentage of the area colored red from five lung sections of each of the different groups was determined.
Statistical analysis.
The GraphPad Prism 5 program was used to analyze the data for these experiments (GraphPad Software, San Diego, CA). The vaccination-challenge protection data, the CD4+ and CD8+ T cell flow cytometry results, and the PCR array data were evaluated by unpaired t-test analysis.
RESULTS
Long-term protective responses induced by i.n. and s.c. BCG immunizations.
To compare the relative effectiveness of i.n. and s.c. BCG vaccinations with that of aerosol infection with M. tuberculosis, C57BL/6 mice were vaccinated with BCG by the i.n. and s.c. routes of administration, challenged from 2 to 10 months after vaccination, and sacrificed 1 to 6 months postchallenge. The lung protection results are summarized in Table 1. At 2 months postvaccination and 1 month postchallenge, typical 1-log10 reductions in mycobacterial burdens were seen in the lungs of s.c. BCG-vaccinated mice (compared to naive controls). However, significantly better (P < 0.05) protection (>2 log10) was detected in the lungs of i.n. BCG-vaccinated animals at the same time point. At the 2-month postvaccination and 3- and 6-month postchallenge time points, the pulmonary protective responses seen in the i.n. BCG-vaccinated mice were also significantly elevated relative to those of s.c. BCG-immunized mice and naive controls. Overall, at 2 months postvaccination, i.n. BCG vaccination induced significantly higher levels of protection in the lungs of C57BL/6 mice than did s.c. BCG immunization.
TABLE 1.
Lung CFU counts after BCG vaccination and aerosol challenge with M. tuberculosis
| Time point and exptl group | Mean postvaccination CFU count ± SEMb |
|||
|---|---|---|---|---|
| 2 mo | 4 mo | 8 mo | 10 mo | |
| 1 mo PCa | ||||
| Naive | 6.04 ± 0.09 | 6.06 ± 0.12 | 5.90 ± 0.09 | 6.03 ± 0.07 |
| BCG s.c. | 4.94 ± 0.12 (−1.10) | 5.06 ± 0.09 (−1.00) | 5.15 ± 0.12 (−0.75) | 5.43 ± 0.05 (−0.60) |
| BCG i.n. | 4.03 ± 0.16 (−2.01)c | 4.86 ± 0.08 (−1.20) | 5.40 ± 0.07 (−0.50) | 5.38 ± 0.07 (−0.65) |
| 3 mo PC | ||||
| Naive | 5.80 ± 0.04 | 5.80 ± 0.09 | 6.55 ± 0.08 | 6.28 ± 0.09 |
| BCG s.c. | 5.20 ± 0.04 (−0.53) | 5.33 ± 0.05 (−0.45) | 5.68 ± 0.15 (−0.87) | 5.42 ± 0.11 (−0.86) |
| BCG i.n. | 4.92 ± 0.09 (−0.88)c | 4.83 ± 0.10 (−0.95)c | 5.77 ± 0.16 (−0.78) | 5.46 ± 0.12 (−0.82) |
| 6 mo PC | ||||
| Naive | 5.89 ± 0.15 | |||
| BCG s.c. | 5.69 ± 0.13 | |||
| BCG i.n. | 5.02 ± 0.19 (−0.87)c | |||
PC, postchallenge.
Values in parenthesis are protection data (naive CFU minus vaccine group CFU) for statistically significant differences (P < 0.05).
The CFU count of the BCG i.n. group is significantly lower than that of BCG s.c. group (P < 0.05).
The superiority of the i.n. immunization route to the s.c. route seen at 2 months postvaccination was generally not seen at 4, 8, and 10 months postvaccination. At these later time points, pulmonary protective responses were significantly increased in both experimental groups relative to those of naive controls, but for five of six time points, no differences were detected in mice vaccinated with BCG by the i.n. or s.c. route. In these longer-term studies, pulmonary protection was significantly increased only in the i.n. versus s.c. BCG vaccine groups at 4 months postvaccination and 3 months postchallenge.
As shown in Table 2, significantly improved protection was seen in the spleens of i.n. BCG-vaccinated mice relative to those of s.c. immunized animals at 2 months postvaccination. At this early postvaccination time point, i.n. immunization induced >2 log10 protection in the spleen at 1 and 6 months postchallenge. Interestingly, increased protection in the spleen was also observed in the i.n. BCG-vaccinated group when the mice were aerogenically infected with M. tuberculosis at 4, 8, and 10 months postvaccination. While the s.c. route-induced protection was somewhat variable at later time points but significantly improved relative to naive controls even at 10 months postvaccination, i.n. BCG immunization evoked protective responses in the spleen that were significantly better than those evoked by s.c. BCG vaccination at all of these later time points. Surprisingly, for three of six later time points (and five of nine total) the splenic protective immune responses elicited by i.n. BCG vaccination exceeded 1.9 log10 protection.
TABLE 2.
Spleen CFU counts after BCG vaccination and aerosol challenge with M. tuberculosis
| Time point and exptl group | Mean postvaccination CFU count ± SEMb |
|||
|---|---|---|---|---|
| 2 mo | 4 mo | 8 mo | 10 mo | |
| 1 mo PCa | ||||
| Naive | 5.00 ± 0.25 | 5.24 ± 0.13 | 4.85 ± 0.04 | 5.15 ± 0.11 |
| BCG s.c. | 3.84 ± 0.11 (−1.16) | 4.29 ± 0.14 (−0.95) | 4.69 ± 0.12 | 4.40 ± 0.06 (−0.75) |
| BCG i.n. | 2.20 ± 0.14 (−2.80)c | 3.89 ± 0.13 (−1.35)c | 3.69 ± 0.09 (−1.16)c | 3.22 ± 0.15 (−1.93)c |
| 3 mo PC | ||||
| Naive | 5.05 ± 0.13 | 5.34 ± 0.13 | 6.06 ± 0.04 | 5.68 ± 0.04 |
| BCG s.c. | 4.77 ± 0.15 | 5.05 ± 0.04 | 5.22 ± 0.19 (−0.84) | 4.80 ± 0.08 (−0.88) |
| BCG i.n. | 4.18 ± 0.12 (−0.87)c | 4.55 ± 0.18 (−0.79)c | 3.98 ± 0.13 (−2.08)c | 3.59 ± 0.35 (−2.09)c |
| 6 mo PC | ||||
| Naive | 6.36 ± 0.11 | |||
| BCG s.c. | 5.40 ± 0.10 (−0.96) | |||
| BCG i.n. | 4.11 ± 0.15 (−2.25)c | |||
PC, postchallenge.
Values in parenthesis are protection data (naive CFU minus vaccine group CFU) for statistically significant differences (P < 0.05).
The CFU count of the BCG i.n. group is significantly lower than that of the BCG s.c. group (P < 0.05).
Lung pathology evaluation after vaccination and challenge.
To further assess the safety and effectiveness of i.n. BCG vaccination, semiquantitative computer-based pathology analysis of H&E-stained lung sections from vaccinated and naive mice was done following aerosol M. tuberculosis infection (24). As shown in Table 3, the inflammation values of the two vaccination groups were nearly identical and significantly lower than those of the naive controls at 2 months postvaccination and 1 month postchallenge. Similarly, the lung pathology inflammation values of the two immunization groups were again lower than those of nonvaccinated controls at 8 months postvaccination and 1 month postchallenge. Overall, although differences in inflammation values were not detected in the lung pathology analysis of the two vaccination groups, there was no evidence of increased inflammation from the i.n. BCG immunization. For representative micrographs of H&E-stained lung sections along with computer-generated images, see Fig. S1 in the supplemental material.
TABLE 3.
Lung inflammation scores following BCG vaccination and aerosol challenge with M. tuberculosis
| Exptl group | Mean postvaccination lung inflammation score ± SEMa |
|
|---|---|---|
| 2 mo | 8 mo | |
| Naive | 26.6 ± 0.62 | 31.9 ± 5.9 |
| BCG s.c. | 11.9 ± 1.3b | 22.2 ± 3.7 |
| BCG i.n. | 11.5 ± 1.1b | 21.6 ± 2.5 |
At 1 month postchallenge.
Significantly lower than that of naive controls (P < 0.05).
Lung immune responses following i.n. and s.c. BCG vaccinations.
To investigate potential mediators of immune pulmonary protection evoked by i.n. BCG vaccination, the CD4+ and CD8+ T cell subsets in the lungs of vaccinated mice were evaluated by multiparameter flow cytometry. In these studies, we focused on the analysis of MFT cells (expressing IFN-γ plus TNF-α and/or IL-2) because earlier studies had suggested that the induction of MFT cells was crucial for controlling infections by intracellular pathogens (25).
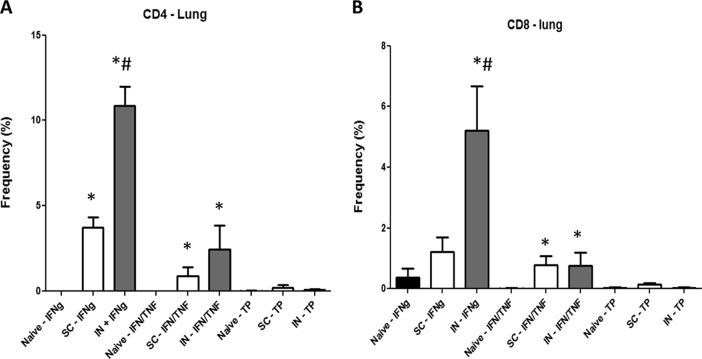
At 3 months postvaccination, cells were removed from the lungs of naive or BCG-immunized mice, stimulated overnight with BCG, and then stained by the intracellular cytokine staining procedures described in Materials and Methods. As shown in Fig. 1A, the frequency of lung CD4+ T cells making only IFN-γ was significantly elevated in both the i.n. (5 × 104-fold increase) and s.c. (1.8 × 104-fold increase) BCG vaccine groups relative to that in naive controls. Similarly, the frequencies of CD4 MFT cells expressing IFN-γ and TNF-α were also significantly higher in i.n. (1.2 × 104) and s.c. (4.5 × 103) immunized mice than in naive controls. Importantly, for IFN-γ+ CD4+ T cells, significantly higher frequencies (about 3-fold) were seen in the lungs of the i.n. vaccinated groups than in s.c. immunized animals.
FIG 1.
Multiparameter flow cytometry analyses of CD4 (A) and CD8 (B) lung T cells. Lung cells from four or five mice immunized with BCG by either the s.c. or the i.n. route were harvested 3 months postvaccination and then stimulated with BCG overnight in vitro. The frequency (percentage) of monofunctional T cells producing only IFN-γ (IFNg) or cells producing both IFN-γ and TNF-α, IFN-γ (IFN/TNF) and IL-2, or TNF-α and IL-2 or all three cytokines (TP) was determined. Significant differences from naive controls (*, P < 0.05) and mice immunized with BCG s.c. (#, P < 0.05) are indicated.
At 3 months postvaccination, increased frequencies of vaccine-induced lung CD8+ T cells were also observed (Fig. 1B). The concentrations of IFN-γ+ CD8+ T cells in i.n. BCG-vaccinated mice were significantly higher (P < 0.05) than in naive controls (15-fold) and s.c. BCG-vaccinated mice (5-fold). Additionally, the relative frequencies of IFN-γ+/TNF-α+ CD8+ T cells were elevated in i.n. (66-fold) and s.c. (52-fold) BCG-immunized mice.
The immune mechanisms associated with the protective responses induced by i.n. and s.c. BCG vaccinations were further evaluated by PCR arrays. At 3 months postvaccination, cells were removed from lungs and stimulated ex vivo with BCG overnight. Then, following RNA extraction, cDNA was generated and applied to PCR arrays designed to assess the expression of cytokine and chemokine genes. Table 4 shows the average gene expression data from at least four different experiments where the gene expression results are represented as values normalized to those of naive controls. In the lung, although only a few genes were consistently upregulated relative to those of controls, significantly elevated levels of IFN-γ, IL-12, IL-21, and lymphotoxin alpha (Ltα) gene expression were seen in the vaccinated animals. Importantly, all of these genes were more highly expressed in i.n. BCG-vaccinated mice than in s.c. BCG-immunized animals.
TABLE 4.
Relative gene expression in the lungs at 3 months postvaccination
| Gene product | Mean gene expression ± SEM |
|
|---|---|---|
| BCG s.c. | BCG i.n. | |
| IFN-γ | 7.3 ± 2.4a | 39.9 ± 14.4b |
| IL-21 | 3.0 ± 0.8 | 17.5 ± 4.0b |
| IL-12 | 1.4 ± 0.8 | 4.8 ± 1.2b |
| Ltα | 1.6 ± 0.6 | 5.2 ± 0.6b |
The relative expression of all of these genes was significantly greater than that of naive controls (P < 0.05).
Gene expression in the BCG i.n. group was significantly greater than that in the BCG s.c. group.
Splenic T cell responses at 3 and 8 months postvaccination.
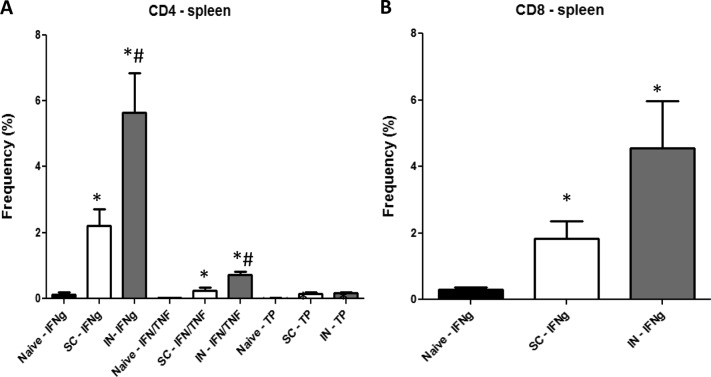
Since long-term increased anti-TB protective responses were seen in the spleens of i.n. BCG-vaccinated mice, we evaluated splenic responses to i.n. and s.c. BCG vaccinations at 3 and 8 months postimmunization by flow cytometry and PCR arrays. Compared to the lung results, a more extensive pattern of CD4 T cell induction was detected in the spleens of vaccinated mice at 3 months postvaccination (Fig. 2A). Again, increased frequencies of CD4+ T cells expressing either IFN-γ or IFN-γ and TNF-α were significantly higher (15- to 40-fold) in BCG-vaccinated mice than in naive controls. The splenic levels of the IFN-γ+ and IFN-γ+/TNF-α+ CD4+ T cells were also significantly higher (2.5- and 3-fold, respectively) in i.n. BCG-immunized mice than in the s.c. BCG vaccination group. Additionally, in the spleens of i.n. and s.c. BCG-vaccinated mice, relatively higher frequencies of CD4+ T cells expressing IFN-γ and IL-2 (75- and 35-fold, respectively) (not shown) and TP IFN-γ+/TNF-α+/IL-2+ (16- and 14-fold, respectively) CD4+ T cells were detected.
FIG 2.
Multiparameter flow cytometry analyses of CD4 (A) and CD8 (B) splenic T cells. Spleen cells from four or five mice immunized with BCG by either the s.c. or the i.n. route were harvested 3 months postvaccination and then stimulated with BCG for about 40 h in vitro. The frequency (percentage) of monofunctional T cells producing only IFN-γ (IFNg) or cells producing both IFN-γ and TNF-α, IFN-γ (IFN/TNF) and IL-2, or TNF-α and IL-2 or all three cytokines (TP) was determined. Significant differences from naive controls (*, P < 0.05) and mice immunized with BCG s.c. (#, P < 0.05) are indicated.
In contrast to the splenic CD4 T cell responses, the vaccine-induced pattern of CD8+ T cell responses at 3 months postvaccination was not complex. Only the frequency of monofunctional CD8+ T cells expressing IFN-γ was significantly higher in i.n. BCG-vaccinated mice than in s.c. BCG-immunized and nonvaccinated animals (15× higher than in naive controls, 2.5× higher than in s.c. BCG-vaccinated mice, P < 0.05) (Fig. 2B).
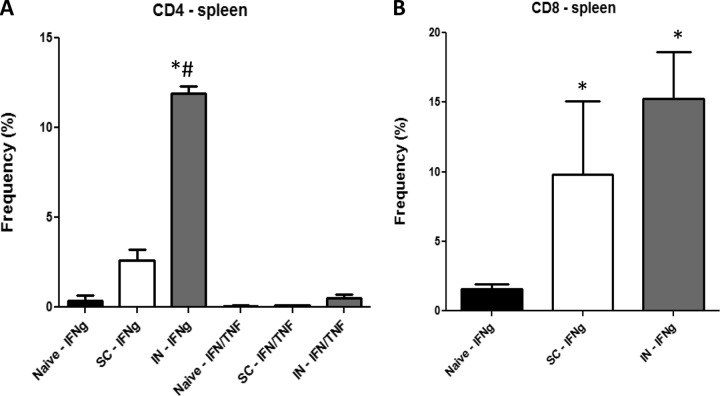
At 8 months postvaccination, exceedingly high frequencies of vaccine-induced monofunctional IFN-γ+ splenic T cells from the BCG i.n. group were detected in both the CD4+ and CD8+ T cell subsets (Fig. 3A and B). Significantly higher frequencies of IFN-γ+ CD4+ T cells were seen in i.n. BCG-vaccinated mice (12%) than in s.c. BCG-immunized (2.5%) and naive (0.4%) mice.
FIG 3.
Multiparameter flow cytometry analyses of CD4 (A) and CD8 (B) splenic T cells. Spleen cells from four or five mice immunized with BCG by either the s.c. or the i.n. route were harvested 8 months postvaccination and then stimulated with BCG for about 40 h in vitro. The frequency (percentage) of monofunctional T cells producing only IFN-γ (IFNg) or cells producing both IFN-γ and TNF-α, IFN-γ (IFN/TNF) and IL-2, or TNF-α and IL-2 or all three cytokines (TP) was determined. Significant differences from naive controls (*, P < 0.05) and mice immunized with BCG s.c. (#, P < 0.05) are indicated.
PCR array experiments showed that a more diverse group of genes were upregulated in the spleen at 3 months after BCG vaccination (Table 5). In addition to increased expression of IFN-γ, IL-2, and Ltα, elevated levels of colony-stimulating factor 2 (CSF2), IL-3, IL-5, IL-9, IL-13, IL-21, IL-24, IL-27, leukemia inhibitory factor (LIF), and tumor necrosis factor superfamily member 15 (TNFS15) were also seen. In contrast to the pulmonary results, no statistically significant differences between the two BCG vaccination groups were observed. Interestingly, at least 10-fold increases (relative to controls) in genes expressing IFN-γ, IL-2, IL-3, IL-9, and IL-21 were detected in one or both vaccination groups.
TABLE 5.
Relative gene expression in the spleen at 3 months postvaccination
| Gene product | Mean fold gene expression increase ± SEMa |
|
|---|---|---|
| BCG s.c. | BCG i.n. | |
| CSF2 | 3.7 ± 0.5 | 4.2 ± 0.6 |
| IFN-γ | 32.1 ± 8.9 | 29.8 ± 11.2 |
| IL-2 | 7.0 ± 0.8 | 10.1 ± 2.3 |
| IL-3 | 23.9 ± 0.5 | 25.7 ± 1.6 |
| Il-5 | 4.6 ± 1.0 | 5.7 ± 0.8 |
| IL-9 | 10.2 ± 2.3 | 8.7 ± 1.3 |
| IL-13 | 3.4 ± 0.6 | 3.0 ± 0.7 |
| IL-21 | 5.8 ± 0.2 | 10.4 ± 1.8 |
| IL-24 | 1.9 ± 0.2 | 3.4 ± 0.5 |
| IL-27 | 4.7 ± 1.2 | 5.5 ± 1.0 |
| LIF | 6.9 ± 3.3 | 5.1 ± 0.4 |
| Ltα | 4.0 ± 1.6 | 3.7 ± 0.2 |
| TNFS15 | 3.1 ± 0.5 | 4.7 ± 1.2 |
The relative expression of all of these genes was significantly greater than that in naive controls (P < 0.05).
To further evaluate the long-term protection observed in the spleen after i.n. BCG vaccination, we analyzed the gene expression patterns in spleen cells at 8 months postvaccination by using PCR arrays. The increased expression of many of the same genes detected at the 3-month time point was seen at this later time. In the spleen cells of i.n. BCG-vaccinated mice, elevated relative expression of IFN-γ, IL-2, IL-3, IL-9, IL-11, IL-13, IL-21, and Ltα was observed (Table 6). Of these genes, the expression of those for IFN-γ, IL-9, IL-11, and IL-21 was significantly higher in the spleen cells of i.n. BCG-vaccinated mice than in those of s.c. BCG-vaccinated mice at 8 months postvaccination.
TABLE 6.
Relative gene expression in the spleen at 8 months postvaccination
| Cytokine | Mean fold mRNA level increase ± SEMa |
|
|---|---|---|
| BCG s.c. | BCG i.n. | |
| IFN-γ | 6.0 ± 1.5 | 21.3 ± 2.9c |
| IL-11 | NSb | 3.2 ± 3.0c |
| IL-13 | 3.1 ± 0.6 | 4.6 ± 0.8 |
| IL-2 | 9.0 ± 2.9 | 6.6 ± 1.7 |
| IL-21 | NS | 9.2 ± 1.5c |
| IL-3 | 8.0 ± 1.8 | 6.6 ± 1.0 |
| IL-9 | 19.8 ± 4.5 | 39.9 ± 6.0c |
| Ltα | 6.7 ± 0.6 | 6.2 ± 1.2 |
Values are relative to those of naive mice.
NS, not significantly elevated relative to naive mice.
P < 0.05 relative to BCG s.c. group.
DISCUSSION
Respiratory vaccination with BCG has been considered a potential alternative to i.d. BCG immunization against TB for nearly 50 years, but large clinical trials designed to demonstrate the effectiveness of aerosolized BCG vaccination have not been pursued. No doubt the lack of a validated delivery device for the administration of aerosolized BCG has hindered the clinical evaluation of this vaccination route. In a review, Tonnis et al. discussed the technical hurdles associated with the delivery of vaccines (including BCG) into the lungs and examined the merits and weaknesses of different inhalation devices (26). Clearly, technological advances are required for the routine pulmonary delivery of BCG; however, the advent of an influenza vaccine licensed for i.n. administration (FluMist) has provided optimism that these technical advances are achievable for other vaccines.
Another reason for the lack of interest in evaluating the clinical efficacy of respiratory BCG vaccination has been the inconsistent results observed in animal models. While several investigators have concluded from preclinical studies that aerosol infection with BCG enhances protection against a subsequent M. tuberculosis challenge, others have reported that respiratory BCG immunization does not induce greater protective responses than standard i.d. or s.c. BCG vaccination (7–14). Differences in study designs, including various vaccination-challenge intervals and contrasting postchallenge evaluation periods, may have contributed to these conflicting results.
Given these confusing data and the recent disappointing clinical results with the MVA85 BCG boosting vaccine, we reexamined the protective activity of the respiratory route of BCG administration in a long-term study (21). In contrast to previous studies, we undertook an exhaustive evaluation of protection by looking at long-term efficacy after vaccination (up to 10 months) and an aerosol challenge (up to 6 months). We also initiated a more comprehensive evaluation of the immune responses in lungs and spleens comparing the s.c. and i.n. immunization routes by using multiparameter flow cytometry and PCR arrays at different time points postimmunization.
Novel to our study, although i.n. delivery of BCG induced only modestly persistent protection in mouse lungs, protective responses in mouse spleens were significantly improved relative to s.c. immunizations over 10 months postvaccination. For example, consistent with short-term studies by Aguilo et al., Chen et al., and Giri et al., who showed that pulmonary administration of BCG was superior to the s.c. route in terms of protection in the lungs at early time points postvaccination, our pulmonary protection results were significantly better for the i.n. route than for the s.c. route at 2 and 4 months postvaccination (14, 16, 27). However, no differences in lung postchallenge protective responses were seen at 8 and 10 months postvaccination. The early pulmonary protection data are consistent with the flow cytometry and gene expression data, since we show that at 3 months postvaccination, significantly higher relative frequencies of CD4 T cells producing IFN-γ or IFN-γ and TNF-α were detected and elevated levels of IFN-γ, IL-12, IL-21, and Ltα were seen in the lungs of i.n. vaccinated mice.
Surprisingly, significantly increased relative protection in the spleen was seen in i.n. BCG-vaccinated mice at all study time points, including at 8 and 10 months postvaccination. The strong splenic protection exceeded 1.9 log10 (relative to naive controls) in five of the nine test periods. Similarly, Nuermberger et al. showed (at 6 weeks after M. tuberculosis infection) that there was virtually no detectable dissemination to the spleen after an M. tuberculosis challenge of mice that had been aerogenically vaccinated with BCG (15). The mechanisms of the enhanced protection in the spleen in BCG-vaccinated mice are uncertain and difficult to interpret. Interestingly, our flow cytometry and PCR array data suggest that i.n. BCG immunization could curtail relative spleen bacterial growth through the improved expression of protective immunity in the spleen itself. At both 3 and 8 months postvaccination, greater cellular responses were detected in the spleens of i.n. BCG-vaccinated mice than in s.c. BCG-immunized animals. In particular, at 8 months postvaccination, when strong persistent protective responses were still observed in the spleen, exceedingly high IFN-γ responses were detected by flow cytometry and PCR analysis in spleen cells of i.n. BCG-vaccinated mice. Two recent studies have suggested how the targeted delivery of vaccines to the lung can lead to improved protection at distal sites. Ciabattini et al. showed that following i.n. immunization of mice, antigen-specific proliferating T cells were observed in the distal lymph nodes and the spleen (28). Importantly, Ruane et al. reported that lung dendritic cells targeted by i.n. immunization induced the migration of protective T cells to the gastrointestinal tract, leading to protection against an enteric Salmonella challenge (29). Similar mechanisms could generate long-term anti-TB immunity in the spleen after i.n. BCG immunization. Furthermore, i.n. BCG immunization may induce better protection at distal sites because respiratory immunization may more efficiently allow trafficking of BCG bacilli to lymph nodes. The presence of BCG in relevant lymph nodes is likely critical for the development of strong and durable protective immunity. Wolf et al. have shown (in an M. tuberculosis infection model) that the initiation of anti-TB adaptive immune responses is dependent on antigen production in the lymph nodes and not the lungs (30). We have recently demonstrated that while the biodistribution of BCG bacilli after s.c. vaccination was highly variable in murine lungs and spleens, live BCG was consistently found in the lymph nodes for at least 1 year after immunization in mice that protected against an M. tuberculosis challenge (S. Derrick, unpublished results). Long-term studies assessing the biodistribution of BCG in relevant organs following i.n. immunization are currently ongoing.
Although most of the upregulated immune response molecules detected in our gene expression studies have been previously reported to be induced by BCG vaccination, surprisingly, substantially increased levels of IL-3 and IL-9 were seen in both vaccination groups. It is unusual to detect IL-3 and IL-9 expression in response to BCG vaccination. IL-3 is referred to as CSF because it stimulates the differentiation of multipotent hematopoietic stem cells and the proliferation of myeloid cells in conjugation with other cytokines. While a role for IL-3 in the pathogenesis of TB has not been established, IL-3 has been shown to support human alveolar macrophage development and human immune responses in the lungs of human IL-3/granulocyte macrophage CSF knock-in mice (31). Similarly, while IL-9 has been associated with impaired Th1 immune responses in TB patients, its role in M. tuberculosis pathogenesis is unknown (32). Generally, IL-9 has been shown to promote the survival of a variety of different cell types, including T cells (33–35). Recently, Turner et al. demonstrated that IL-9 mediated the survival of innate lymphoid cells, which contributed to the restoration of tissue integrity and the reduction in lung inflammation observed after a helminth infection (36). Given that IL-3 and IL-9 may influence lung function, specific studies focusing on the impact of these cytokines on BCG vaccination of M. tuberculosis infection is clearly warranted.
Taken together, our data show that i.n. BCG vaccination induces a complex immune response and protection profile. Administration of BCG to mice by the i.n. route clearly evokes long-lived protective responses that reduce M. tuberculosis infections at extrapulmonary sites. However, while elevated anti-TB protection in murine lungs is seen early after i.n. BCG vaccination, the enhanced pulmonary protection (relative to that afforded by s.c. immunization) is not persistent. Given the undetermined clinical benefit, the possible risks associated with aerosolized BCG administration should be carefully and thoroughly considered before the initiation of clinical trials. However, since the advent of improved aerosol delivery devices should reduce potential safety concerns, the increased overall potency of the i.n. route of BCG immunization should encourage further investigation of this vaccination approach.
Supplementary Material
Footnotes
Published ahead of print 20 August 2014
Supplemental material for this article may be found at http://dx.doi.org/10.1128/CVI.00394-14.
REFERENCES
- 1.World Health Organization. 2014. Tuberculosis fact sheet no. 104. World Health Organization, Geneva, Switzerland: http://www.who.int/mediacentre/factsheets/fs104/en. [Google Scholar]
- 2.World Health Organization. 2014. Immunization, vaccines, and biologicals: tuberculosis. World Health Organization, Geneva, Switzerland: http://www.who.int/immunization/diseases/tuberculosis/en. [Google Scholar]
- 3.Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, Mosteller F. 1994. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA 271:698–702. [PubMed] [Google Scholar]
- 4.Colditz GA, Berkey CS, Mosteller F, Brewer TF, Wilson ME, Burdick E, Fineberg HV. 1995. The efficacy of bacillus Calmette-Guerin vaccination of newborns and infants in the prevention of tuberculosis: meta-analyses of the published literature. Pediatrics 96:29–35. [PubMed] [Google Scholar]
- 5.Mangtani P, Abubakar I, Ariti C, Beynon R, Pimpin L, Fine PE, Rodrigues LC, Smith PG, Lipman M, Whiting PF, Sterne JA. 2014. Protection by BCG vaccine against tuberculosis: a systemic review of randomized controlled trials. Clin. Infect. Dis. 58:470–480. 10.1093/cid/cit790. [DOI] [PubMed] [Google Scholar]
- 6.Giri PK, Khuller GK. 2008. Is intranasal vaccination a feasible solution for tuberculosis? Expert Rev. Vaccines 7:1341–1356. 10.1586/14760584.7.9.1341. [DOI] [PubMed] [Google Scholar]
- 7.Middlebrook G. 1961. Immunological aspects of airborne infection: reactions to inhaled antigens. Bacteriol. Rev. 24:331–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barclay WR, Busey WM, Dalgard DW, Good RC, Janicki BW, Kasik JE, Ribi E, Ulrich CE, Wolinsky E. 1973. Protection of monkeys against airborne tuberculosis by aerosol vaccination with bacillus Calmette-Guerin. Am. Rev. Respir. Dis. 107:351–358. [DOI] [PubMed] [Google Scholar]
- 9.Lefford MJ. 1977. Induction and expression of immunity after BCG immunization. Infect. Immun. 18:646–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schell RF, Ealey WF, Harding GE, Smith DW. 1974. The influence of vaccination on the course of experimental airborne tuberculosis in mice. J. Reticuloendothel. Soc. 16:131–138. [PubMed] [Google Scholar]
- 11.Orme IM, Collins FM. 1986. Aerogenic vaccination of mice with Mycobacterium bovis BCG. Tubercle 67:133–140. 10.1016/0041-3879(86)90007-3. [DOI] [PubMed] [Google Scholar]
- 12.Lagranderie M, Ravisse P, Marchal G, Gheorghiu M, Balasubramanian V, Weigeshaus EH, Smith DW. 1993. BCG-induced protection in guinea pigs vaccinated and challenged via the respiratory route. Tuber. Lung Dis. 74:38–46. 10.1016/0962-8479(93)90067-8. [DOI] [PubMed] [Google Scholar]
- 13.Goonetilleke NP, McShane H, Hannan CM, Anderson RJ, Brookes RH, Hill AV. 2003. Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacille Calmette-Guerin vaccine using mucosal administration and boosting with a recombinant modified vaccinia virus Ankara. J. Immunol. 171:1602–1609. 10.4049/jimmunol.171.3.1602. [DOI] [PubMed] [Google Scholar]
- 14.Chen L, Wang J, Zganiacz A, Xing Z. 2004. Single intranasal mucosal Mycobacterium bovis BCG vaccination confers improved protection compared to subcutaneous vaccination against pulmonary tuberculosis. Infect. Immun. 72:238–246. 10.1128/IAI.72.1.238-246.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nuermberger EL, Yoshimatsu T, Tyagi S, Bishai WR, Grosset JH. 2004. Paucibacillary tuberculosis in mice after prior aerosol immunization with Mycobacterium bovis BCG. Infect. Immun. 72:1065–1071. 10.1128/IAI.72.2.1065-1071.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giri PK, Verma I, Khuller GK. 2006. Protective efficacy of intranasal vaccination with Mycobacterium bovis BCG against airway Mycobacterium tuberculosis challenge in mice. J. Infect. 53:350–356. 10.1016/j.jinf.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 17.Rosenthal SR, McEnery JH, Raisys N. 1968. Aerogenic BCG vaccination against tuberculosis in animal and human subjects. J. Asthma Res. 5:309–323. 10.3109/02770906809100348. [DOI] [PubMed] [Google Scholar]
- 18.Cusumano CL, Jernigan JA, Waldman RH. 1975. Aerosolized BCG (Tice strain) treatment of bronchogenic carcinoma: phase I study. J. Natl. Cancer Inst. 55:275–279. [PubMed] [Google Scholar]
- 19.Garner FB, Meyer CA, White DS, Lipton A. 1975. Aerosol BCG treatment of carcinoma metastatic to the lung: a phase I study. Cancer 35:1088–1094. . [DOI] [PubMed] [Google Scholar]
- 20.Dalmia N, Ramsay AJ. 2012. Prime-boost approaches to tuberculosis vaccine development. Expert Rev. Vaccines 11:1221–1233. 10.1586/erv.12.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tameris MD, Hatherill M, Landry BS, Scriba TJ, Snowden MA, Lockhart S, Shea JE, McClain JB, Hussey GD, Hanekom WA, Mahomed H, McShane H, MVA85 Trial Study Team A 2013. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomized, placebo-controlled phase 2b trial. Lancet 381:1021–1028. 10.1016/S0140-6736(13)60177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Derrick SC, Dao D, Yang A, Kolibab K, Jacobs WR, Morris SL. 2012. Formulation of a mmaA4 gene deletion mutant of Mycobacterium bovis BCG in cationic liposomes significantly enhances protection against tuberculosis. PLoS One 7(3):e32959. 10.1371/journal.pone.0032959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeon BY, Derrick SC, Lim J, Kolibab K, Dheenadhayalan V, Yang AL, Kreiswirth B, Morris SL. 2008. Mycobacterium bovis BCG immunization induces protective immunity against nine different Mycobacterium tuberculosis strains in mice. Infect. Immun. 76:5173–5180. 10.1128/IAI.00019-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Derrick SC, Perera LP, Dheenadhayalan V, Yang A, Kolibab K, Morris SL. 2008. The safety of post-exposure vaccination of mice infected with Mycobacterium tuberculosis. Vaccine 26:6092–6098. 10.1016/j.vaccine.2008.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Darrah PA, Patel DT, De Luca PM, Lindsay RW, Davey DF, Flynn BJ, Hoff ST, Andersen P, Reed SG, Morris SL, Roederer M, Seder RA. 2007. Multifunctional TH1 cells define a correlate of vaccine mediated protection against Leishmania major. Nat. Med. 13:843–850. 10.1038/nm1592. [DOI] [PubMed] [Google Scholar]
- 26.Tonnis WF, Lexmond AJ, Frijlink HW, de Boer AH, Hinrichs LJ. 2013. Devices and formulations for pulmonary vaccination. Expert Opin. Drug Deliv. 10:1383–1397. 10.1517/17425247.2013.810622. [DOI] [PubMed] [Google Scholar]
- 27.Aguilo N, Toledo AM, Lopez-Roman EM, Perez-Herran E, Gormley E, Rullas-Trincado J, Angulo-Barturen I, Martin C. 2014. Pulmonary Mycobacterium bovis BCG vaccination confers dose-dependent superior protection compared to that of subcutaneous vaccination. Clin. Vaccine Immunol. 21:594–597. 10.1128/CVI.00700-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ciabattini A, Pettini E, Fiorino F, Prota G, Pozzi G, Medaglini D. 2011. Distribution of primed T cells and antigen-loaded antigen presenting cells following intranasal immunization in mice. PLoS One 6(4):e19346. 10.1371/journal.pone.0019346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruane D, Brane L, Reis BS, Cheong C, Poles J, Do Y, Zhu H, Velinzon K, Choi J-H, Studt N, Mayer L, Lavelle EC, Steinman RM, Mucida D, Mehandru S. 2013. Lung dendritic cells induce migration of protective T cells to the gastrointestinal tract. J. Exp. Med. 210:1871–1888. 10.1084/jem.20122762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolf AJ, Desvignes L, Linas B, Banaiee N, Tamura T, Takatsu K, Ernst JD. 2008. Initiation of the adaptive immune response to Mycobacterium tuberculosis depends on antigen production in the local lymph node, not the lungs. J. Exp. Med. 205:105–115. 10.1084/jem.20071367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Willinger T, Rongvaux A, Takizawa H, Yancopoulos GD, Valenzuela DM, Murphy AJ, Auerbach W, Eynon EE, Steven S, Manz MG, Flavell RA. 2011. Human IL-3/GM-CSF knock-in mice support human alveolar macrophage development and human immune responses in the lung. Proc. Natl. Acad. Sci. U. S. A. 108:2390–2395. 10.1073/pnas.1019682108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wu B, Huang C, Kato-Maeda M, Hopewell PC, Daley CL, Krensky AM, Clayberger C. 2008. IL-9 is associated with an impaired Th1 immune response in patients with tuberculosis. Clin. Immunol. 126:202–210. 10.1016/j.clim.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 33.Gounni AS, Gregory B, Nutku GB, Aris F, Latifa K, Minshall E, North J, Tavernier J, Levit R, Nicolaides N, Robinson D, Hamid Q. 2000. Interleukin-9 enhances interleukin-5 receptor expression, differentiation, and survival of human eosinophils. Blood 96:163–171. [PubMed] [Google Scholar]
- 34.Fontaine RH, Cases O, Lelievre V, Mesples B, Renauld JC, Loron G, Degos V, Dournaud P, Baud O, Gressens P. 2008. IL-9/IL-9 receptor signaling selectively protects cortical neurons against developmental apoptosis. Cell. Death Differ. 15:1542–1552. 10.1038/cdd.2008.79. [DOI] [PubMed] [Google Scholar]
- 35.Elyaman W, Bradshaw EM, Uyttenhove C, Dardalhon V, Awasthi A, Imitola J, Bettelli E, Oukka M, van Snick J, Renauld JC, Kuchroo VK, Khoury SJ. 2009. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc. Natl. Acad. Sci. U. S. A. 106:12885–12890. 10.1073/pnas.0812530106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turner J-E, Morrison PJ, Wilhelm C, Wilson M, Ahlfors H, Renauld J-C, Panzer U, Helmby J, Stockinger B. 2013. IL-9-mediated survival of type 2 innate lymphoid cells promotes damage control in helminth-induced lung inflammation. J. Exp. Med. 210:2951–2965. 10.1084/jem.20130071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.