Abstract
Background
Fractional exhaled NO (FENO) is a marker of airway inflammation. For successful use of this marker it is important to have reference ranges from different healthy populations. The aim of this study was to establish these in healthy Tibetan adults who had always lived at high altitude on the Qinghai-Tibet Plateau.
Materia/Methods
The study included 145 healthy Tibetan subjects, aged 18 to 75 years, who were non-smokers. FENO was measured at a flow rate of 50 mL/s using a chemiluminescence analyzer. Residential altitude was classified as: Grade 1 (3678–3800 m), Grade 2 (3800–4200 m), or Grade 3 (>4200 m). Correlations between subject characteristics (age, sex, height, and weight), altitude, and FENO were investigated.
Results
The geometric mean FENO (95% CI) was 15.4 (7.0, 35.0) parts per billion (ppb). The 95% upper limit of the log-transformed data was 33.0 ppb, which was slightly lower than that for Han Chinese, and much lower than in the Northwest Han population. Mean FENO values were higher in males (16.8 ppb) than females (14.3 ppb) and inversely related to altitude. Multiple linear regression analysis showed that FENO was predicted by the equation Ln (FENO)=[2.844+0.161 × sex (1 for male; 0 for female) −0.111 × altitude grade]. The residual standard deviation (SD) was 0.048, and the explanatory value was 7%.
Conclusions
The upper limit of FENO in healthy Tibetan adults is 33 ppb. This value can be predicted on the basis of sex and altitude.
MeSH Keywords: Altitude Sickness, Nitric Oxide Synthase Type III, Tibet
Background
Measurement of fractional exhaled NO (FENO) is a useful marker of airway inflammation [1–3]. Previous studies have shown that FENO levels are increased in inflammatory lung diseases such as asthma [1–3]. Establishing FENO reference ranges in healthy individuals would be a useful aid for the interpretation of data in the clinical context [4]. To this end, the European Respiratory Society and the American Thoracic Society have published guidelines for the measurement of FENO to allow comparisons to be made between data from different studies and different populations. However, these guidelines do not make provision for subjects living at high altitude.
A previous multicenter study showed that the geometric mean FENO in 2229 healthy Han Chinese adults was 16 ppb. The 95% confidence interval (CI) showed evidence of a skewed distribution, with upper and lower limits of 5 and 30 ppb, respectively [5]. However, it has been demonstrated that subjects from the Tibetan and the Andean plateaus, who live at high altitude, exhale more NO than populations living at low altitude [6], and long-term residence at high altitude results in increased exhalation of NO, especially in subjects who are not prone to high-altitude edema [7]. For these reasons, it has been proposed that FENO values measured at high altitude may need to be corrected to account for the physiological effects of living at high altitude [8].
Previous studies have demonstrated that the determinants of FENO include age, sex, body height, atopy, and smoking status [9–11]. The effects of high altitude are less well documented. A small, previously published study estimated the mean partial pressure of NO in 20 male and 37 female Tibetan subjects living at >4500 m [12]. However, the study made no attempt to estimate FENO at different altitude levels. The present study was, therefore, undertaken to establish reference values for FENO at an exhalation flow rate of 50 mL/s in a healthy adult Tibetan population. We also investigated the factors that determined FENO in this population, and discussed the relationship between FENO and residence at different altitudes.
Material and Methods
Study population
This single-center controlled study was conducted in the Lhasa and NaQu District of Xizang Autonomous Region of China between from July to August, 2012. The enrollment protocol was based on the Multicenter Study of FENO Normal Values in Healthy Chinese Subjects [5].
The subjects included students, manual workers, farmers, herdsman, commercial service workers, and civil servants. None were athletes. All subjects answered a questionnaire that provided information on smoking status and past history of disease. The subjects were between 18 and 75 years of age, with a normal body mass index (16 to 26 kg/m2). None of them had previously consumed more than 20 cigarettes a day and none of them had smoked at all in the previous year. In addition, none had suffered a respiratory, cardiac, or renal disease or organ dysfunction.
All subjects underwent a simple physical examination including blood pressure, heart rate, height, weight, external nasal inspection, and chest auscultation. A skin-pick test (Allergopharma, Reinbek, Germany) was performed to test for allergy to walnuts (production serial number (SN) 554), poultry meat (SN059), goat skin (SN322), animal fur (SN033), mutton (SN501), grass (No.015), beef (SN506), and weeds (SN014) [13].
To facilitate analysis, residential altitude was graded as follows: Grade 1 (3678–3800 m), Grade 2 (3800–4200 m), and Grade 3 (>4200 m) based on the geographic characteristics of Tibet [14].
The study was approved by the Ethics Committee of the General Hospital of the People’s Liberation Army (Beijing, China). Informed consent was obtained from each participant.
Measurement of FENO
FENO was measured in a room maintained at a temperature of 20–28°C, and at 20–60% humidity. The room was free from flowers, dirt, and perfumes. The subjects had fasted for at least 1 h before the measurements.
An electrochemical NO analyzer (NIOX MINO; Aerocrine AB, Stockholm, Sweden) was used to measure FENO, in accordance with recommendations of the Multicenter Study of FENO Normal Values in Healthy Chinese Subjects [5] and the guidelines of the American Thoracic Society and European Respiratory Society. Measurements were performed in the sitting position in accordance with the manufacturer’s guidelines. Participants were asked to exhale into the device through a mouthpiece at a constant flow rate of 50 mL/s for 10 s. The device included a display to help subjects maintain a rate of constant exhalation. Gas exhaled during the last 4 s was analyzed. Measurements were performed twice and the average of the 2 values was used for the analysis. Lung function tests FEV1 (forced expiratory volume in 1 s), and FVC (forced vital capacity) were performed using a dry, round spirometer (Minispir; MIR, Rome, Italy) [4].
Statistical analysis
Statistical analysis was performed using SPSS version 18.0 software (SPSS Inc., Chicago, IL, USA). FENO values showed a skewed distribution. Analysis was therefore performed on logarithmic transformed data. The data were back-transformed for presentation [5]. Reference ranges for FENO are presented as geometric means and 95% CI and as the 95% upper limit of the log transformation of the data [5]. Comparisons of mean FENO values between sex and altitude grade were performed using Mann-Whitney U test or Kruskal-Wallis test. A P value <0.05 was considered statistically significant. A multiple linear regression model was used to determine factors influencing FENO. Variables were included in the model using forward stepwise regression analysis.
Results
The initial study population included 170 healthy Tibetan subjects. Nine subjects were excluded following a positive reaction to the skin prick test. An additional 16 subjects failed to complete the FENO and lung function measurements and were also excluded from the analysis.
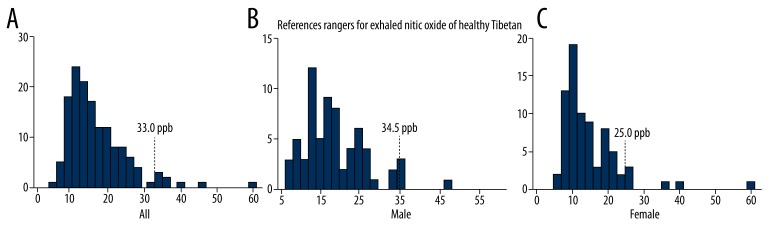
Table 1 presents the baseline characteristics of the 145 subjects included in the analysis. The geometric mean FENO for the whole population was 15.4 ppb (95% CI: 7.0–35.0 ppb). Mean values were higher in subjects living at altitude level 1 than in those living at altitude levels 2 and 3 (Table 2). Subgroup analysis showed that FENO values decreased as altitude increased, especially among male participants (Table 3). As shown in Table 3, FENO values in male subjects were higher than in females. The upper 95% confidence limits were 33.0 ppm in the total population compared with 34.5 ppm in men and 25.0 ppm in women (Figure 1).
Table 1.
Baseline characteristics.
| Variable | All subjects (n=145) | Male (n=68) | Female (n=77) |
|---|---|---|---|
| Age, years | 40.1±13.5 | 38.9±13.6 | 41.1±13.5 |
| Height, cm | 163.3±7.8 | 166.9±8.0 | 160.2±6.2* |
| Weight, kg | 60.2±12.8 | 66.3±13.8 | 54.9±9.0* |
| Altitude, n | |||
| Grade 1 | 70 | 36 | 34 |
| Grade 2 | 54 | 17 | 37 |
| Grade 3 | 21 | 15 | 6 |
| FEV1 (L) | 3.93±0.96 | 3.53±0.90 | 2.70±0.65* |
| FVC (L) | 3.09±0.88 | 4.43±0.94 | 3.49±0.74* |
Values are means ±SD or number of subjects (n). Grade 1 (3678–3800 m), Grade 2 (3800–4200 m) or Grade 3 (>4200 m).
P<0.01.
Table 2.
FENO in healthy Tibetan adults at different altitude grades.
| Altitude | N | Mean ±SD | 95% CI | P-value* |
|---|---|---|---|---|
| Grade 1 | 70 | 16.9±1.5 | 8.0–35.0 | 0.0001 |
| Grade 2 | 54 | 14.0±1.4 | 8.0–26.0 | |
| Grade 3 | 21 | 14.4±1.7 | 6.0–46.0 |
FENO values are geometric means±SD of logarithm-transformed values. Altitude Grade 1, 3678–3800 m; Grade 2, 3800–4200 m; Grade 3, >4200 m;
Kruskal-Wallis H test.
Table 3.
Geometric mean FENO values according to sex and altitude grade a.
| Group | N | Geometric mean ±SD | 95% CI | P value |
|---|---|---|---|---|
| All | 145 | 15.4 (1.5) | 7.0–35.0 | |
| Female | ||||
| All | 77 | 14.3 (1.5)* | 8.0–35.0 | 0.023 |
| Altitude 1 | 34 | 16.4 (1.8) | 8.0–35.0 | |
| Altitude 2 | 37 | 13.9 (1.4) | 8.0–26.0 | |
| Altitude 3 | 6 | 14.3 (1.4) | 9.0–24.8 | |
| Male | ||||
| All | 68 | 16.8 (1.5)* | 7.0–34.0 | 0.023 |
| Altitude 1 | 36 | 19.1 (1.7) | 9.0–34.0 | <0.0001 |
| Altitude 2 | 17 | 13.1 (1.5) | 8.0–24.0 | |
| Altitude 3 | 15 | 12.9 (1.4) | 6.0–46.0 | |
Values of FENO are the means ±SD of logarithm-transformed values. Grade 1, 3678–3800 m; Grade 2, 3800–4200 m; Grade 3, >4200 m.
Figure 1.
Distribution of FENO values in all subjects (A), male subjects (B), and female subjects (C). Dotted lines show 95% upper CI limits of the reference range for logarithm-transformed FENO values in each subgroup.
Forward and backward stepwise selections were used to test for factors that affected FENO in order to develop reference equations. The results from both procedures were the same. The analyses indicated that age, height and weight, FVC, and FEV1 did not contribute significantly to the variance. Sex and altitude grade were selected as explanatory variables. FENO was defined by the equation:
The residual SD was 0.048, and the explanatory value was 7%.
Discussion
The present study is one of the first community-based studies to investigate the effect of altitude on reference values of FENO in healthy Tibetan adults. A previous study found that Tibetan subjects living at a fixed altitude of 4200 m had >10-fold higher circulating concentration of NO products than U.S. subjects living at 206 m. It was proposed that this was an adaptive mechanism to living at high altitude without developing symptoms of hypoxia [15].
Subjects were enrolled in the study on the basis of the criteria used in the MSSTNVHCS study. We used a simple skin prick test to exclude atopy, but due to the lack of medical resources we were unable to quantitate allergen levels or undertake IgE tests [5]. In accordance with Multicenter Study of FENO Normal Values in Healthy Chinese Subjects, the study included subjects who had formerly smoked <20 cigarettes a day. As in similar studies in other regions, we excluded subjects with symptoms of lung disease, those receiving inhaled medications, and those with allergic rhinitis. These steps ensured that the population included in this study was comparable to those in other areas of the world.
It has previously been proposed that an NO analyzer should be used to measure the partial pressure of NO [8]. As with other metabolically produced or consumed gases (such as CO2, CO and O2), there are a number of reasons why the standard FENO procedure may lead to different results at high altitude. Molecular oxygen is necessary for the synthesis of NO and it has been shown that the partial pressure of exhaled NO decreases during the first hours at altitude. For this reason, measurement of FENO at high altitude using a NIOX MINO analyzer may produce misleading results, necessitating corrections to ensure that the data are comparable with those measured at lower altitudes [16]. By keeping the mass flow constant between altitudes, the dilution effect of the carrier gas can be standardized, which results in comparable fractional NO concentrations at different altitudes. For this reason, constant flow was adopted in our study.
In a previous study, FENO was shown to be reduced after exposure to intermittent hypoxia in well-acclimatized mine workers in Kyrgyzstan [17]. Another study showed that long residence time at high altitude can result in increased elimination of NO, especially in subjects not prone to high altitude edema [7]. However, there is lack of consensus regarding FENO values in lifelong high-altitude dwellers in comparison with subjects living at sea level. Natives of Tibet living at a fixed altitude of 4105 m were shown to exhale 18.6 ppb NO (range: 5.5–55.7 ppb), which was more than twice as high as values seen in low-altitude residents in the U.S. (7.4 ppb, range: 4.5–14.6 ppb) [6], but the study did not document the type of analyzer used or its flow speed.
The published literature includes few studies providing reference values for FENO at altitude that were measured according to current guidelines [7,18,19]. Our results are similar to those from studies in Sweden (16.6 ppb, 95% CI: 5.87–47.14 ppb) and Japan (15.4 ppb, mean ±2SD 6.5–36.8 ppb) [9,10], and to a 2001 study from Tibet [6]. Values in our study were lower than those reported in male (33.9±14.3 ppb) and female (24.1±10.6 ppb) Korean subjects. In all cases, values were higher than those obtained at low altitude from U.S. subjects. Interestingly FENO values were found to be higher at low altitude in European children than in European adults [20].
The present study is the first to statistically associate altitude level with FENO. We demonstrated an inverse relationship between FENO and altitude, which is consistent with previous findings [6]. In our study, all participants lived at an altitude of 3678 m or above, and most lived in the Lhasa district at an altitude of 3678–3800 m [14]. Altitude was an independent explanatory variable in the multiple linear regression analysis. This is especially relevant in Tibet, where altitude changes play an important role in the lifestyle of its inhabitants.
Previous studies have shown that there are several determinants of FENO levels, such as age, sex, body height, atopy, smoking, and diet [5]. Lack of correlation between age and FENO has been reported previously. Our results also suggest that normal ranges for FENO are dependent on sex, which is similar to results seen in Korean and Chinese Han populations [5,11], but different from results obtained in Japan and Sweden [9,10]. In our population, FENO was 1.17-times higher in men than in women. The sex difference remained significant after adjusting for other variables such as body weight, height, FVC, and FEV1. One reason for the sex difference may be related to the fact that males are more influenced by exposure to a passive smoking environment than women [21]. Other sex-specific life-style traits may also contribute to the differences. Environmental factors such as climate changes and pollution levels also determine FENO values. For example, research has also shown that traffic pollution increases the respiratory load of children with asthma.
Based on our questionnaire, a history of smoking was more common in Tibetan men than in women. Because of our limited medical resources, we recorded smoking history using a questionnaire and were unable to verify smoking information by test procedures in advance of the study. However, we speculate that passive smoking is one of the main causes of the sex difference.
The FENO values in Tibetan adults were slightly lower than those seen in Chinese Han populations [6]. Both studies demonstrated a sex difference, and in Han Chinese adults, FENO was significantly higher in the subgroup living in the northwest of China than in other regions. This difference was attributed to climatic and environmental factors. As the climate of northwest China is similar to that in Tibet, we attribute the observed difference between the 2 districts to altitude and genetic characteristics.
A shortcoming of the present study is that the number of subjects included from areas at altitude Grade 3 was relatively small, possibly resulting in bias. In addition, no comparable data are available for Han Chinese subjects who have always lived at high altitude or for Tibetans who live at altitudes well below 3678 m. Furthermore, the study was predominantly undertaken in July, which was during the pollen season in Tibet, and this may have resulted in higher than normal FENO in some participants [10].
Conclusions
Our results indicate that the upper limit of FENO in healthy Tibetan adults is 33 ppb. This value can be predicted on the basis of sex and altitude grade. Values at high altitude were lower than those at low altitude, indicating that FENO is inversely correlated to altitude level.
Acknowledgments
The author wish to thank the participants of the study and the staff of the People’s Hospital of Lhasa, the Hospital of the Autonomous District of Tibet, and the Hospital of Dulongdeqi County, Lhasa.
Abbreviations
- FENO
fraction of exhaled nitric oxide
- NO
nitric oxide
- ppb
parts per billion
- RI
reference interval
Footnotes
Conflicts of interest
The authors declare that they have no conflicts of interest.
Source of support: The study was supported by the Public Welfare Industry Special Funds Scientific Research Projects of the Ministry of Health (2013–2015)
References
- 1.Kharitonov S, Alving K, Barnes PJ. Exhaled and nasal nitric oxide measurements: recommendations. The European Respiratory Society Task Force. Eur Respir J. 1997;10:1683–93. doi: 10.1183/09031936.97.10071683. [DOI] [PubMed] [Google Scholar]
- 2.Gustafsson LE, Leone AM, Persson MG, et al. Endogenous nitric oxide is present in the exhaled air of rabbits, guinea pigs and humans. Biochem Biophys Res Commun. 1991;181:852–57. doi: 10.1016/0006-291x(91)91268-h. [DOI] [PubMed] [Google Scholar]
- 3.Alving K, Weitzberg E, Lundberg JM. Increased amount of nitric oxide in exhaled air of asthmatics. Eur Respir J. 1993;6:1368–70. [PubMed] [Google Scholar]
- 4.American Thoracic S, European Respiratory S. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171:912–30. doi: 10.1164/rccm.200406-710ST. [DOI] [PubMed] [Google Scholar]
- 5.Guangzhou Institute of Respiratory Disease. Multicenter study of FENO normal values in healthy Chinese subjects. Chin J Gen Pract. 2013;11:341–45. [Google Scholar]
- 6.Beall CM, Laskowski D, Strohl KP, et al. Pulmonary nitric oxide in mountain dwellers. Nature. 2001;414:411–12. doi: 10.1038/35106641. [DOI] [PubMed] [Google Scholar]
- 7.Duplain H, Sartori C, Lepori M, et al. Exhaled nitric oxide in high-altitude pulmonary edema: role in the regulation of pulmonary vascular tone and evidence for a role against inflammation. Am J Respir Crit Care Med. 2000;162:221–24. doi: 10.1164/ajrccm.162.1.9908039. [DOI] [PubMed] [Google Scholar]
- 8.Hemmingsson T, Horn A, Linnarsson D. Measuring exhaled nitric oxide at high altitude. Respir Physiol Neurobiol. 2009;167:292–98. doi: 10.1016/j.resp.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Matsunaga K, Hirano T, Kawayama T, et al. Reference ranges for exhaled nitric oxide fraction in healthy Japanese adult population. Allergol Int. 2010;59:363–67. doi: 10.2332/allergolint.10-OA-0197. [DOI] [PubMed] [Google Scholar]
- 10.Olin AC, Bake B, Toren K. Fraction of exhaled nitric oxide at 50 mL/s: reference values for adult lifelong never-smokers. Chest. 2007;131:1852–56. doi: 10.1378/chest.06-2928. [DOI] [PubMed] [Google Scholar]
- 11.Kim SH, Kim TH, Sohn JW, et al. Reference values and determinants of exhaled nitric oxide in healthy Korean adults. J Asthma. 2010;47:563–67. doi: 10.3109/02770901003702840. [DOI] [PubMed] [Google Scholar]
- 12.Hoit BD, Dalton ND, Erzurum SC, et al. Nitric oxide and cardiopulmonary hemodynamics in Tibetan highlanders. J Appl Physiol (1985) 2005;99:1796–801. doi: 10.1152/japplphysiol.00205.2005. [DOI] [PubMed] [Google Scholar]
- 13.Li-hua C, Wei LI. Food allergen skin prick tests in patients with allergic diseases in Northwest of China. Chin J Allergy Clin Immunol. 2012;6:218–22. [Google Scholar]
- 14.The Information Center of Xizang autonomous district of China. Tibetan Geography information of China. [Accessed 11.13]. Available at www.xinhuanet.com.
- 15.Erzurum SC, Ghosh S, Janocha AJ, et al. Higher blood flow and circulating NO products offset high-altitude hypoxia among Tibetans. Proc Natl Acad Sci USA. 2007;104:17593–98. doi: 10.1073/pnas.0707462104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemmingsson T, Horn A, Linnarsson D. Measuring exhaled nitric oxide at high altitude. Respiratory physiology & neurobiology. 2009;167:292–8. doi: 10.1016/j.resp.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Vinnikov D, Brimkulov N, Redding-Jones R, Jumabaeva K. Exhaled nitric oxide is reduced upon chronic intermittent hypoxia exposure in well-acclimatized mine workers. Respir Physiol Neurobiol. 2011;175:261–64. doi: 10.1016/j.resp.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 18.Brown DE, Beall CM, Strohl KP, Mills PS. Exhaled nitric oxide decreases upon acute exposure to high-altitude hypoxia. Am J Hum Biol. 2006;18:196–202. doi: 10.1002/ajhb.20489. [DOI] [PubMed] [Google Scholar]
- 19.Stuber T, Sartori C, Salmon CS, et al. Respiratory nitric oxide and pulmonary artery pressure in children of aymara and European ancestry at high altitude. Chest. 2008;134:996–1000. doi: 10.1378/chest.08-0854. [DOI] [PubMed] [Google Scholar]
- 20.Olivieri M, Talamini G, Corradi M, et al. Reference values for exhaled nitric oxide (reveno) study. Respir Res. 2006;7:94. doi: 10.1186/1465-9921-7-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dressel H, de la Motte D, Reichert J, et al. Exhaled nitric oxide: independent effects of atopy, smoking, respiratory tract infection, gender and height. Respir Med. 2008;102:962–69. doi: 10.1016/j.rmed.2008.02.012. [DOI] [PubMed] [Google Scholar]