Abstract
Background
A screening strategy combining rapid HIV-1/2 (HIV) antibody testing with pooled HIV-1 RNA testing increases identification of HIV infections, but may have other limitations that restrict its usefulness to all but the highest incidence populations.
Objective
By combining rapid antibody detection and pooled nucleic acid amplification testing (NAAT) testing, we sought to improve detection of early HIV-1 infections in an urban Newark, NJ hospital setting.
Study design
Pooled NAAT HIV-1 RNA testing was offered to emergency department patients and out-patients being screened for HIV antibodies by fingerstick-rapid HIV testing. For those negative by rapid HIV and agreeing to NAAT testing, pooled plasma samples were prepared and sent to the University of Washington where real-time reverse transcription-polymerase chain reaction (RT-PCR) amplification was performed.
Results
Of 13,226 individuals screened, 6381 had rapid antibody testing alone, and 6845 agreed to add NAAT HIV screening. Rapid testing identified 115 antibody positive individuals. Pooled NAAT increased HIV-1 case detection by 7.0% identifying 8 additional cases. Overall, acute HIV infection yield was 0.12%. While males represent only 48.1% of those tested by NAAT, all samples that screened positive for HIV-1 RNA were obtained from men.
Conclusion
HIV-1 RNA testing of pooled, HIV antibody-negative specimens permits identification of recent infections. In Newark, pooled NAAT increased HIV-1 case detection and provided an opportunity to focus on treatment and prevention messages for those most at risk of transmitting infection. Although constrained by client willingness to participate in testing associated with a need to return to receive further results, use of pooled NAAT improved early infection sensitivity.
Keywords: HIV testing algorithms, NAAT, Rapid HIV tests, HIV screen, HIV seronegativity, Nucleic acid amplification tests
1. Background
In New Jersey, more than 100,000 individuals [1] are screened annually for human immunodeficiency virus (HIV) using rapid tests that detect antibodies to HIV. Unfortunately, antibody assays possess a limited ability to detect acute HIV infection (AHI) [2].
AHI [3] is characterized by active viral replication, shedding [4], increases in viral load in blood [5] and genital fluids [6] and a high degree of infectivity [7,8]. During the first 5 months of infection, the probability of transmission (per coital act) has been estimated to be 8–10 times higher than during asymptomatic infection [9].
Primary infection is 26 times more infectious than the asymptomatic phase [10] that follows. By some estimates, AHI accounts for the transmission of up to 50% of all new HIV infections [11].
Screening for AHI provides opportunities for early linkage to care and treatment, with benefits ranging from immune system preservation to decreased onward transmission of HIV [12]. Since many HIV-infected individuals are known to take active measures to reduce their risk of infecting others upon learning of their infection [13], the identification of those in the midst of infection can have important consequences.
First used to detect the presence of HIV RNA during the seronegative period among blood donors [14], pooled nucleic acid amplification testing (NAAT) has been used in conjunction with several generations of HIV-1 immunoassays [15–18], to identify acutely infected individuals and reportedly increases case detection beyond that of even the most sensitive, third generation immunoassays [19]. Unfortunately, pooled NAAT testing is also labor intensive, time-consuming, costly and complex [20].
Newark is the epicenter of the HIV epidemic in New Jersey. Minorities account for 76% of adult/adolescent cumulative HIV/AIDS cases and 77% of all persons living with HIV/AIDS. In the US, African-Americans have among the highest racial or ethnic HIV prevalence [21] and the highest incidence [22] (68.9 new cases per 100,000 populations) – 7.9 times the rate in whites.
Among African-American females, heterosexual contact with a person known to have, or to be at high risk for HIV infection is associated with an estimated 87% of all new infections. The rate of new HIV infections for black females (38.1 per 100,000) is 20.1 times the rate for white females (1.9 per 100,000) [10].
Among males in the US, African-American male-to-male sexual contact is responsible for an estimated 72% of new HIV infections and accounted for 45% of new HIV infections (ages 13–24). The rate of new HIV infections (103.6 per 100,000 populations) was 6.6 times the rate for white males (15.8 per 100,000 populations) and the highest of any racial/ethnic subgroup [10].
Injection drug use and their sexual contacts have traditionally been a major mode of HIV exposure in Newark. While the proportion exposed through injection drug use (IDU) is lower than in the past, the proportion of cases exposed through sexual contact is increasing [23].
Among factors affecting HIV transmission [24], a high viral load [25] particularly during the earliest phase of the infection, before the appearance of any significant antibody, response is important [26–28]. Pilcher and Cohen [29] estimate the risk of heterosexual transmission per coital act to be between 1/30 and 1/200 during the acute phase of an HIV infection compared to a risk of 1/1000 and 1/10,000 during the later, asymptomatic phase of the infection.
2. Objectives
Utilizing a second generation rapid HIV test and NAAT pooling, we sought to assess the frequency of AHI in an emergency room and an outpatient testing site in Newark, NJ.
3. Study design
Routine HIV testing was offered to patients in the emergency department (ED) daily from 7 am to 11 pm and those who walked in and requested testing. The following patients were not routinely screened: <13 years of age, psychiatric illness, trauma, mental status changes, critically ill patients (e.g. chest pain, difficulty breathing, etc.).
All individuals underwent initial screening for HIV antibodies (13,226) utilizing a rapid HIV-1/HIV-2 antibody test, the Clearview HIV 1/2 STAT-PAK (Alere North America, Inc., Princeton, NJ). Between February 2010 and January 2012, pooled NAAT testing was also offered to emergency department (ED) patients and out-patients (OP) seen at University Hospital, a large, urban hospital in Newark, NJ.
For those negative by rapid HIV and agreeing to NAAT testing (6845–52.2%), plasma samples were collected, centrifuged and stored frozen until a 27 sample batch could be pooled and transported, frozen, to the University of Washington Department of Laboratory Medicine. Real-time reverse transcription-polymerase chain reaction (RT-PCR) amplification was performed at the University of Washington, to assess HIV RNA (dynamic range for HIV RNA detection by Real-Time RT-PCR, 30–1,000,000 copies/mL).
4. HIV-1 RNA pooling by matrix method
Four 125 mcL aliquots from each of 27 seronegative plasma specimens were placed into a single tube representing the total pool and into three respective sub-pool tubes from among nine sub-pool tubes representing a three-dimensional matrix comprised of three rows (x = 1, 2, 3), three columns (y = 1, 2, 3) and three levels (z = 1,2, 3); whereby each of the nine sub-pool tubes represent the sum of nine aliquots from each row, column and level. Thus, each unique specimen with its (x, y, z) coordinate could be identified from among the expected patterns of tube reactivity for this size of matrix. For example, a specimen with the matrix-pool coordinate (1, 2, 3) would contribute 125-mcL aliquots to the 27-member pool tube and into each of three sub-pool tubes representing row 1, column 2 and level 3, respectively. Should this specimen have detectable HIV-1 RNA, then these three tubes would be reactive for HIV-1 RNA and the pattern of this tube reactivity would deconstruct to identify the unique specimen with the matrix coordinates (1, 2, 3). The expected pattern of reactivity for up to three positive samples for this size of matrix-pool should deconstruct accordingly, otherwise a pre-analytical dilution error was suspected and the dilution schema considered invalid and required repeating. In this schema, a reactive specimen was considered presumptively positive for HIV-1 RNA and the original specimen was re-tested to confirm the matrix-pool result. This matrix-pooling method and earlier variants for detection of AHI have been in place at the University of Washington Retrovirus Laboratory and Public Health – Seattle King County, Seattle, WA for several years [30].
Typically, the turn-around-time from specimen acquisition to result delivery took 7–10 days.
AHI case definition
All RT-negative/NAAT-positive results that had detectable viral loads were considered presumptive AHI cases. Persons with presumptive AHI had specimens drawn for follow-up confirmatory testing on the day they received their test results. Follow-up plasma specimens underwent EIA, WB, and viral load testing at University Hospital, Newark, NJ.
AHI case notification
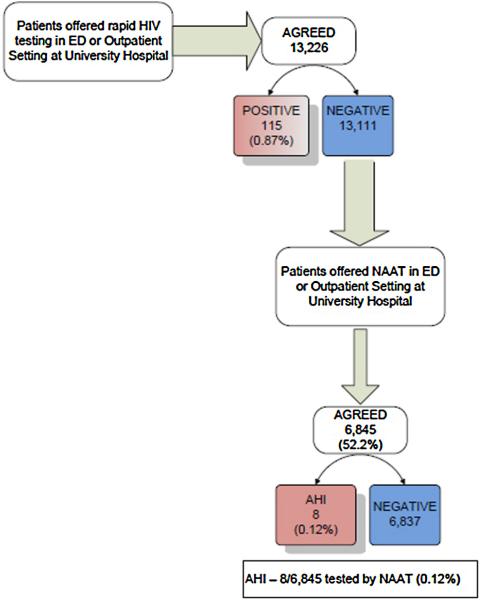
NJ-certified counselors notified presumptive AHI cases of their results and arranged follow-up visits with infectious disease specialists (Fig. 1).
Fig. 1.
Testing flowchart – patients were approached in the Emergency Department and in an Outpatient Clinic at University Hospital, Newark, NJ and offered rapid HIV screening. After rapid HIV screening, using the StatPak Rapid HIV 1/2 test (Alere North America, Inc., Princeton, NJ), those determined to be negative (13,111) were offered an opportunity to participate in pooled nucleic acid amplification testing (NAAT). Plasma samples were collected, centrifuged and stored frozen from those agreeing (6845) until a 27 sample batch could be pooled and transported to the University of Washington, Center for AIDS Research (CFAR) for analysis.
5. Results
Of 13,226 individuals screened, 6381 (48.2%) agreed to rapid HIV testing alone (3587 female, 2794 male), while 6845 (51.8%), (3554 female 3291 male), agreed to rapid testing plus additional NAAT screening of seronegative specimens. More females than males agreed to additional testing (51.9% vs. 48.1%).
As shown in Table 1, rapid testing alone identified 115 antibody positive individuals (0.87%). Of the total, 75 (65.2%) were male and 40 (34.8%) were female. Almost half of the men, 33/75 (44.0%), testing positive by rapid HIV reported male-to-male sexual activity (MSM) as a transmission risk factor. Overall, 33/115 (28.7%) of all positive rapid HIV tests were associated with MSM as a stated risk factor. Pooled NAAT increased HIV case detection by 7.0% identifying 8 additional cases – 6 of which reported MSM as a risk factor. Overall, AHI yield was 0.12%. While representing only 48.1% of those tested by NAAT, all NAAT-positive screens were male.
Table 1.
Summary – rapid HIV screening supplemented with pooled NAAT testing (B/W reproduction).
| Rapid HIV screening supplemented by pooled NAAT testing February 2010-January 2012 | |||||||
|---|---|---|---|---|---|---|---|
| Description | Rapid tested | Rapid and NAAT tested | AHI | HIV Ab+ | %HIVAb+ | %Increase in yield | %Yield AHI |
| HIV Ab– adults receiving testing and counseling at a high risk urban hospital in Newark, NJ | 13,226 | 6845 | 8 | 115 | 0.87% | 7.0% | 0.12% |
Five of eight clients (62.5%) identified as having AHI were connected to care immediately. Three clients did not return. Notification Assistance Personnel (NJ Department of Health) attempted to contact them. One client was located and connected to care. A total of 6/8 (75%) were connected to care.
The distribution of risk factors by testing format is shown in Table 2. In Newark, heterosexual sex was the dominant risk factor regardless of gender or format, accounting for nearly 90% of male risk: 89.0% of male (Rapid Only) risk or 89.3% of male (NAAT plus Rapid) risk; and 99.8% of female risk: 99.7% (Rapid Only) vs. 99.9% (NAAT plus Rapid). Among factors associated with HIV antibody positive results, 31/75 (41.3%) reported MSM risk. Six of 8 acute HIV infections (75%) were associated with MSM risk.
Table 2.
The distribution of risk factors associated with testing format and forthose identified as AHI (NAAT+ HIV antibody–) or HIV+ antibody (B/W reproduction).
| Raw numbers | Percentage | |||
|---|---|---|---|---|
| Risk factor | Male | Female | Male | Female |
| Distribution of risk factors | ||||
| Rapid only | ||||
| Male-to-male | 299 | 0 | 10.3% | 0 |
| Heterosexual sex | 2595 | 3457 | 89.0% | 99.7% |
| Injection drug use | 21 | 9 | 0.7% | 0.3% |
| Total 6381 | 2915 | 3466 | 100.0% | 100% |
| NAAT+rapid | ||||
| Male-to-male | 376 | 0 | 10.1% | 0 |
| Heterosexual sex | 3313 | 3131 | 89.3% | 99.9% |
| Injection drug use | 21 | 4 | 0.6% | 0.1% |
| Total: 6845 | 3710 | 3135 | 100.0% | 100% |
| Acute HIV infection (AHI) | ||||
| Male-to-male | 6 | 0 | 75.0% | 0.0% |
| Heterosexual sex | 2 | 0 | 25.0% | 0.0% |
| Injection drug use | 0 | 0 | 0.0% | 0.0% |
| Total 8 | 8 | 0 | 100.0% | 0.0% |
| HIV+Ab | ||||
| Male-to-male | 31 | 0 | 41.3% | 0.0% |
| Heterosexual sex | 41 | 40 | 54.6% | 100.0% |
| Injection drug use | 3 | 0 | 4.1% | 0.0% |
| Total: 115 | 75 | 40 | 100.0% | 100.0% |
6. Discussion
To our knowledge, this is the first use of a pooled NAAT strategy to look for AHI in New Jersey. The increase in case identification is consistent with reports from other high-prevalence communities including: Los Angeles (7.14%) [31], Atlanta (6.06%) [32] and Seattle-King Country (6.17%) [33], but remains less than that observed in San Francisco (10.48%) [26]. As shown in Table 3, case identification is substantially higher than that observed in settings where universal screening has been implemented, e.g. Baltimore (0.40%) [34] and North Carolina (3.80%) [35], but less than sites that employed strategic use of pooled NAAT based upon clinical suspicions and referral [36].
Table 3.
Summary – rapid HIV screening supplemented with pooled NAAT testing (B/W reproduction).
| Identification of acute HIV infection in urban US cities using pooled NAAT of HIV seronegative plasma | |||||||
|---|---|---|---|---|---|---|---|
| Description | Rapid tested alone | Rapid and NAATtested | AHI | HIV Ab+ | %HIV Ab+ | %Increase in yield | %Yield AHI |
| Los Angelesa | 1698 | 1 | 14 | 0.82% | 7.14% | 0.06% | |
| Newark, NJ | 12,390 | 6785 | 8 | 116 | 0.94% | 6.90% | 0.12% |
| Seattle King Countyb | 3439 | 5 | 81 | 2.36% | 6.17% | 0.15% | |
| Atlantac | 2136 | 4 | 66 | 3.09% | 6.06% | 0.19% | |
| San Franciscoa | 2722 | 11 | 105 | 3.86% | 10.48% | 0.40% | |
| Baltimored | 60,695 | 58,925 | 7 | 1766 | 2.90% | 0.40% | 0.01%-0.02%* |
| Rhode Islande | 113 | 6 | 5.31% | ||||
| North Carolinaf | 109,250 | 23 | 606 | 0.55% | 3.80% | 0.02% | |
When reflex RNA screening of indeterminant Western blots were included an additional 4 AHI specimens were identified (0.02%).
Ref. [31].
Ref. [33].
Priddy F, et al. NAAT-based screening for acute HIV infection in an urban HIV counselling and testing population in the Southeastern United States. In: Program and abstracts, 12th Conf Retroviruses Opp Infect, Boston, February 2005: Abstract 964.
Ref. [34].
Ref. [36].
Ref. [35].
Following second-generation, rapid testing (Clearview HIV 1/2 STAT-PAK); pooled NAAT increased HIV case detection by 7.0%. It has been suggested that 50–75% [37,38] (4–6/8) of these would potentially have been identified using a third-generation (IgM-and IgG-sensitive EIA using an antigen-sandwich format), or even more using a fourth generation antigen–antibody combination test. Among currently FDA-approved rapid tests only the Uni-Gold Recombigen HIV test can potentially detect both IgG and IgM, as it uses a sandwich-based capture and detection system [39]. A 4th generation, point-of-care, RT [40,41], the Alere Determine Combo is currently undergoing FDA review.
7. Limitations
This program was not a universal NAAT screening program. These results may not be generalizable due to self-selection bias in agreeing to participate. Despite the implementation of routine testing and efforts to convince clients of the usefulness of pooled NAAT, a bare majority (52.2%) were willing to have blood drawn and sent for additional testing. No effort was made to determine why clients refused additional NAAT testing, but the explanation could include many different motivations: unwillingness to return for results; an unwillingness to have phlebotomy; failure to adequately understand the value associated with the increased sensitivity of the assay; or perhaps a failure to understand the meaning of their own risk events. Anecdotal information suggests that delays associated with NAAT referral, processing and reporting (typically 7–10 days) including the need to return to receive the results of the testing weighed upon client willingness to continue with a second, more sensitive screening procedure. Such observations would potentially favor the use of more sensitive, point-of-care based 4th generation rapid HIV tests to achieve better acceptance, a reduction in the number of infections missed using current antibody-based technologies and an expedited linkage into care.
Despite almost equal proportions of men and women agreeing to NAAT testing, no women were identified as being infected during this study. The significance of this finding is unclear. Given that 34.8% of the NAAT tested cohorts were female, AHI appears less prevalent among woman in Newark; but the sample size was limited. In North Carolina, 443 pregnant women were identified as HIV Ab+, while an additional 15 were identified as HIV Ab− and pooled NAAT+ (AHI) [42]. Women in the age group 18–44 years old are more likely to test for HIV than are men [43]; as such, it is reasonable that the same holds true for HIV-1 NAAT. In our sample, MSM constitute only 4.7% of clients being screened but were responsible for 28.7% of all positive rapid HIV antibody tests and 75.0% of all clients identified having AHI. Whether this rate is consistent with national reports of increased diagnoses rates among MSM [44]; or an increase in the number of MSM in Newark is unclear. According to a recent NJ epidemiological report [45] MSM were responsible for 30% of existing HIV/AIDS infection in 2008–2010, and 53% of all new infections.
Previous studies have shown that a combination of antibody-based screening and NAAT testing of pools of negative specimens can improve detection of AHI. However, in many of these studies, the initial antibody testing was performed using an earlier generation of enzyme immunoassay (EIA). Patel et al. [2] established that the yield of pooled NAAT for HIV diagnosis is lower using a third-generation EIA than first- or second-generation EIAs, because third-generation EIA detects a higher proportion of early infections. In their study, HIV detection was increased using a pooled NAAT by 8.2% when a first-generation EIA was used, but by only 2.2% when a third-generation EIA was used.
Use of pooled NAAT testing combined with a rapid screening test facilitates diagnosis of early HIV infection. Unfortunately, the utility of the strategy is impacted by client unwillingness to return for results later, logistic complexities and cost effectiveness [46]. Laboratory diagnostics continue to evolve. The availability of sensitive 4th generation, combination HIV antigen/antibody screening assays and newer diagnostic algorithms may facilitate identification of acutely infected individuals. It may not immediately resolve the issue of client reluctance to return for follow-up results and linkage into care.
While able to detect 80% of acute infections [34] or more [47], 4th generation, laboratory-based assays still necessitate the availability of laboratory resources, personnel and complicated logistics to complete a definitive result [48] expeditiously.
Fourth generation lab-based tests and new diagnostic algorithms may facilitate AHI identification with less delay than has been associated with a traditional, pooled NAAT testing strategy; but optimally an AHI sensitive, 4th generation, point-of-care, assay would permit on-site patient navigators to immediately link individuals into care, more quickly permitting treatment with antiretroviral agents with the expectation of viral load and transmission risk reduction. A better understanding of why clients fail to return to receive results and why they are unwilling to have additional testing will better inform future efforts to eliminate HIV transmission.
Acknowledgements
We gratefully acknowledge the contributions of study staff at University Hospital, Newark and the clinical contributions and support of Drs. Michael Jaker (Internal Medicine) [2] and Sandra Scott (Emergency Medicine) [2] at UMDNJ – New Jersey Medical School. In addition, we are most grateful for the organizational efforts of Joanne Corbo, MBA, MT (ASCP), the HIV Program Manager at UMDNJ – Robert Wood Johnson Medical School (RWJMS), the technologists of the NJ HIV Program at RWJMS who prepared specimen pools from plasma collected by study staff at University Hospital; and Ms. Joan Dragavon, MLS Retrovirology Core Manager at the University of Washington Center for AIDS Research (CFAR) as well as the staff of CFAR who performed Real Time PCR used in this study.
Funding: NJ Department of Health & Senior Services 10-778-AIDE-0 (EMC, EGM, GS); AI 38858 (RWC), AI 27757 (RWC).
Footnotes
Publisher's Disclaimer: This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution and sharing with colleagues. Other uses, including reproduction and distribution, or selling or licensing copies, or posting to personal, institutional or third party websites are prohibited. In most cases authors are permitted to post their version of the article (e.g. in Word or Tex form) to their personal website or institutional repository. Authors requiring further information regarding Elsevier's archiving and manuscript policies are encouraged to visit: http://www.elsevier.com/authorsrights
On July 1, 2013, the New Jersey Medical and Health Sciences Education Restructuring Act took effect, integrating Rutgers, The State University of New Jersey, with all units of the University of Medicine and Dentistry of New Jersey (formerly UMDNJ).
Conflict of interest
Competing interests: None declared.
Ethical approval: UMDNJ–IRB protocol: 02200800007: Follow-up Positive HIV Screening Results.
References
- 1.NJ HIV/AIDS Report. NJ Department of Health; Jun 30, 2012. Available at: http://www.state.nj.us/health/aids/documents/hiv_aids_report123111.pdf. [Google Scholar]
- 2.Patel P, et al. Detecting acute human immunodeficiency virus infection using 3 different screening immunoassays and nucleic acid amplification testing for human immunodeficiency virus RNA, 2006–2008. Arch Intern Med 2010 January. 170:66. doi: 10.1001/archinternmed.2009.445. [DOI] [PubMed] [Google Scholar]
- 3.Pilcher CD, Tien HC, Eron JJ, Jr, Vernazza PL, Leu S-Y, et al. Brief but efficient: acute HIV infection and the sexual transmission of HIV. J Infect Dis. 2004;189(10):1785–92. doi: 10.1086/386333. [DOI] [PubMed] [Google Scholar]
- 4.Quinn TC. Acute primary HIV infection. JAMA. 1997;278(1):58–62. [PubMed] [Google Scholar]
- 5.Clark SJ, Saag MS, Decker WD, et al. High titers of cytopathic virus in plasma of patients with symptomatic primary HIV-1 infection. N Engl J Med. 1991;324(14):954–60. doi: 10.1056/NEJM199104043241404. [DOI] [PubMed] [Google Scholar]
- 6.Tindall B, Evans L, Cunningham P, et al. Identification of HIV-1 in semen following primary HIV-1 infection. AIDS. 1992;6(9):949–52. doi: 10.1097/00002030-199209000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Daar ES, Moudgil T, Meyer RD, Ho DD. Transient high levels of viremia in patients with primary human immunodeficiency virus type 1 infection. N Engl J Med. 1991;324(14):961–4. doi: 10.1056/NEJM199104043241405. [DOI] [PubMed] [Google Scholar]
- 8.Pilcher CD, Shugars DC, Fiscus SA, Miller WC, Menezes P, Giner J, et al. HIV in body fluids during primary HIV infection: implications for pathogenesis, treatment and public health. AIDS. 2001;15(7):837–45. doi: 10.1097/00002030-200105040-00004. [DOI] [PubMed] [Google Scholar]
- 9.Maria JW, Ronald HG, Nelson KS, David S, Xianbin L, Oliver L, et al. Quinn rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191(9):1403–9. doi: 10.1086/429411. http://dx.doi.org/10.1086/429411. [DOI] [PubMed] [Google Scholar]
- 10.Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. J Infect Dis. 2008;198(5):687–93. doi: 10.1086/590501. [DOI] [PubMed] [Google Scholar]
- 11.Brenner BG, Roger M, Routy JP, Moisi D, Ntemgwa M, Matte C, et al. High rates of forward transmission events after acute/early HIV-1 infection. J Infect Dis. 2007;195(7):951–9. doi: 10.1086/512088. http://dx.doi.org/10.1086/512088. [DOI] [PubMed] [Google Scholar]
- 12.Cohen MH, Gay CL, Busch MP, Hecht FM. The detection of acute HIV infection. J Infect Dis. 2010;202(Suppl, 2):S270–7. doi: 10.1086/655651. [DOI] [PubMed] [Google Scholar]
- 13.Kilmarx PH, Hamers FF, Peterman TA. Living with HIV: experiences and perspectives of HIV-infected sexually transmitted disease clinic patients after post-test counselling. Sex Trans Dis. 1998;25(1):28–37. doi: 10.1097/00007435-199801000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Stramer SL, Glynn SA, Kleinman SH, et al. National Heart, Lung, and Blood Institute Nucleic Acid Test Study Group. Detection of HIV-1 and HCV infections among antibody-negative blood donors by nucleic acid-amplification testing. N Engl J Med. 2004;351(8):760–8. doi: 10.1056/NEJMoa040085. [DOI] [PubMed] [Google Scholar]
- 15.Sherlock M, Zetola NM, Klausner JD. Routine detection of acute HIV infection through RNA pooling: Survey of current practices in the United States [1]. Sex Transm Dis. 2007;34(5):314–6. doi: 10.1097/01.olq.0000263262.00273.9c. [DOI] [PubMed] [Google Scholar]
- 16.Pilcher CD, McPherson JT, Leone PA, Smurzynski M, Owen-O'Dowd, Peace-Brewer AL, et al. Real-time, universal screening for acute HIV infection in a routine HIV counseling and testing population. JAMA. 2002;288(2):216–21. doi: 10.1001/jama.288.2.216. [DOI] [PubMed] [Google Scholar]
- 17.Busch MP, Hecht FM. Nucleic acid amplification testing for diagnosis of acute HIV infection. Has the time come? AIDS. 2005;19(12):1317–9. doi: 10.1097/01.aids.0000180103.65640.d8. [DOI] [PubMed] [Google Scholar]
- 18.Granich RM, Gilks CF, Dye C, deCock KM, Williams BG. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009 Jan;373(9657):48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 19.Patel P, Mackellar D, Simmons P, Uniyal A, Gallagher K, Bennett B, et al. Detecting acute human immunodeficiency virus infection using 3 different screening immunoassays and nucleic acid amplification testing for human immunodeficiency virus RNA, 2006–2008. Arch Intern Med. 2010 Jan;170(1):66–74. doi: 10.1001/archinternmed.2009.445. http://dx.doi.org/10.1001/archinternmed.2009.445. [DOI] [PubMed] [Google Scholar]
- 20.Westreich DJ, Hudgens MG, Fiscus SA, Pilcher CD. Immunodeficiency virus infection with pooled nucleic acid amplification tests. J Clin Microbiol. 2008;46(5):1785–92. doi: 10.1128/JCM.00787-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.CDC Diagnoses of HIV infection and AIDS in the United States and dependent areas, 2010. HIV surveillance report. 2010;22 2012 [available at http://www.cdc.gov/hiv/surveillance/resources/reports/2010report/index.htm] [Google Scholar]
- 22.CDC Estimated HIV incidence in the United States, 2007–2010. HIV surveil-lance supplemental report. 2012;17 2012 [available at http://www.cdc.gov/hiv/surveillance/resources/reports/2010supp_vol17no4] [Google Scholar]
- 23.NJ HIV/AIDS Report. 2012 Jun; Available at: http://www.state.nj.us/health/aids/documents/hiv_aids_report123111.pdf.
- 24.Hall HI, Holtgrave DR, Tang T, Rhodes P. HIV transmission in the United States: considerations of viral load, risk behavior and health disparities. AIDS Behav. 2013;17(5):1632–6. doi: 10.1007/s10461-013-0426-z. [DOI] [PubMed] [Google Scholar]
- 25.Novitsky V, Essex M. Using HIV viral load to guide treatment-for-prevention interventions. Curr Opin HIV AIDS. 2012;7(2):117–24. doi: 10.1097/COH.0b013e32834fe8ff. [DOI] [PubMed] [Google Scholar]
- 26.Anderson RM, May R. Epidemiological parameters of HIV transmission. Nature. 1988;333:514–22. doi: 10.1038/333514a0. [DOI] [PubMed] [Google Scholar]
- 27.de Vincenzi I, European Study Group on Heterosexual Transmission of HIV A longitudinal study of human immunodeficiency virus transmission by heterosexual partners. N Engl J Med. 1994;331:341–6. doi: 10.1056/NEJM199408113310601. [DOI] [PubMed] [Google Scholar]
- 28.Leynaert B, Downs AM, de Vincenzi I, European Study Group on Heterosexual Transmission of HIV Heterosexual transmission of human immunodeficiency virus: variability of infectivity throughout the course of infection. Am J Epidemiol. 1998;148:88–96. doi: 10.1093/oxfordjournals.aje.a009564. [DOI] [PubMed] [Google Scholar]
- 29.Cohen MS, Pilcher CD, Amplified HIV. Transmission and new approaches to HIV prevention. [26.03.13];JID. 2005 191:1391–3. doi: 10.1086/429414. [available at: http://www.who.int/hiv/events/artprevention/cohen_amplified.pdf] [DOI] [PubMed] [Google Scholar]
- 30.Stekler JD, Swenson PD, Coombs RW, Dragavon J, Thomas KK, Brennan CA, et al. HIV testing in a high-incidence population: is antibody testing alone good enough? Clin Infect Dis. 2009;49:444–53. doi: 10.1086/600043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Patel P, Klausner JD, Bacon OM, Liska S, Taylor M, Gonzalez A, et al. Detection of acute HIV infections in high-risk patients in California. [07.07.13];J Acquir Immune Defic Syndr. 2006 May;42(1):75–9. doi: 10.1097/01.qai.0000218363.21088.ad. [available at: http://journals.lww.com/jaids/Fulltext/2006/05000/Detection_of_Acute_HIV_Infections_in_High_Risk.10.aspx] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Priddy FH, Pilcher CD, Moore RH, Tambe P, Park MN, Fiscus SA, et al. Detection of acute HIV infections in an urban HIV counselling and testing population in the United States. J Acquir Immune Defic Syndr. 2007 Feb;44(2):196–202. doi: 10.1097/01.qai.0000254323.86897.36. [DOI] [PubMed] [Google Scholar]
- 33.Stekler J, Swenson PD, Wood RW, Handsfield HH, Golden MR. Targeted screening for primary HIV infection through pooled HIV–RNA testing in MSM. AIDS. 2005;19(12):1323–4. doi: 10.1097/01.aids.0000180105.73264.81. [DOI] [PubMed] [Google Scholar]
- 34.Temkin E, Marsiglia VC, Hague C, Erbelding E. Screening for acute human immunodeficiency virus infection in Baltimore public testing sites. Sex Trans Dis. 2011;38(5):374–7. doi: 10.1097/OLQ.0b013e31820279bd. [DOI] [PubMed] [Google Scholar]
- 35.Pilcher CD, Fiscus SA, Nguyen TQ, Foust E, Wolf L, et al. Detection of acute infections during HIV testing in North Carolina. NEJM. 2005;352(18):1873–83. doi: 10.1056/NEJMoa042291. [DOI] [PubMed] [Google Scholar]
- 36.Beckwith CG, Cornwall AH, Dubrow R, Chapin K, Ducharme R, et al. Identifying acute HIV in Rhode Island. Med Health RI. 2009;92(7):231–3. [PMC free article] [PubMed] [Google Scholar]
- 37.Hecht FM, Busch MP, Rawal B, et al. Use of laboratory test and clinical symptoms for identification of primary HIV infection. AIDS. 2002;16(8):1119–29. doi: 10.1097/00002030-200205240-00005. [DOI] [PubMed] [Google Scholar]
- 38.Louie B, Wong E, Klausner JD, et al. Assessment of rapid tests for detection of human immunodeficiency virus-specific antibodies in recently infected individuals. J Clin Microbiol. 2008;46(4):1494–7. doi: 10.1128/JCM.01945-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Louie B, Wong E, Klausner JD, Liska S, Hecht F, Dowling T, et al. Assessment of rapid tests for detection of human immunodeficiency virus-specific antibodies in recently infected individuals. J Clin Microbiol. 2008 Apr;46(4):1494–7. doi: 10.1128/JCM.01945-07. http://dx.doi.org/10.1128/JCM.01945-07 [published online 30.01.08] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faraoni S, Rocchetti S, Gotta F, Ruggiero T, Orofino G, Bonora S, et al. Evaluation of a rapid antigen and antibody combination test in acute HIV infection. J Clin Virol. 2013;57(1):84–7. doi: 10.1016/j.jcv.2013.01.007. http://dx.doi.org/10.1016/j.jcv.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 41.Beelaert G, Fransen K. Evaluation of a rapid and simple fourth-generation HIV screening assay for qualitative detection of HIV p24 antigen and/or antibodies to HIV-1 and HIV-2. J Virol Meth. 2010;168:218–22. doi: 10.1016/j.jviromet.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 42.Patterson KB, Leone PA, Fiscus SA, Kuruc L, McCoy SI, et al. Frequent detection of acute HIV in pregnant woman. AIDS. 2007;21(17):2303–8. doi: 10.1097/QAD.0b013e3282f155da. [DOI] [PubMed] [Google Scholar]
- 43.MMWR QuickStats percentage of adults aged >18 years who had ever been tested for human immunodeficiency virus (HIV). Age Group and Sex – Nat Health Interv Survey, United States 2007. 2009;58(3):62. [Google Scholar]
- 44.Centers for Disease Control [10.04.13];HIV among Gay and Bisexual Men. Available at: http://www.cdc.gov/hiv/topics/msm.
- 45.Sadashige C, New Jersey HIV/AIDS Planning Group . New Jersey HIV/AIDS epidemiologic profile – 2011. New Brunswick; New Jersey: Apr, 2013. [Google Scholar]
- 46.Hutchinson AB, Patel P, Sansom SL, Farnham PG, Sullivan TJ, Bennett B, et al. Cost-effectiveness of pooled nucleic acid amplification testing for acute HIV infection after third-generation HIV antibody screening and rapid testing in the United States: a comparison of three public health settings. PLoS Med. 2010;7(9):e1000342. doi: 10.1371/journal.pmed.1000342. http://dx.doi.org/10.1371/journal.pmed.1000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pandori M, Hackett JH, Louie B, Vallari A, Dowling T, Liska S, et al. Assessment of the ability of a fourth-generation immunoassay for human immunodeficiency virus (HIV) antibody and p24 antigen to detect both acute and recent HIV infections in a high-risk setting. J Clin Microbiol. 2009;47(8):2639–42. doi: 10.1128/JCM.00119-09. http://dx.doi.org/10.1128/JCM.00119-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morbidity and Mortality Weekly Report (MMWR) Detection of acute HIV infection in two evaluations of a new HIV diagnostic testing algorithm – United States, 2011–2013. 2013;62(24):489–94. [PMC free article] [PubMed] [Google Scholar]