Abstract
Individuals with human immunodeficiency virus (HIV) infection have increased susceptibility to invasive disease caused by Salmonella enterica serovar Typhimurium. Studies from Africa have suggested that this susceptibility is related in part to the development of a high level of lipopolysaccharide (LPS)-specific IgG that is able to inhibit the killing of S. Typhimurium by bactericidal antibodies in healthy individuals. To explore this issue further, we examined the bactericidal activity against S. Typhimurium using serum and plasma samples from healthy controls and various clinical subgroups of HIV-infected adults in the United States. We found that the bactericidal activity in the samples from HIV-positive elite controllers was comparable to that from healthy individuals, whereas it was significantly reduced in HIV-positive viremic controllers and untreated chronic progressors. As demonstrated previously for healthy controls, the bactericidal activity of the plasma from the elite controllers was inhibited by preincubation with S. Typhimurium LPS, suggesting that it was mediated by anti-LPS antibodies. S. Typhimurium LPS-specific IgG was significantly reduced in all subgroups of HIV-infected individuals. Interestingly, and in contrast to the healthy controls, plasma from all HIV-positive subgroups inhibited in vitro killing of S. Typhimurium by plasma from a healthy individual. Our results, together with the findings from Africa, suggest that multiple mechanisms may be involved in the HIV-induced dysregulation of humoral immunity to S. Typhimurium.
INTRODUCTION
Invasive disease caused by nontyphoidal Salmonella (NTS) strains, mainly Salmonella enterica serovar Typhimurium, has become a significant public health problem in sub-Saharan Africa (1, 2). Children <2 years of age constitute one population that is particularly susceptible to NTS sepsis, and a recent study from Malawi has helped to clarify the immunological basis of this susceptibility. This study showed in an in vitro assay that serum from most healthy adults and older children had antibodies that were able to kill S. Typhimurium, whereas most infants below the age of about 16 months lacked such bactericidal antibodies (3). These findings, which correlated inversely with the pattern of incidence of invasive salmonellosis (i.e., high in infants and low in older children and adults) suggested an important role for antibody-mediated protection in invasive NTS disease. The initial analysis indicated that the bactericidal antibodies present in healthy adults in Malawi were directed against Salmonella outer membrane proteins, but recent work from our laboratory, as well as that from other investigators, has focused on the O antigen of lipopolysaccharide (LPS) as the relevant antigenic target (4–8). Our experiments demonstrated that serum and plasma from the majority of healthy adults in the United States had bactericidal activity against S. Typhimurium and that this activity was mediated by antibodies to an epitope in the outer polysaccharide of LPS (5). We also found that the bactericidal activity was less robust in children <5 years of age, correlating with lower levels of S. Typhimurium LPS-specific IgM and IgG in this age group (5). In addition to young children, HIV-infected adults represent a second population that has a high risk of invasive NTS disease (9–12). The results from Malawi suggest that this susceptibility is at least partly related to an HIV-associated abnormality of the humoral response to S. Typhimurium. The abnormality manifests as high levels of anti-LPS IgG antibodies that are able to inhibit the killing of S. Typhimurium by bactericidal antibodies from healthy individuals (4). Interestingly, although these LPS-specific IgG antibodies inhibited bacterial killing when present at high concentrations, they were able to kill S. Typhimurium in the in vitro bactericidal assay when they occurred at the lower levels found in healthy individuals or in a subset of HIV-positive individuals (4, 6).
The circulating viral load is a major determinant of disease progression in HIV infection. Viral loads of <1,000 to 2,000 RNA copies per ml of serum correlate with long-term AIDS-free survival (13, 14). A small minority of HIV-infected individuals have remained without overt disease for many years (>30 years in some cases) by controlling viral replication and keeping viral loads at a low level even in the absence of treatment. Such individuals have been classified into 2 subgroups: elite controllers (estimated to be about 1 in 300 infected people), who maintain viral loads below the level of detection by the currently available ultrasensitive assays (<50 to 75 copies/ml), and viremic controllers (about 7% of infected people), who maintain viral loads of 50 to 2,000 copies/ml (15, 16). These numbers are in contrast to individuals with chronic progressive disease, who typically have viral loads of >10,000 copies/ml without treatment. Despite their very low circulating viral loads, it is becoming clear from recent studies that even elite controllers have reservoirs of latently infected CD4+ T cells, and some controllers will go on to develop declining CD4+ T-cell numbers and AIDS-defining illnesses, possibly as a result of abnormalities of lymphopoiesis and thymic function (17–20).
It is not known whether the dysregulation of humoral immunity to S. Typhimurium observed in HIV-infected adults in Africa applies to other populations or whether it is also seen in the clinical subgroups of infection that are associated with low viral loads. Clarifying these issues might provide insight into the immunological abnormalities induced by HIV infection, and it might also have implications for developing and applying strategies to prevent invasive NTS disease in vulnerable HIV-positive populations. Accordingly, we tested serum and plasma samples from HIV-infected individuals and HIV-negative healthy controls in the United States for the presence of bactericidal activity against S. Typhimurium. The HIV-infected group included elite controllers, viremic controllers, and untreated and treated chronic progressors. The results of our analysis confirm that HIV infection is associated with abnormalities of anti-Salmonella bactericidal activity and antibody responses. However, notable differences between our findings and those reported from Africa suggest that the mechanisms underlying the observed impaired bactericidal activity may vary depending on the geographic location and clinical characteristics of the HIV-infected population under consideration.
MATERIALS AND METHODS
Serum and plasma samples.
Deidentified serum and plasma samples from healthy adults from the United States were collected during routine health maintenance visits to clinics at the Massachusetts General Hospital. The criteria used for their selection have been described in detail earlier (5). Plasma samples from HIV-positive individuals were collected in clinics at hospitals in the Boston area and elsewhere in the United States, and they were part of a collection maintained by the Ragon Institute. As described previously (15), the HIV-positive patients were categorized into subgroups on the basis of viral load (elite controllers, viremic controllers, and chronic progressors, both untreated and treated with antiretroviral therapy for various periods). All samples were stored at −80°C until use. Samples from a total of 13 HIV-negative healthy controls and 52 HIV-positive individuals (12 elite controllers, 13 viremic controllers, 15 untreated chronic progressors, and 12 treated chronic progressors) were characterized. All experiments with human samples were approved by the Human Research Committee of Massachusetts General Hospital.
Bactericidal assays.
The killing of Salmonella by serum or plasma was assessed essentially as described previously (3, 5). In brief, 5 μl of a phosphate-buffered saline (PBS) suspension of S. Typhimurium strain SL1344 containing approximately 104 CFU was incubated with 22.5 μl of serum or plasma and 22.5 μl of PBS at 37°C for 1 h. The number of bacteria surviving at the end of that period was determined by serial dilution of the reaction mixture and plating on Luria-Bertani (LB) agar, and the results are displayed in the figures as log10 CFU. Bacteria mixed with 45 μl of PBS alone served as the negative control. The limit of detection of the bactericidal assay was 50 CFU. Competition with LPS was carried out as described previously (4, 5) by preincubating serum or plasma samples for 60 min at room temperature with 100 μg/ml of purified LPS from S. Typhimurium (List Biological Laboratories, Campbell, CA). To test for inhibitory activity, 22.5 μl of plasma from healthy or HIV-infected individuals was mixed with an equal volume of plasma from a single specified healthy individual and then incubated at 37°C for 1 h with 5 μl of a suspension of 104 S. Typhimurium bacteria. The number of surviving bacteria was determined as described above.
Enzyme-linked immunosorbent assays.
To detect S. Typhimurium LPS-specific antibodies, we used procedures described in detail earlier (5, 21). Immulon II microtiter plates were coated with S. Typhimurium LPS diluted in PBS at a concentration of 10 μg/ml, washed, blocked, and then incubated overnight at 4°C with triplicate aliquots of serum or plasma diluted 500-fold in PBS. The plates were developed with horseradish peroxidase-conjugated secondary antibodies specific for human IgM or IgG (BD Biosciences, San Jose, CA), followed by o-phenylenediamine substrate. The intensity of color change was measured at 490 nm in a plate reader. In a subset of samples, the endpoint titers of LPS-specific IgG were determined by carrying out an enzyme-linked immunosorbent assay (ELISA) with serial 2-fold dilutions of the samples. The titer was defined as the reciprocal of the highest dilution that yielded an absorbance 3 times as high as the background (the background being the absorbance when the sample was replaced with PBS, with all other steps of the ELISA being the same). The total serum IgG was measured using an ELISA kit from eBioscience (San Diego, CA), according to their guidelines.
Hemolytic complement assay.
As described in detail earlier, we estimated the hemolytic complement activity in our samples using a published microassay that has been shown to have good correlation with the traditional CH50 titration method (the CH50 measures the total hemolytic activity of a test sample and is the reciprocal of the dilution of serum complement needed to lyse 50% of a standardized suspension of sheep erythrocytes coated with antierythrocyte antibody) (5, 22). In brief, 25-μl aliquots of serum or plasma were incubated with 75 μl of antibody-coated sheep erythrocytes suspended at 5 × 108 cells per ml in gelatin-Veronal buffer (Complement Technology, Inc., Tyler, TX) for 30 min at 37°C in a microtiter plate. Twenty-five microliters of human serum complement (Sigma-Aldrich, St. Louis, MO; derived from pooled normal human plasma, with a nominal activity of 41 CH50 units/ml) was assayed in parallel to provide reference hemolytic activity. The negative control consisted of 25 μl of gelatin-Veronal buffer instead of serum or plasma, while 25 μl of serum or plasma plus 75 μl of buffer was used as the blank. At the end of 30 min, the plate was centrifuged briefly and the absorbance at 540 nm (A540) of the supernatants used as a measure of the amount of hemoglobin released from the lysed erythrocytes. The hemolytic activity of the individual serum or plasma samples was calculated as a percentage of the activity of the reference sample as follows: (A540 of sample − A540 of negative control)/(A540 of reference − A540 of negative control) × 100.
Statistical analysis.
The results from the groups of serum or plasma samples are displayed as scattergrams, with each symbol representing an individual sample and the error bars indicating the mean ± standard deviation (SD). The intergroup differences were analyzed by the Mann-Whitney U test using Prism version 6.0c (GraphPad Software, Inc., La Jolla, CA). A P value of <0.05 was considered to be significant.
RESULTS
Using an assay essentially identical to that described in previously published studies from Africa (3, 4) and our own work from the United States (5), we tested serum and plasma samples from groups of healthy HIV-negative adults and from HIV-infected adults for the presence of bactericidal activity against S. Typhimurium. As we reported earlier (5), all the healthy control samples significantly reduced the number of surviving bacteria by 1 to 2 logs relative to PBS over the course of 1 h (Fig. 1). In contrast, the samples from the HIV-infected individuals had various bactericidal activities that differed between the clinical subgroups (Fig. 1). The elite controllers had bactericidal activity that was comparable to that of the healthy individuals, whereas the viremic controllers and untreated chronic progressors had a significantly reduced ability to kill S. Typhimurium. Interestingly, antiretroviral treatment appeared to improve bactericidal activity to some extent, although the effect did not reach statistical significance (Fig. 1).
FIG 1.
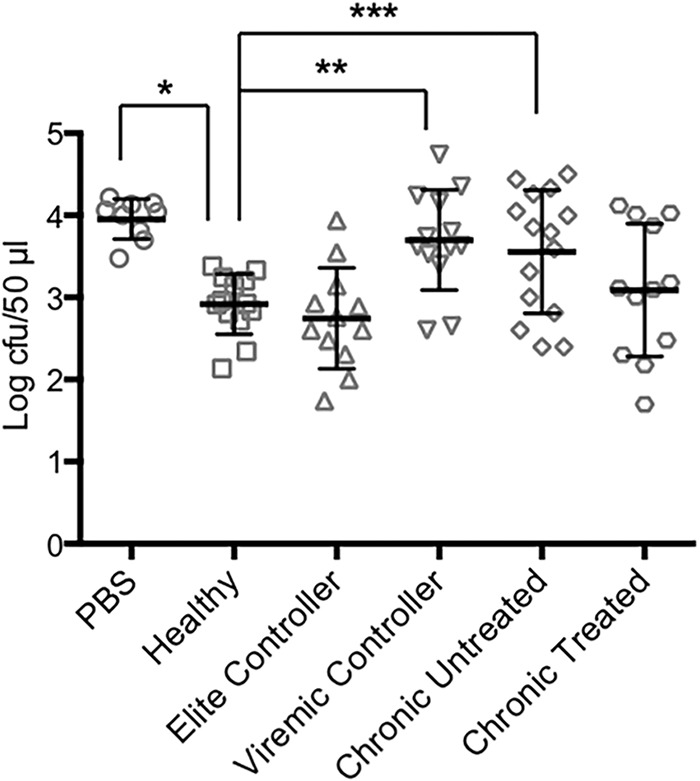
Bactericidal activity in serum and plasma samples from healthy and HIV-positive individuals. The bactericidal assays were carried out using PBS or samples from healthy or HIV-positive individuals. The numbers of bacteria surviving at the end of the 1-h incubation with the indicated samples are shown (mean ± SD). *, P < 0.0001; **, P = 0.0008; ***, P = 0.025.
Earlier work has shown that serum bactericidal activity against S. Typhimurium is antibody mediated and complement dependent (3–5). The bactericidal activity of the samples from the elite controllers was also complement dependent, as indicated by its abrogation by heating to 56°C for 30 min (data not shown). Our experiments with serum and plasma from healthy individuals in the United States demonstrated that the antigenic target of the activity was LPS (5). To determine if the bactericidal activity detected in the samples from the elite controllers was also directed against LPS, we preincubated a subset of these samples with an excess of S. Typhimurium LPS before carrying out the bactericidal assay. As shown in Fig. 2, the LPS preincubation significantly inhibited bactericidal activity, suggesting that LPS was the target of the activity. Based on this finding, we sought to elucidate the mechanism responsible for the reduced bactericidal activity of the HIV-positive samples by examining their levels of S. Typhimurium LPS-specific antibodies. The samples from all subgroups of the HIV-infected individuals had LPS-specific IgG levels that were significantly lower than those in the healthy controls, as indicated by the relative absorbances detected using a standard ELISA (Fig. 3A). We also carried out endpoint titer measurements using a subset of the samples. The results demonstrated a pattern similar to that observed in the standard ELISA, i.e., the titers of LPS-specific IgG in all the HIV-positive subgroups were lower than those in the healthy controls, with the difference reaching statistical significance in the elite controllers and the untreated chronic progressors (Fig. 3B). This HIV-associated abnormality of humoral immunity was antigen and isotype specific, since the total IgG levels in the HIV-infected individuals were comparable to those in the healthy controls, and the levels of LPS-specific IgM varied considerably across the HIV-positive subgroups (data not shown). Somewhat surprisingly, the level of S. Typhimurium LPS-specific IgG was significantly reduced in the elite controllers relative to that in the healthy individuals, despite the relatively normal bactericidal activity of this HIV-positive subgroup. This finding suggests that the impaired bactericidal activities in the other HIV-positive subgroups (which have S. Typhimurium LPS-specific IgG levels similar to those of the elite controllers) might involve additional abnormalities. Accordingly, we tested the samples for complement activity to determine if differences in this parameter play a role. Based on the ability to lyse sensitized sheep red blood cells, we found that the complement activity in the elite controllers was similar to that in the healthy individuals, whereas the activity of viremic controllers was significantly lower than that of the healthy individuals (Fig. 4). The complement activity in the untreated chronic progressors was also reduced relative to the healthy controls, although the difference did not reach statistical significance (P = 0.086, Fig. 4). These findings suggest that the reduced complement activity associated with HIV infection may be an additional factor contributing to the attenuation of bactericidal activity against S. Typhimurium and might help explain why samples from the elite controllers had relatively normal bactericidal activity while those from the viremic controllers did not, even though the levels of S. Typhimurium LPS-specific IgG are similar in the two subgroups. Interestingly, the plasma samples from the chronic progressors on antiretroviral treatment had a clear trend toward higher complement activity relative to that of the untreated group (P = 0.07, Fig. 4), consistent with their slightly greater bactericidal activity (Fig. 1).
FIG 2.
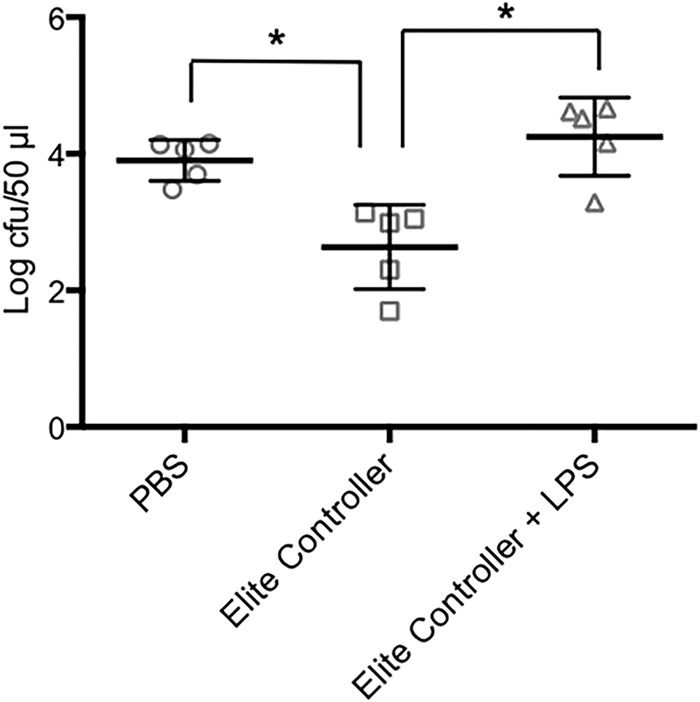
Effect of LPS competition on bactericidal activity. The bactericidal assays were carried out with PBS or plasma samples from elite controllers with and without preincubation with 100 μg/ml of S. Typhimurium LPS. The numbers of bacteria surviving at the end of 1 h are shown (mean ± SD). *, P = 0.008.
FIG 3.
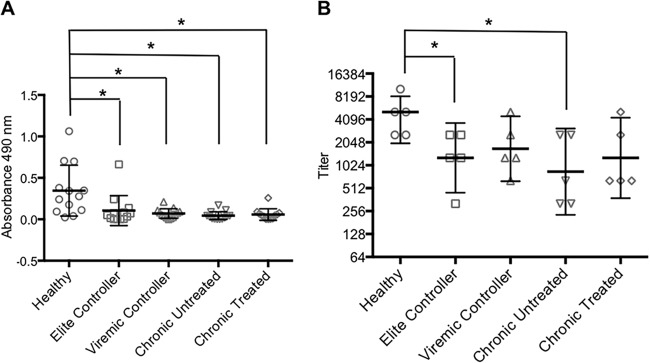
(A) S. Typhimurium LPS-specific IgG in samples from healthy and HIV-positive individuals (mean ± SD). *, P < 0.005. (B) Endpoint titers were determined in a subset of the samples in panel A (mean ± SD). *, P = 0.048.
FIG 4.
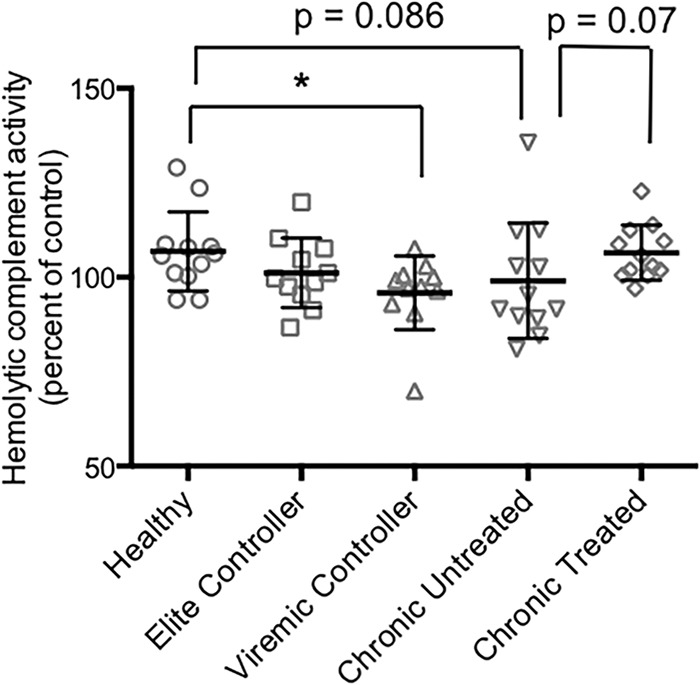
Hemolytic complement activity in samples from healthy and HIV-positive individuals. The complement activity was determined and expressed as a percentage of the reference sample (mean ± SD). *, P = 0.008.
Studies in Africa have demonstrated that one of the striking abnormalities of anti-Salmonella humoral immunity in HIV-infected individuals is the appearance of an activity that inhibits the ability of serum or plasma from healthy individuals to kill S. Typhimurium (4). To determine if this phenomenon also occurs in our population from the United States, we tested the effect of serum or plasma from the healthy or HIV-infected individuals on the bactericidal activity of plasma from a healthy control. When the samples from the healthy controls were mixed with plasma from the healthy individual, the anti-S. Typhimurium bactericidal activity of the plasma from the healthy individual was not diminished and even was significantly enhanced (Fig. 5). In contrast, the plasma from all subgroups of HIV-positive individuals, including the elite controllers, significantly inhibited the bactericidal activity of the healthy sample (Fig. 5). Thus, even well-controlled HIV infection is associated with an abnormality that results in the inhibition of antibody- and complement-mediated killing of S. Typhimurium.
FIG 5.
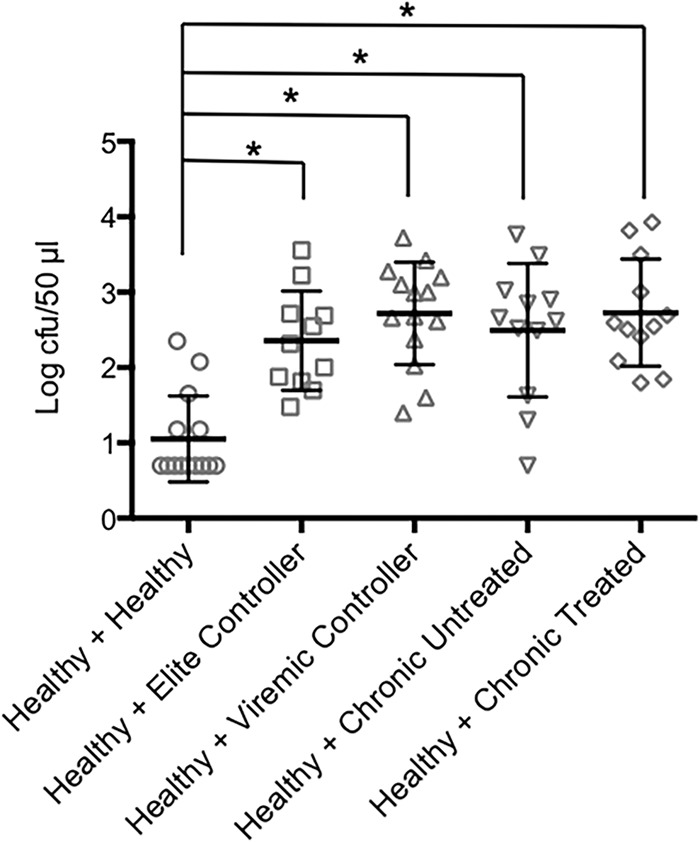
Inhibition of bactericidal activity by HIV-positive samples. The bactericidal assays were carried out with plasma from a healthy individual mixed with samples from either healthy or HIV-positive individuals. The numbers of surviving bacteria (mean ± SD) are shown. *, P ≤ 0.0001.
DISCUSSION
The findings described here indicate that HIV-infected adults in the United States, with the exception of the elite controller and treated chronic progressor subgroups, have a significant impairment in plasma bactericidal activity against S. Typhimurium compared to healthy adults (Fig. 1). The mechanism of this impairment appears to involve a significant decrease in the circulating levels of IgG against S. Typhimurium LPS (Fig. 3), the antigen that we have shown is the target of this bactericidal activity in healthy individuals (5) and in elite controllers (Fig. 2). However, the decrease in LPS-specific IgG cannot be the only factor since, despite this abnormality, the elite controllers have bactericidal activity comparable to that of the healthy controls. A decrease in complement activity may be an additional factor contributing to reduced bactericidal activity: unlike elite controllers, viremic controllers were found to have significantly reduced hemolytic complement activity compared to that of healthy individuals, and the untreated chronic progressors displayed a similar trend (Fig. 4). In further support of this idea, antiretroviral treatment reversed the decline in complement activity in the chronic progressors (Fig. 4) and also improved their bactericidal activity to some extent (Fig. 1). Thus, our data suggest that it may be a combination of low levels of LPS-specific IgG and reduced complement activity that results in impaired bactericidal activity against S. Typhimurium. We cannot completely exclude the possibility that decreases in complement activity may be related to storage conditions. However, we think it is unlikely, since the samples from all the HIV-positive subgroups were stored and handled similarly. Interestingly, HIV infection has been associated with disease severity-dependent activation of the complement pathway (23, 24), providing a plausible explanation for the reduced complement activity in our plasma samples from the viremic controllers and untreated chronic progressors.
The presence of inhibitory activity that prevents the killing of S. Typhimurium by healthy plasma may be another factor contributing to impaired bactericidal activity in HIV infection (Fig. 5). Currently, we can only speculate on the nature of the inhibitory activity present in the samples from the HIV-positive individuals. In HIV-infected patients in Malawi, the inhibition of bactericidal activity was dependent on high levels of LPS-specific IgG (4). This mechanism is unlikely to apply to our population, since the LPS-specific IgG levels in the HIV-infected individuals were lower than those in the healthy controls (Fig. 3). It is possible that subtle differences in the epitope targeted by the anti-LPS IgG between healthy and HIV-positive individuals might lead to bacterial killing or the inhibition of bacterial killing, respectively. Such epitope-specific differences in the functional outcome of antibody binding, including complement activation, have been described in the case of antibodies directed against the capsule of Cryptococcus neoformans (25, 26). It is also possible that difference in the glycosylation of LPS-specific IgG between healthy and HIV-infected individuals affects complement activation. Glycosylation of the Fc region has been shown to contribute to the ability of some anti-Neisseria meningitidis IgG to block bactericidal activity (27), and IgG glycosylation is known to be influenced by HIV infection (28, 29). LPS-specific antibodies of isotypes other than IgG might also play a role in the inhibitory activity. For instance, it is possible that an HIV infection-associated increase in the relative level of LPS-specific IgA is a factor, since IgA does not activate complement and might block the binding of complement-fixing IgG or IgM. There are precedents for this type of IgA-mediated blocking of complement activation in meningococcal infection (30–33). We found that LPS-specific IgM was increased in some of the HIV-positive subgroups in our study (data not shown), raising the possibility that high levels of such antibodies inhibit bacterial killing in a manner similar to the high levels of LPS-specific IgG in HIV-infected individuals in Malawi (4). All the potential mechanisms discussed above merit further investigation. Definitive proof of their involvement in HIV-associated inhibition of bactericidal activity will require a more focused investigation in which sufficient volumes of serum or plasma are collected to allow the use of depletion and purification strategies. Currently, the sample volumes available to us are not adequate for such studies, which, in any case, may be premature given that we do not yet have evidence that the inhibition is antibody mediated.
It is interesting that a decrease in S. Typhimurium LPS-specific IgG was observed in all the HIV-positive subgroups in our study. This abnormality might be related to HIV-induced impairments of CD4+ T-cell function, since T-cell help is required for switching from IgM to other isotypes. Thus, our findings suggest that CD4+ T lymphocytes from elite and viremic controllers might have functional deficiencies that impair their ability to provide help for B-cell isotype switching, even though their numbers were relatively normal in these subgroups (1,004 ± 395 cells per μl blood and 698 ± 117 cells per μl blood in the elite and viremic controllers, respectively).
Our results provide insights into the potential functional importance of bactericidal antibodies against S. Typhimurium. Although the clinical status of elite and viremic controllers may ultimately deteriorate (16), they are not known to be unusually susceptible to opportunistic infections during the period that they are able to suppress viral replication. That being the case, our observations that viremic controllers have decreased plasma bactericidal activity against S. Typhimurium (Fig. 1) and that the plasma of both elite and viremic controllers inhibits killing of S. Typhimurium by healthy plasma (Fig. 5) raise questions about the relevance of bactericidal antibodies in the protection against this pathogen. Long-term follow-up studies of HIV-positive controllers will be required to clarify this issue.
There are similarities and contrasts between our observations and those from Malawi (4). Both studies clearly demonstrate that HIV infection is associated with impaired bactericidal activity against S. Typhimurium. However, the mechanisms involved in the United States and Africa appear to be quite different. In contrast to our findings, HIV-infected adults in Malawi have high levels of S. Typhimurium LPS-specific IgG, which were shown to inhibit the bactericidal activity of serum from healthy individuals (4). The explanation for this striking difference in circulating LPS-specific IgG between HIV-infected individuals in the United States and Malawi is not clear. One possibility is that the high rates of gastrointestinal infection in Africa might chronically compromise the intestinal epithelial barrier, leading to increased LPS translocation and immune activation (34–37). Our results, together with those from Malawi, suggest that there are multiple ways in which anti-S. Typhimurium humoral immunity can be compromised by HIV infection. The exact mechanisms involved are likely to depend on the geographic and clinical context. Further clarification of these mechanisms will help shed light on how HIV infection impairs B- and T-cell responses and predisposes to invasive NTS disease.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant R01 AI089700 to B.J.C. from the National Institute of Allergy and Infectious Diseases, and by the Nutrition and Obesity Research Center at Harvard (P30DK040561 to W. Allan Walker).
Footnotes
Published ahead of print 13 August 2014
REFERENCES
- 1.Graham SM. 2010. Nontyphoidal salmonellosis in Africa. Curr. Opin. Infect. Dis. 23:409–414. 10.1097/QCO.0b013e32833dd25d. [DOI] [PubMed] [Google Scholar]
- 2.Feasey NA, Dougan G, Kingsley RA, Heyderman RS, Gordon MA. 2012. Invasive nontyphoidal salmonella disease: an emerging and neglected tropical disease in Africa. Lancet 379:2489–2499. 10.1016/S0140-6736(11)61752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacLennan CA, Gondwe EN, Msefula CL, Kingsley RA, Thomson NR, White SA, Goodall M, Pickard DJ, Graham SM, Dougan G, Hart CA, Molyneux ME, Drayson MT. 2008. The neglected role of antibody in protection against bacteremia caused by nontyphoidal strains of Salmonella in African children. J. Clin. Invest. 118:1553–1562. 10.1172/JCI33998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacLennan CA, Gilchrist JJ, Gordon MA, Cunningham AF, Cobbold M, Goodall M, Kingsley RA, van Oosterhout JJ, Msefula CL, Mandala WL, Leyton DL, Marshall JL, Gondwe EN, Bobat S, López-Macías C, Doffinger R, Henderson IR, Zijlstra EE, Dougan G, Drayson MT, MacLennan IC, Molyneux ME. 2010. Dysregulated humoral immunity to nontyphoidal Salmonella in HIV-infected African adults. Science 328:508–512. 10.1126/science.1180346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trebicka E, Jacob S, Pirzai W, Hurley BP, Cherayil BJ. 2013. Role of antilipopolysaccharide antibodies in serum bactericidal activity against Salmonella enterica serovar Typhimurium in healthy adults and children in the United States. Clin. Vaccine Immunol. 20:1491–1498. 10.1128/CVI.00289-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.MacLennan CA, Tennant SM. 2013. Comparing the roles of antibodies to nontyphoidal Salmonella enterica in high- and low-income countries and implications for vaccine development. Clin. Vaccine Immunol. 20:1487–1490. 10.1128/CVI.00465-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rondini S, Lanzilao L, Necchi F, O'Shaughnessy CM, Micoli F, Saul A, MacLennan CA. 2013. Invasive African Salmonella Typhimurium induces bactericidal antibodies against O-antigens. Microb. Pathog. 63:19–23. 10.1016/j.micpath.2013.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Micoli F, Ravenscroft N, Cescutti P, Stefanetti G, Londero S, Rondini S, Maclennan CA. 2014. Structural analysis of O-polysaccharide chains extracted from different Salmonella Typhimurium strains. Carbohydr. Res. 385:1–8. 10.1016/j.carres.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 9.Gilks CF, Brindle RJ, Otieno LS, Simani PM, Newnham RS, Bhatt SM, Lule GN, Okelo GB, Watkins WM, Waiyaki PG, Were JBO, Warrell DA. 1990. Life-threatening bacteremia in HIV-1 seropositive adults admitted to hospital in Nairobi, Kenya. Lancet 336:545–549. 10.1016/0140-6736(90)92096-Z. [DOI] [PubMed] [Google Scholar]
- 10.Levine WC, Buehler JW, Bean NH, Tauxe RV. 1991. Epidemiology of nontyphoidal Salmonella bacteremia during the human immunodeficiency virus epidemic. J. Infect. Dis. 164:81–87. 10.1093/infdis/164.1.81. [DOI] [PubMed] [Google Scholar]
- 11.Gruenewald R, Blum S, Chan J. 1994. Relationship between human immunodeficiency virus infection and salmonellosis in 20- to 59-year-old residents of New York City. Clin. Infect. Dis. 18:358–363. 10.1093/clinids/18.3.358. [DOI] [PubMed] [Google Scholar]
- 12.Gordon MA, Banda HT, Gondwe M, Gordon SB, Boeree MJ, Walsh AL, Corkill JE, Hart CA, Gilks CF, Molyneux ME. 2002. Non-typhoidal Salmonella bacteraemia among HIV-infected Malawian adults: high mortality and frequent recrudescence. AIDS 16:1633–1641. 10.1097/00002030-200208160-00009. [DOI] [PubMed] [Google Scholar]
- 13.O'Brien TR, Blattner WA, Waters D, Eyster E, Hilgartner MW, Cohen AR, Luban N, Hatzakis A, Aledort LM, Rosenberg PS, Miley WJ, Kroner BL, Goedert JJ. 1996. Serum HIV-1 RNA levels and time to development of AIDS in the Multicenter Hemophilia Cohort Study. JAMA 276:105–110. 10.1001/jama.276.2.105. [DOI] [PubMed] [Google Scholar]
- 14.Giorgi JV, Lyles RH, Matud JL, Yamashita TE, Mellors JW, Hultin LE, Jamieson BD, Margolick JB, Rinaldo CR, Jr, Phair JP, Detels R, Multicenter AIDS Cohort Study 2002. Predictive value of immunologic and virologic markers after long or short duration of HIV-1 infection. J. Acquir. Immune Defic. Syndr. 29:346–355. 10.1097/00126334-200204010-00004. [DOI] [PubMed] [Google Scholar]
- 15.Pereyra F, Addo MM, Kaufmann DE, Liu Y, Miura Y, Rathod A, Baker B, Trocha A, Rosenberg R, Mackey E, Ueda P, Lu Z, Cohen D, Wrin T, Petropoulos CJ, Rosenberg ES, Walker BD. 2008. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J. Infect. Dis. 197:563–571. 10.1086/526786. [DOI] [PubMed] [Google Scholar]
- 16.Walker BD, Yu XG. 2013. Unravelling the mechanisms of durable control of HIV-1. Nat. Rev. Immunol. 13:487–498. 10.1038/nri3478. [DOI] [PubMed] [Google Scholar]
- 17.Blankson JN, Bailey JR, Thayil S, Yang HC, Lassen K, Lai J, Gandhi SK, Siliciano JD, Williams TM, Siliciano RF. 2007. Isolation and characterization of replication-competent human immunodeficiency virus type 1 from a subset of elite suppressors. J. Virol. 81:2508–2518. 10.1128/JVI.02165-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graf EH, Mexas AM, Yu JJ, Shaheen F, Liszewski MK, Di Mascio M, Migueles SA, Connors M, O'Doherty U. 2011. Elite suppressors harbor low levels of integratred HIV DNA and high levels of 2-LTR circular HIV DNA compared to HIV+ patients on and off HAART. PLoS Pathog. 7:e1001300. 10.1371/journal.ppat.1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sauce D, Larsen M, Fastenackels S, Pauchard M, Ait-Mohand H, Schneider L, Guihot A, Boufassa F, Zaunders J, Iguertsira M, Bailey M, Gorochov G, Duvivier C, Carcelain G, Kelleher AD, Simon A, Meyer L, Costagliola D, Deeks SG, Lambotte O, Autran B, Hunt PW, Katlama C, Appay V. 2011. HIV disease progression despite suppression of viral replication is associated with exhaustion of lymphopoiesis. Blood 117:5142–5151. 10.1182/blood-2011-01-331306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Y, Al-Mozaini M, Buzon MJ, Beamon J, Ferrando-Martinez S, Ruiz-Mateos E, Rosenberg ES, Pereyra F, Yu XG, Lichterfeld M. 2012. CD4 T-cell regeneration in HIV-1 elite controllers. AIDS 26:701–706. 10.1097/QAD.0b013e3283519b22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harrington L, Srikanth CV, Antony R, Rhee SJ, Mellor AL, Shi HN, Cherayil BJ. 2008. Deficiency of indoleamine 2,3-dioxygenase enhances commensal-induced antibody responses and protects against Citrobacter rodentium-induced colitis. Infect. Immun. 76:3045–3053. 10.1128/IAI.00193-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu CC, Young JD. 1988. A semi-automated microassay for complement activity. J. Immunol. Methods 114:33–39. 10.1016/0022-1759(88)90150-0. [DOI] [PubMed] [Google Scholar]
- 23.Senaldi G, Peakman M, McManus T, Davies ET, Tee DE, Vergani D. 1990. Activation of the complement system in human immunodeficiency virus infection: relevance of the classical pathway to pathogenesis and disease severity. J. Infect. Dis. 162:1227–1232. 10.1093/infdis/162.6.1227. [DOI] [PubMed] [Google Scholar]
- 24.Lian YC, Della-Negra M, Rutz R, Ferriani V, de Moraes Vasconcelos D, da Sliva Duarte AJ, Kirschfink M, Grumach AS. 2004. Immunological analysis in paediatric HIV patients at different stages of the disease. Scand. J. Immunol. 60:615–624. 10.1111/j.0300-9475.2004.01492.x. [DOI] [PubMed] [Google Scholar]
- 25.Nussbaum G, Cleare W, Casadevall A, Scharff MD, Valadon P. 1997. Epitope location in the Cryptococcus neoformans capsule is a determinant of antibody efficacy. J. Exp. Med. 185:685–694. 10.1084/jem.185.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.MacGill TC, MacGill RS, Casadevall A, Kozel TR. 2000. Biological correlates of capsular (quellung) reactions of Cryptococcus neoformans. J. Immunol. 164:4835–4842. 10.4049/jimmunol.164.9.4835. [DOI] [PubMed] [Google Scholar]
- 27.Ray TD, Lewis LA, Gulati S, Rice PA, Ram S. 2011. Novel blocking human IgG directed against the pentapeptide repeat motifs of Neisseria meningitidis Lip/H. 8 and Laz lipoproteins. J. Immunol. 186:4881–4894. 10.4049/jimmunol.1003623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moore JS, Wu X, Kulhavy R, Tomana M, Novak J, Moldoveanu Z, Brown R, Goepfert PA, Mestecky J. 2005. Increased levels of galactose-deficient IgG in sera of HIV-1-infected individuals. AIDS 19:381–389. 10.1097/01.aids.0000161767.21405.68. [DOI] [PubMed] [Google Scholar]
- 29.Ackerman ME, Crispin M, Yu X, Baruah K, Boesch AW, Harvey DJ, Dugast AS, Heizen EL, Ercan A, Choi I, Streeck H, Nigrovic PA, Bailey-Kellogg C, Scanlan C, Alter G. 2013. Natural variation in Fc glycosylation of HIV-specific antibodies impacts antiviral activity. J. Clin. Invest. 123:2183–2192. 10.1172/JCI65708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Griffiss JM. 1975. Bactericidal activity of meningococcal antisera. Blocking by IgA of lytic antibody in human convalescent sera. J. Immunol. 114:1779–1784. [PubMed] [Google Scholar]
- 31.Griffiss JM, Bertram MA. 1977. Immunoepidemiology of meningococcal disease in military recruits. II. Blocking of serum bactericidal activity by circulating IgA early in the course of invasive disease. J. Infect. Dis. 136:733–739. [DOI] [PubMed] [Google Scholar]
- 32.Griffiss JM, Goroff DK. 1983. IgA blocks IgM and IgG-initiated immune lysis by separate molecular mechanisms. J. Immunol. 130:2882–2885. [PubMed] [Google Scholar]
- 33.Hamadeh RM, Estabrook MM, Zhou P, Jarvis GA, Griffiss JM. 1995. Anti-Gal binds to pili of Neisseria meningitidis: the immunoglobulin A isotype blocks complement-mediated killing. Infect. Immun. 63:4900–4906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kelly P, Davies SE, Mandanda B, Veitch A, McPhail G, Zulu I, Drobniewski F, Fuchs D, Summerbell C, Luo NP, Pobee JO, Farthing MJ. 1997. Enteropathy in Zambians with HIV-related diarrhoea: regression modelling of potential determinants of mucosal damage. Gut 41:811–816. 10.1136/gut.41.6.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campbell DI, Elia M, Lunn PG. 2003. Growth faltering in rural Gambian infants is associated with impaired small intestinal barrier function, leading to endotoxemia and systemic inflammation. J. Nutr. 133:1332–1138. [DOI] [PubMed] [Google Scholar]
- 36.Kelly P, Menzies I, Crane R, Zulu I, Nickols C, Feakins R, Mwansa J, Mudenda V, Katubulushi M, Greenwald S, Farthing M. 2004. Responses of small intestinal architecture and function over time to environmental factors in a tropical population. Am. J. Trop. Med. Hyg. 70:412–419. [PubMed] [Google Scholar]
- 37.Kelly P, Shawa T, Mwanamakondo S, Soko R, Smith G, Barclay GR, Sanderson IR. 2010. Gastric and intestinal barrier impairment in tropical enteropathy and HIV: limited impact of micronutrient supplementation during a randomised controlled trial. BMC Gastroenterol. 10:72. 10.1186/1471-230X-10-72. [DOI] [PMC free article] [PubMed] [Google Scholar]


