SUMMARY
Importance
In older adults reduced mobility is common and is an independent risk factor for morbidity, hospitalization, disability, and mortality. Limited evidence suggests that physical activity may help prevent mobility disability; however, there are no definitive clinical trials examining if physical activity prevents or delays mobility disability.
Objective
To test the hypothesis that a long-term structured physical activity program is more effective than a health education program (also referred to as a successful aging program) in reducing the risk of major mobility disability.
Design, Setting, and Participants
The Lifestyle Interventions and Independence for Elders (LIFE) study was a multicenter, randomized trial that enrolled participants between February 2010 and December 2011, who participated for an average of 2.6 years. Follow-up ended in December 2013. Outcome assessors were blinded to the intervention assignment. Participants were recruited from urban, suburban and rural communities at 8 field centers throughout the US. We randomized a volunteer sample of 1,635 sedentary men and women aged 70–89 years who had physical limitations, defined as a score on the Short Physical Performance Battery of 9 or below, but were able to walk 400 m.
Interventions
Participants were randomized to a structured moderate intensity physical activity program (n=818) done in a center and at home that included including aerobic, resistance and flexibility training activities or to a health education program (n=817) consisting of workshops on topics relevant to older adults and upper extremity stretching exercises.
Main Outcomes and Measures
The primary outcome was major mobility disability objectively defined by loss of ability to walk 400 m.
Results
Incident major mobility disability occurred in 30.1% (n=246/818) of physical activity and 35.5% (n=290/817) of health education participants (HR=0.82, 95%CI=0.69–0.98, p=0.03). Persistent mobility disability was experienced by 120/818 (14.7%) physical activity and 162/817 (19.8%) health education participants (HR=0.72; 95%CI=0.57–0.91; p=0.006). Serious adverse events were reported by 404/818 (49.4%) of the physical activity and 373/817 (45.7%) of the health education participants (Risk Ratio=1.08; 95%CI=0.98–1.20).
Conclusions and Relevance
A structured moderate intensity physical activity program, compared with a health education program, reduced major mobility disability over 2.6 years among older adults at risk of disability. These findings suggest mobility benefit from such a program in vulnerable older adults.
Registration
ClinicalsTrials.gov identifier NCT01072500.
INTRODUCTION
The life expectancy of older Americans continues to increase, with persons aged 65 years or older representing the fastest growing segment of the U.S. population.1 While prolongation of life remains an important public health goal, of even greater significance is preservation of the capacity to live independently and to function well during late life.2 Identification of proven interventions to prevent disability is an important public health challenge.3
Mobility - the ability to walk without assistance - is a critical characteristic for functioning independently.4;5 Those who lose mobility have higher rates of morbidity, disability, and mortality,6–13 and yet are often excluded from clinical trials. Preserving the ability to walk 400 m, an excellent proxy for community ambulation, is central to maintaining a high quality of life and independence in the community.
To our knowledge, no trial has conclusively tested that physical activity can prevent or delay the onset of mobility disability over an extended follow-up. Therefore, we conducted the Lifestyle Interventions and Independence for Elders (LIFE) Pilot study from 2004 to 2006 to plan for the Phase 3 randomized trial.14 As hypothesized, the LIFE Pilot study (N=424) showed significant improvements in walking speed and physical performance measures. The pilot was not powered for a disability endpoint, but showed a non-significant reduction in risk of major mobility disability in the physical activity group, compared with the health education group, also referred to as the successful aging group. In the LIFE study we hypothesized that compared with a health education program, a long-term structured physical activity program would reduce the risk of major mobility disability.
METHODS
Trial design and participants
The LIFE study was a multicenter, single-blinded, parallel randomized trial conducted at 8 field centers across the U.S. (University of Florida, Gainesville and Jacksonville, Florida; Northwestern University, Chicago, Illinois; Pennington Biomedical Research Center, Baton h21-Rouge, Louisiana; University of Pittsburgh, Pittsburgh, Pennsylvania; Stanford University, Stanford, California; Tufts University, Boston, Massachusetts; Wake Forest School of Medicine, Winston-Salem, North Carolina; and Yale University, New Haven, Connecticut) between February 2010 and December 2013. The Administrative Coordinating Center was located at the University of Florida and the Data Management, Analysis, and Quality Control Center at Wake Forest School of Medicine. The field centers included rural, suburban and urban communities.
Details of the methods were published previously.15 Briefly, the eligibility criteria consisted of men and women aged 70–89 years who (a) were sedentary (reporting <20 min/week in the past month performing regular physical activity and <125 min/week of moderate physical activity); (b) were at high risk for mobility disability based on lower extremity functional limitations measured by the Short Physical Performance Battery (SPPB)16 score ≤9 out of a of 12 (45% of participants were targeted to have a score <8); (c) could walk 400 m in ≤15 minutes without sitting, leaning, or the help of another person or walker; (d) had no major cognitive impairment (Modified Mini-Mental State Examination17 [3MSE] 1.5 standard deviations below education- and race-specific norms); and (e) could safely participate in the intervention as determined by medical history, physical exam and resting ECG.
Targeted mass mailings to the community was the primary recruitment strategy.18
The study protocol was approved by the institutional review boards at all participating sites. Written informed consent was obtained from all study participants. The trial was monitored by a data and safety monitoring board appointed by the National Institute on Aging. The trial is registered at ClinicalsTrials.gov with the identifier NCT01072500.
Randomization
Participants were randomized to a physical activity or to a health education program, via a secure web-based data management system using a permuted block algorithm (with random block lengths) stratified by field center and gender. Both groups received an initial individual 45-minute face-to-face introductory session by a health educator who described the intervention, communicated expectations, and answered questions.
Interventions
The physical activity intervention involved walking, with a goal of 150 min/week, strength, flexibility, and balance training.15 The intervention included attendance at two center-based visits per week and home-based activity 3–4 times per week for the duration of the study. A protocol was in place to restart the intervention for the participants who suspended the physical activity for medical reasons. The physical activity sessions were individualized and progressed towards a goal of 30 min of walking daily at moderate intensity, 10 min of primarily lower extremity strength training by means of ankle weights (2 sets of 10 repetitions), 10 min of balance training, and large muscle group flexibility exercises. The participants began with lighter intensity and gradually increased intensity over the first 2–3 weeks of the intervention. The Borg’s scale of self-perceived exertion19 that ranges from 6 to 20, was used to measure intensity of activity. Participants were asked to walk at an intensity of 13 (activity perception “somewhat hard”), and lower extremity strengthening exercises were performed at an intensity of 15 to 16.
The health education program focused on successful aging, and which has been termed the successful aging arm of the study in previous publications. The health education group attended weekly workshops of health education during the first 26 weeks, and then monthly sessions thereafter (bi-monthly attendance was optional). Workshops included topics, other than physical activity, relevant to older adults, such as how to effectively negotiate the health care system, how to travel safely, preventive services and screenings recommended at different ages, where to go for reliable health information, nutrition, etc. The program also included a 5- to 10-minute instructor-led program of gentle upper extremity stretching or flexibility exercises.
Measurements
Participants were assessed every six months at clinic visits. Home, telephone, and proxy assessments were attempted if the participants could not come to the clinic. The assessment staff was blinded to the intervention and remained separate from the intervention team. Participants were asked not to disclose their assigned group and not to talk about their interventions during the assessment. Self-reported physical activity was ascertained by a separate set of un-blinded assessors.
The main baseline assessments included self-reported demographic and contact information, medical and hospitalization history, medication inventory, ECG, physical exam, Quality of Well-Being questionnaire,20 health care utilization, physical activity assessed with the Community Healthy Activities Model Program for Seniors (CHAMPS) questionnaire,21 and with accelerometry over 7-day periods (Actigraph Inc., Pensacola FL), cognitive testing, 400 m walk test,22 the SPPB; body weight, blood pressure, and pulse rate. These measures were repeated during follow-up at varied intervals. Details of these measures and their frequency are described elsewhere.15 The SPPB consisted of 4 m walk at usual pace, a timed repeated chair stand, and three increasingly difficult standing balance tests.16,23 Each measure was assigned a categorical score ranging from 0 (inability to complete the test) to 4 (best performance). A summary score ranging from 0 (worst performers) to 12 (best performers) was calculated by summing the three component scores. Race and ethnicity were reported by the participants and were collected according to NIH requirements. To minimize reporting bias, adverse events originating from the blinded assessments are presented.
Outcome assessment
The primary outcome of major mobility disability was defined as the inability to complete a 400 m walk test within 15 min without sitting and without the help of another person or walker.15 Use of a cane was acceptable. Participants were asked to walk 400 m at their usual pace, without over exerting, on a 20 m course for 10 laps (40 m per lap). Participants were allowed to stop for up to 1 minute for fatigue or related symptoms. When major mobility disability could not be objectively measured because of the inability of the participant to come to the clinic and absence of a suitable walking course at the participant’s home, institution or hospital, an alternative adjudication of the outcome was based on objective inability to walk 4 m in ≤10 sec, or self-, proxy-, or medical record-reported inability to walk across a room. If participants met these alternative criteria, they would not be able to complete the 400 m walk within 15 minutes. Reports of death were tracked through regular surveillance. Two consecutive major mobility disability assessments, or major mobility disability followed by death defined persistent mobility disability. Censoring was defined at the time of the last definitive assessment for major mobility disability.
At each contact, participants (or proxies if the participant was not available) were questioned about outcomes and hospitalizations since the last visit. All records for hospitalizations were obtained and outcomes were reviewed and adjudicated independently by two experts who were blinded to the group randomization. If the two reviewers disagreed, the information was forwarded to the adjudication committee and a determination was made by consensus.
Statistical Considerations
Power calculations for the primary outcome, time until the first post-randomization occurrence of major mobility disability, were based on a log-rank test with a 2-sided, 0.05 significance level. Based on the LIFE Pilot study,14 the annual incidence rate of major mobility disability in the health education group was assumed to increase from 18% in the first year to 21% after two years. We further assumed that recruitment would be uniform over 21 months, follow-up would average 31 months, and loss to follow-up would be 8%/year. Under these assumptions, randomization of 1600 participants provides 80% power to detect a 21% reduction, and 90% power to detect 24% reduction in the hazard for major mobility disability in the physical activity participants. These effect size targets were determined based on consistency with effects derived from observational research, the LIFE Pilot experience, clinical relevance (around 20% reduction) and available funding resources.
Baseline characteristics were summarized by intervention group using mean (SD) or percentages. Intervention adherence was calculated as the percentage of scheduled intervention sessions attended by participants. Self-reported minutes of activity and minutes spent in activity associated with >760 counts/minute (by accelerometry)24 were analyzed using mixed effects ANCOVA models for repeatedly measured outcomes with an unstructured parameterization for longitudinal covariance. Models contained the following terms: field center and gender (both used to stratify randomization), baseline value of the relevant physical activity measure, intervention, clinic visit and intervention-by-visit interaction. Least squares means were obtained from these models and contrasts were used to estimate the average effects (95% CI) over follow-up. Risk ratios (95% CI) were calculated to determine the relative effect of the intervention on the proportion of participants reporting adverse effects. A test of equality of the risk ratios for hospitalization between baseline subgroups defined by SPPB levels (< 8 vs ≥ 8) was performed using Poisson regression.
The effect of the intervention on the primary outcome (i.e. time until the initial ascertainment of major mobility disability) was tested based on a two-tailed significance of 0.05 using the intention to treat approach in which participants are grouped according to randomization assignment. To compare intervention, we used a likelihood ratio test from a Cox regression model, stratified by field center and gender. Failure time was measured from the time of randomization; follow-up was censored at the last successfully completed 400m walk test. For participants who did not have any outcome assessments, we assigned one hour of follow-up time, since we knew that they completed the 400m walk at baseline. An assessment for non-proportionality of hazards was made with the addition of the interaction between log(time) and intervention.25 Interaction terms were entered into these Cox models and likelihood ratio tests were used to assess the consistency of the intervention effect across levels of baseline subgroups (ethnicity/race, gender, cardiovascular disease, diabetes, walking speed, and physical performance). The secondary endpoints were analyzed using the same approach as used for the primary outcome.
Sensitivity analyses were performed to investigate the effect of loss to follow-up on major mobility disability. These analyses used stabilized inverse probability weights that were a function of baseline covariates hypothesized to be predictive of loss-to-follow-up (i.e. gender, race-ethnicity, age (80+), history of diabetes, gait speed <0.8 m/sec, low SPPB score (<8), 3MS<90, clinical site, and living alone (yes/no)) and follow-up gait speed and SPPB scores to explore how the estimated hazard ratios and confidence intervals may have been altered under these missing data assumptions. Statistical analyses were performed in SAS 9.3 and R.26
RESULTS
Study participants
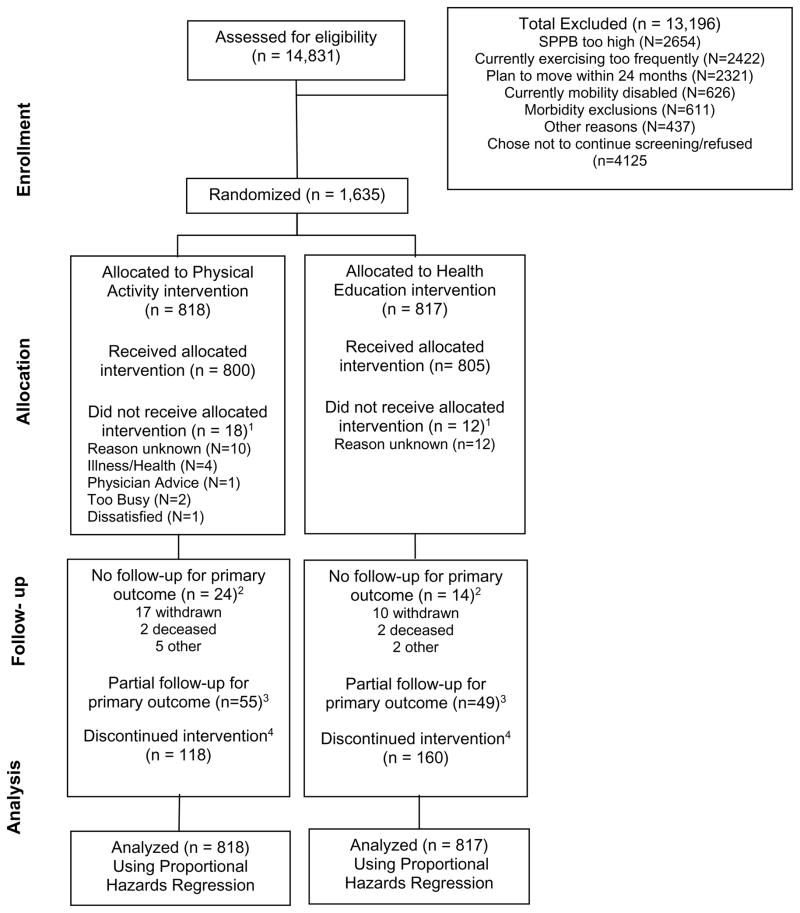
From February 2010 to December 2011, we screened 14,831 participants; of these, 1,635 were eligible and randomized (818 to physical activity and 817 to health education; Figure 1). Details regarding screening, recruitment yields and baseline characteristics have been published.18 Baseline characteristics were similar in the two groups (Table 1). The mean age was 78.9 years, 67.2% were women, 17.6% were African American, the average body mass index was 30.2 kg/m2, and the average SPPB score was 7.4. The mean follow-up for any contact (including telephone) was 2.6 years (median=2.7 years; Inter-Quartile Range [IQR]=2.3–3.1 years). The trial ended in December 2013 as planned in the study protocol.
Figure 1.
Study flow
1Participants who did not receive the allocated intervention, i.e. attended no intervention sessions.
2For participants who did not have any MMD assessments, we assigned one hour of follow-up time, since we knew that they were able to do the 400m walk at baseline.
3Partial follow-up indicates participants who had censoring times prior to the last planned follow-up visit.
4Discontinuation of the intervention was operationalized as participants who did not attend at least one intervention session during their last 6-months of follow-up prior to the last planned follow-up visit date. Deaths and intervention withdrawals are included in these numbers. As an example, a participant may have discontinued the intervention in the initial six month of follow-up due to illness and then died prior to the 6-months assessment for the primary outcome. This participant would be reflected as missing the primary outcome due to death and also discontinuing the intervention.
Table 1.
Baseline characteristics of the participants
| Characteristic | Physical Activity N=818 |
Health education N=817 |
|---|---|---|
| Age (years) | 78.7 ± 5.2 | 79.1 ± 5.2 |
| Women | 547 (66.9%) | 551 (67.4%) |
| Ethnicity: Hispanic | 31 (3.8%) | 30 (3.7%) |
| Race: Caucasian | 604 (73.8%) | 635 (77.7%) |
| Race: African American | 163 (19.9%) | 125 (15.3%) |
| Short Physical Performance Battery score | 7.4 ± 1.6 | 7.3 ± 1.6 |
| Short Physical Performance Battery score <8 | 353 (43.3%) | 378 (46.2%) |
| 400 m walking speed (m/sec) | 0.83 (0.17) | 0.82 (0.17) |
| Body mass index (kg/m2) | 30.1 ± 5.7 | 30.3 ± 6.2 |
| Self-reported minutes per week in walking/weight training activities | 75.1 ± 125.6 Median=0 IQR=0–105 |
86.7 ± 134.5 Median=30 IQR=0–105 |
| Accelerometry minutes per week of moderate physical activity | 193.7 ± 155.3 Median=161 IQR=80–257 (N=590) |
202.1 ± 186.5 Median=153 IQR=85–266 (N=581) |
| Modified Mini-Mental State Examination (3MSE) score (0–100 scale) | 91.5 ± 5.5 | 91.6 ± 5.3 |
| Self-reported hypertension | 573 (70.5%) (N=813) | 578 (71.5%) (N=808) |
| Self-reported diabetes | 199 (24.4%) (N=815) | 216 (26.6%) (N=813) |
| Self-reported heart attack, myocardial infarction | 60 (7.4%) (N=815) | 69 (8.5%) (N=812) |
| Self-reported stroke | 57 (7.0%) (N=814) | 52 (6.4%) (N=814) |
| Self-reported cancer | 178 (21.9%) (N=814) | 192 (23.6%) (N=815) |
| Self-reported chronic pulmonary disease | 130 (16.0%) (N=815) | 123 (15.2%) (N=812) |
Intervention adherence
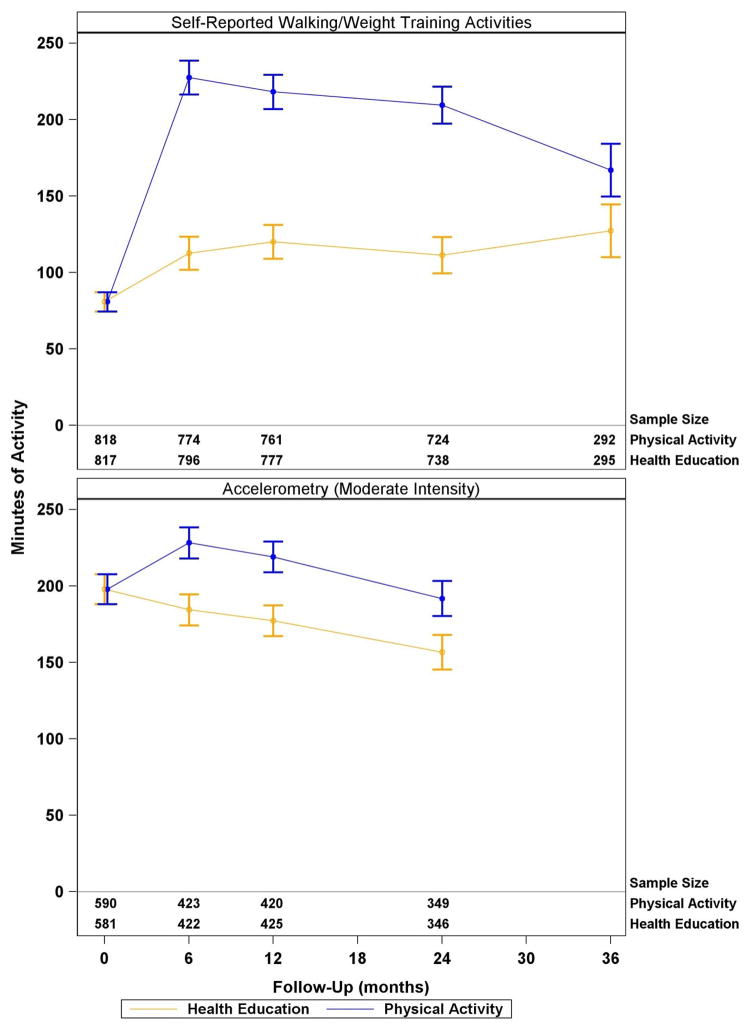
The physical activity group attended 63%, SD=27% (median=71%; IQR=50–83) of the scheduled sessions after excluding medical leave. A total of 479 (58.6%) participants went on medical leave at least once and 210 (25.7%) more than once. The mean duration of medical leave was 135±203 days (median=49 days; IQR=21–140). Health education participants attended 73±25% (median=82%; IQR=63–90). Based on CHAMPS questionnaires, through the 24 month follow-up visit (the minimum planned intervention duration for all participants), the physical activity group maintained an average of 218 min/week (95%CI 210–227; average change from baseline=138 min, 95%CI=129–146) in walking/weight training activities; whereas, the health education group maintained an average of 115 min/week (95%CI=106–123; average change from baseline=34 min, 95%CI=24–42) (Figure 2). Thus, the physical activity intervention maintained a 104 min (95%CI=92–116; p<0.001) difference in walking/weight training activities compared with the health education group during the initial two years where all participants were followed.
Figure 2.
Self-reported and accelerometry derived physical activity by treatment group in the LIFE study participants. Plotted values represent least squares means (95% CI) from a mixed effects model adjusting for clinical site and gender (both used to stratify randomization) and the baseline self-reported walking/weight training activities or accelerometry counts. In addition to the above mentioned terms, the model contained a term for intervention group, follow-up clinic visit (i.e. 6, 12, 18, … months) and the intervention by visit interaction. All participants had expected follow-up through 24 months and approximately 47% of randomized participants had expected visits at 36 months. Accelerometry data were not collected at the 36 month visit. Baseline values represent the overall mean of both groups combined: this is the assumed value for both groups when obtaining least squares means at follow-up using mixed effects ANCOVA. The baseline, pre-randomization value, is reflected by follow-up time 0. P-values represent tests of the average intervention effect across all visits.
Based on accelerometry using a definition of >760 counts/minute for moderate activity,24 through follow-up, on average, the physical activity group participated in 213 min/week (95%CI=205–221; average change from baseline=15 min, 95%CI 7–23) of moderate activity; whereas, the health education group maintained 173 min/week (95%CI=165–181; average change from baseline= −25 min, 95%CI=−33, −17 min) (Figure 2). Thus, the physical activity intervention maintained a 40 min/week (95%CI=29–52; p<0.001) difference in moderate physical activity assessed with accelerometry, compared with the health education group during two years of follow-up.
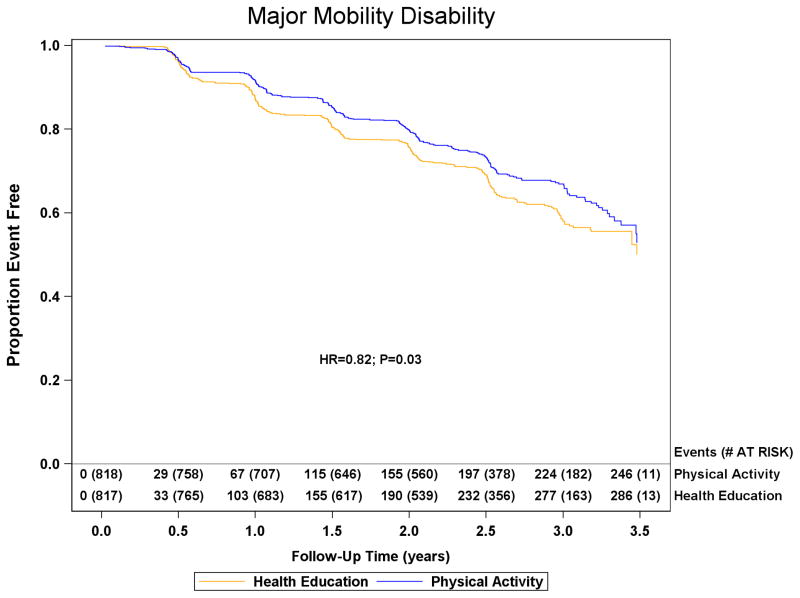
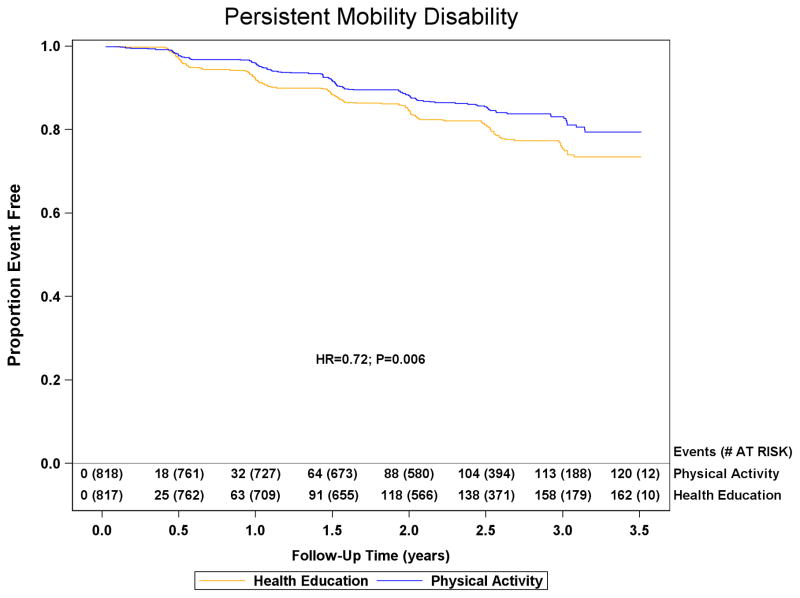
Major mobility disability
Data for major mobility disability were obtained for 794/818 (97.1%) physical activity and 803/817 (98.3%) health education participants. Loss to follow-up was 4.0% annually. Major mobility disability was experienced by 246/818 (30.1%) physical activity participants and 290/817 (35.5%) health education participants (HR=0.82; 95%CI=0.69–0.98; p=0.03, Figure 3). Of the 246 and 290 physical activity and health education participants classified with major mobility disability, 42 (17%) and 32 (11%) resulted from alternative adjudications in each group, respectively. The sensitivity analyses exploring the effect of loss to follow-up on conclusions altered the estimates of the hazard ratio and confidence limits by less than 0.016 for all analyses (see online appendix table). Persistent mobility disability was experienced by 120/818 (14.7%) physical activity and 162/817 (19.8%) health education participants (HR=0.72; 95%CI=0.57–0.91; p=0.006). Major mobility disability or death was experienced by 264/818 (32.3%) physical activity and 309/817 (37.8%) health education participants (HR=0.82; 95%CI=0.70–0.97; p=0.02).
Figure 3.
The effect of a moderate physical activity intervention on the onset of major mobility disability and persistent mobility disability: The Life Study. Kaplan Meier plot of major mobility disability occurrence and persistent mobility disability occurrence are presented in the top and bottom panels, respectively. The graph for major mobility disability was truncated at 3.5 years and the health education group had 4 additional failures between 3.5 and 3.6 years of follow-up. Number of events represents cumulative events and adjusted hazard ratios and p-values are from proportional hazards regression models defined in the methods.
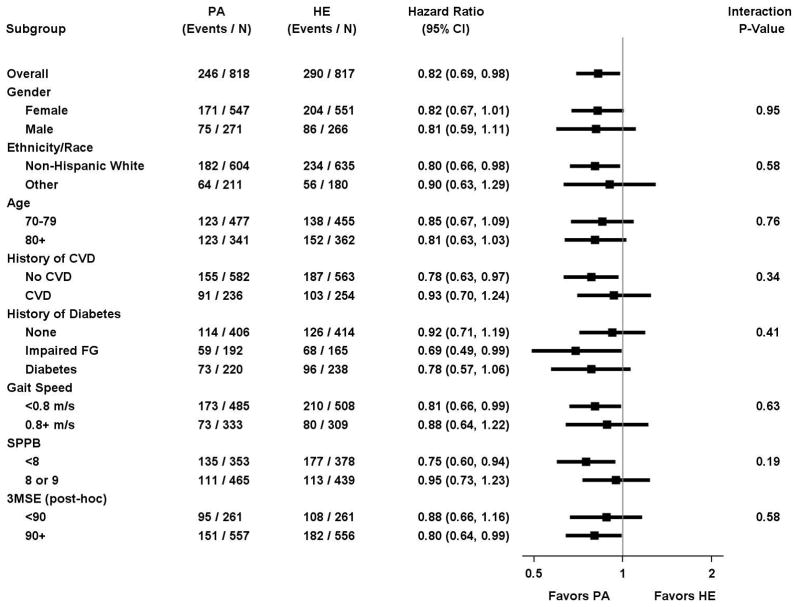
In pre-specified subgroup analyses, results for major mobility disability did not significantly differ when participants were categorized by ethnicity/race, gender, history of cardiovascular disease, history of diabetes, baseline walking speed, and baseline physical performance (Figure 4). The subgroup with lower physical function at baseline (SPPB<8), representing 44.7% of the study population yet 71% (283 of 536 total events) of major mobility disability events, received considerable benefit (HR=0.81). In post-hoc analyses, the benefit of physical activity on major mobility disability was similar in participants with 3MSE score <90 and ≥90 (Figure 4).
Figure 4.
Forest plot of the hazard ratio of major mobility disability for physical activity vs. health education according to sub-groups (PA= Physical Activity; HA = Health education; FG = Fasting Glucose). P-values were obtained from likelihood ratios tests of the interaction terms added to the Cox regression model.
Safety Serious adverse events were reported by 404/818 (49.4%) of the physical activity and 373/817 (45.7%) of the health education participants (Risk Ratio=1.08; 95%CI=0.98–1.20, Table 2). For inpatient hospitalizations, 396/818 (48.4%) physical activity and 360/817 (44.1%) health education participants reported an event (Risk Ratio=1.10; 95%CI=0.99–1.22). The reasons for hospitalization were highly heterogeneous, and most of them deemed unrelated to the intervention. Among those with SPPB < 8 the Risk Ratio was 1.04 (95%CI=0.90–1.20); and among those with SPPB ≥ 8 the Risk Ratio was 1.17 (95%CI=1.00–1.36). The test of equality of risk ratios for hospitalization for physical activity vs. health education between the two baseline SPPB subgroups was not significant (p=0.44).
Table 2.
All Deaths and number of participants reporting adverse events at blinded assessments
| Event Type | Physical Activity (N=818) | Health education (N = 817) | Risk Ratio1 (95% CI) | ||
|---|---|---|---|---|---|
| # Pts. (%) | # of Events | # Pts. (%) | # Events | ||
| Serious Adverse Events | |||||
| • All serious adverse events | 404 (49.4%) | 879 | 373 (45.7%) | 774 | 1.08 (0.98, 1.20) |
| • Death | 48 (5.9%) | 48 | 42 (5.1%) | 42 | 1.14 (0.76, 1.71) |
| • Life threatening event | 11 (1.3%) | 11 | 8 (1.0%) | 8 | 1.37 (0.56, 3.40) |
| • Persistent disability/incapacity | 33 (4.0%) | 51 | 26 (3.2%) | 45 | 1.27 (0.77, 2.10) |
| • All inpatient hospitalizations | 396 (48.4%) | 777 | 360 (44.1%) | 681 | 1.10 (0.99, 1.22) |
| • Any other serious events | 7 (0.9%) | 8 | 8 (1.0%) | 10 | 0.87 (0.32, 2.40) |
| Most frequent hospitalization diagnoses | |||||
| • Infection | 74 (.9.0%) | 95 | 57 (7.0%) | 68 | 1.30 (0.93, 1.81) |
| • Surgical Procedure | 68 (8.3%) | 76 | 73 (8.9%) | 84 | 0.93 (0.68, 1.28) |
| • Fall, Syncope, Dizziness, Vertigo | 54 (6.4%) | 58 | 53 (6.5%) | 62 | 1.02 (0.71, 1.49) |
| • Atrial Fibrillation/Flutter | 24 (2.9%) | 28 | 20 (2.4%) | 23 | 1.20 (0.67, 2.15) |
| • Heart Failure | 18 (2.2%) | 21 | 14 (1.7%) | 20 | 1.28 (0.64, 2.56) |
| • Stroke/TIA/Intracranial Hemorrhage | 29 (3.5%) | 33 | 28 (3.4%) | 34 | 1.03 (0.62, 1.72) |
| • MI/Chest Pain/Acute Coronary Syndrome | 33 (4.0%) | 42 | 25 (3.1%) | 27 | 1.32 (0.79, 2.20) |
| • Fracture | 27 (3.3%) | 29 | 26 (3.2%) | 27 | 1.04 (0.61, 1.76) |
| • Neoplasm | 17 (2.1%) | 17 | 17 (2.1%) | 20 | 1.00 (0.51, 1.94) |
| • Arthritis/Back, Neck, or Bone Pain | 30 (3.7%) | 31 | 33 (4.0%) | 35 | 0.91 (0.56, 1.47) |
| Symptoms Resulting in At Least 1 Week of Restricted Activity2 | |||||
| • All Cases | 198 (24.2%) | 253 | 198 (24.2%) | 249 | 1.00 (0.84, 1.19) |
| • Fall | 47 (5.7%) | 53 | 71 (8.7%) | 81 | 0.66 (0.46, 0.94) |
| • Fatigue | 38 (4.6%) | 46 | 41 (5.0%) | 45 | 0.93 (0.60, 1.42) |
| • Muscle or joint aching | 32 (3.9%) | 37 | 40 (4.9%) | 43 | 0.80 (0.51, 1.26) |
| • Back pain | 36 (4.4%) | 41 | 33 (4.0%) | 35 | 1.09 (0.69, 1.73) |
| • Muscle or joint stiffness | 26 (3.2%) | 30 | 33 (4.0%) | 35 | 0.79 (0.48, 1.30) |
| • Foot pain | 17 (2.1%) | 17 | 18 (2.2%) | 18 | 0.94 (0.49, 1.82) |
| • Dizziness | 18 (2.2%) | 19 | 14 (1.7%) | 15 | 1.28 (0.64, 2.56) |
| • Shortness of breath | 15 (1.8%) | 16 | 20 (2.4%) | 22 | 0.75 (0.39, 1.45) |
| • Fainting | 16 (2.0%) | 18 | 10 (1.2%) | 11 | 1.60 (0.73, 3.50) |
| • Abnormal heart rhythm | 9 (1.1%) | 9 | 8 (1.0%) | 8 | 1.12 (0.44, 2.90) |
| • Other Symptom | 84 (10.3%) | 96 | 71 (8.7%) | 75 | 1.18 (0.87, 1.60) |
Risk ratio compares the proportion of participants reporting any events in the physical activity group versus the health education group, with 95% asymptotic confidence intervals.
Symptoms resulting in at least one week of restricted activity may also lead to serious adverse events. Thus, events reported in this section of the Table may also be reflected as serious adverse events or hospitalizations.
DISCUSSION
The LIFE study showed that, over 2.6 years of follow-up, the physical activity intervention, compared with the health education intervention, significantly reduced major mobility disability (HR=0.82, p=0.03), persistent mobility disability (HR=0.72, p=0.006) and the combined outcome of major mobility disability or death (HR=0.82, p=0.02). The subgroup with lower physical function at baseline (SPPB<8), representing 44.7% of the study population yet 71% (283 of 536 total events) of major mobility disability events, received considerable benefit (HR=0.81). These results suggest the potential for structured physical activity as a feasible and effective intervention to reduce the burden of disability among vulnerable older persons, in spite of functional decline in late life. To our knowledge, the LIFE study is the largest and longest duration randomized trial of physical activity in older persons.
The LIFE study has important strengths, including the objectively measured primary outcome of major mobility disability that is a reliable,22 well-validated and important clinical and public health outcome in older people.11 Participants at high risk of disability were recruited from 8 field centers spanning the US, including urban, suburban and rural settings, and included a high proportion of older adults from African American/Hispanic backgrounds. Although highly prevalent and increasing in size, the older, more vulnerable population has been understudied and typically is not included in large randomized trials. Retention throughout the follow-up was excellent. The adherence rates to the physical activity intervention were similar or higher than those achieved in other much shorter studies involving older adults.27–29 The physical activity program was likely successful in part because of the adherence and lifestyle motivation procedures put in place.30 The participants were reimbursed for their transportation costs, which added to the cost of the intervention, but likely contributed to the high levels of attendance. According to initial cost data collected in the LIFE study, the physical activity intervention cost, including transportation, was approximately $4,900 per participant over the 2.6 years of average participation ($1,815 per year). The physical activity intervention was designed to be simple for widespread implementation in a variety of communities and settings, as it does not require any special equipment.
The LIFE study has limitations. We could not ascertain whether participants who were excluded because of their high level of physical function or severe cognitive deficits, would also benefit from physical activity. The participants were recruited from the community, but may have been self-referred, so they may not be fully representative of all people in the community. The average follow-up duration of 2.6 years was relatively short vs. the estimated average 9 year life-expectancy of the LIFE cohort.31 Ideally, it would be useful to assess the effect of the intervention on the quality of the remaining years of life. The study, which was powered based on assumptions of 21%–24% risk reduction, achieved a hazard ratio of 0.82 and an absolute risk difference of 5.4%. While the effect size was slightly lower than planned, we believe that it is clinically relevant given the major health impact of mobility disability and the lack of proven interventions to avert mobility disability in vulnerable older populations. In addition, persistent mobility disability was significantly reduced by a larger degree in the physical activity group (HR=0.72), indicating that physical activity not only prevents the onset of major mobility disability, but also favors improved recovery in those who lose mobility.
Based on observational cohorts,32 we expected a lower hospitalization rate in the physical activity group. In the LIFE study, Physical activity did not decrease the hospitalizations rate. We found a higher rate of hospitalizations in the physical activity group that did not reach statistical significance. The hospitalizations comprised a range of heterogeneous diagnoses mostly deemed unrelated to the intervention. Our finding may have several explanations. First, physical activity may unmask symptoms resulting in earlier detection of underlying medical conditions. For example, sedentary older persons with subclinical left ventricular dysfunction may observe heart failure symptoms when they start moderate physical activity. Second, the physical activity group’s more frequent contact, and testing of vital signs at each intervention session may have led to a higher rate of recognition of health events. Third, the stress of exercise in the context of lowered homeostatic reserve in vulnerable participants,33 may have led to a higher risk of adverse events. However, our data do not support this explanation. The hospitalization results were not significantly different among those with SPPB score <8, and those with score 8 or 9. Finally, there may be no causal association between physical activity and hospitalizations.
Physical activity did not decrease the death rate. We found a higher rate of mortality in the physical activity group that did not reach statistical significance, and which was compatible with benefit or harm of physical activity (Table 2). Given the small number of events the data regarding mortality are inconclusive. Further studies are needed to assess the effects of physical activity on mortality and hospitalizations in vulnerable older adults.
Conclusion
A structured moderate intensity physical activity program, compared with a health education program, reduced major mobility disability over 2.6 years among older adults at risk of disability. These findings suggest mobility benefit from such a program in vulnerable older adults.
Acknowledgments
The Lifestyle Interventions and Independence for Elders Study is funded by a National Institutes on Health/National Institute on Aging Cooperative Agreement UO1AG22376 and a supplement from the National Heart, Lung and Blood Institute 3U01AG022376-05A2S, and was sponsored in part by the Intramural Research Program, National Institute on Aging, NIH. The NIH sponsor was a voting member (1 vote out of 12 votes) of the LIFE Steering Committee, which approved the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, and approval of the manuscript; and decision to submit the manuscript for publication.
We are indebted to Evan C. Hadley, MD, and Sergei Romashkan, MD, PhD from the National Institute on Aging, Bethesda, MD, for their substantial intellectual contribution to the development and implementation of the LIFE study. Dr. Hadley and Dr. Romashkan are federal employees fully paid by the NIH. They did not receive any additional compensation from the study.
The research is partially supported by the Claude D. Pepper Older Americans Independence Centers at the University of Florida (1 P30 AG028740), Wake Forest University (1 P30 AG21332), Tufts University (1P30AG031679), University of Pittsburgh (P30 AG024827), and Yale University (P30AG021342) and the NIH/NCRR CTSA at Stanford University (UL1 RR025744), at University of Florida (U54RR025208) and at Yale University (UL1 TR000142) Tufts University is also supported by the Boston Rehabilitation Outcomes Center (1R24HD065688-01A1).
LIFE investigators are also partially supported by the following:
Dr. Thomas Gill (Yale University) is the recipient of an Academic Leadership Award (K07AG3587) from the National Institute on Aging.
Dr. Carlos Fragoso (Spirometry Reading Center, Yale University) is the recipient of a Career Development Award from the Department of Veterans Affairs.
Dr. Roger Fielding (Tufts University) is partially supported by the U.S. Department of Agriculture, under agreement No. 58-1950-0-014. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Dept of Agriculture.
Appendix: Research Investigators for the LIFE Study
The Lifestyle Interventions and Independence for Elders Study is funded by a National Institutes of Health/National Institute on Aging Cooperative Agreement #UO1 AG22376 and a supplement from the National Heart, Lung and Blood Institute 3U01AG022376-05A2S, and sponsored in part by the Intramural Research Program, National Institute on Aging, NIH.
The research is partially supported by the Claude D. Pepper Older Americans Independence Centers at the University of Florida (1 P30 AG028740), Wake Forest University (1 P30 AG21332), Tufts University (1P30AG031679), University of Pittsburgh (P30 AG024827), and Yale University (P30AG021342) and the NIH/NCRR CTSA at Stanford University (UL1 RR025744),
Tufts University is also supported by the Boston Rehabilitation Outcomes Center (1R24HD065688-01A1).
LIFE investigators are also partially supported by the following:
Dr. Thomas Gill (Yale University) is the recipient of an Academic Leadership Award (K07AG3587) from the National Institute on Aging.
Dr. Carlos Fragoso (Spirometry Reading Center, Yale University) is the recipient of a Career Development Award from the Department of Veterans Affairs.
Dr. Roger Fielding (Tufts University) is partially supported by the U.S. Department of Agriculture, under agreement No. 58-1950-0-014. Any opinions, findings, conclusion, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Dept of Agriculture.
Administrative Coordinating Center, University of Florida, Gainesville, FL
Marco Pahor, MD – Principal Investigator of the LIFE Study
Jack M. Guralnik, MD, PhD – Co-Investigator of the LIFE Study (University of Maryland School of Medicine, Baltimore, MD)
Christiaan Leeuwenburgh, PhD
Connie Caudle
Lauren Crump, MPH
Latonia Holmes
Jocelyn Lee, PhD
Ching-ju Lu, MPH
Data Management, Analysis and Quality Control Center, Wake Forest University, Winston Salem, NC
Michael E. Miller, PhD – DMAQC Principal Investigator
Mark A. Espeland, PhD – DMAQC Co-Investigator
Walter T. Ambrosius, PhD
William Applegate, MD
Daniel P. Beavers, PhD, MS
Robert P. Byington, PhD, MPH, FAHA
Delilah Cook, CCRP
Curt D. Furberg, MD, PhD
Lea N. Harvin, BS
Leora Henkin, MPH, Med
John Hepler, MA
Fang-Chi Hsu, PhD
Laura Lovato, MS
Wesley Roberson, BSBA
Julia Rushing, BSPH, MStat
Scott Rushing, BS
Cynthia L. Stowe, MPM
Michael P. Walkup, MS
Don Hire, BS
W. Jack Rejeski, PhD
Jeffrey A. Katula, PhD, MA
Peter H. Brubaker, PhD
Shannon L. Mihalko, PhD
Janine M. Jennings, PhD
National Institutes of Health, Bethesda, MD
Evan C. Hadley, MD (National Institute on Aging)
Sergei Romashkan, MD, PhD (National Institute on Aging)
Kushang V. Patel, PhD (National Institute on Aging)
National Heart, Lung and Blood Institute, Bethesda, MD
Denise Bonds, MD, MPH
Field Centers
Northwestern University, Chicago, IL
Mary M. McDermott, MD – Field Center Principal Investigator
Bonnie Spring, PhD – Field Center Co-Investigator
Joshua Hauser, MD – Field Center Co-Investigator
Diana Kerwin, MD – Field Center Co-Investigator
Kathryn Domanchuk, BS
Rex Graff, MS
Alvito Rego, MA
Pennington Biomedical Research Center, Baton Rouge, LA
Timothy S. Church, MD, PhD, MPH – Field Center Principal Investigator
Steven N. Blair, PED (University of South Carolina)
Valerie H. Myers, PhD
Ron Monce, PA-C
Nathan E. Britt, NP
Melissa Nauta Harris, BS
Ami Parks McGucken, MPA, BS
Ruben Rodarte, MBA, MS, BS
Heidi K. Millet, MPA, BS
Catrine Tudor-Locke, PhD, FACSM
Ben P. Butitta, BS
Sheletta G. Donatto, MS, RD, LDN, CDE
Shannon H. Cocreham, BS
Stanford University, Palo Alto, CA
Abby C. King, PhD – Field Center Principal Investigator
Cynthia M. Castro, PhD
William L. Haskell, PhD
Randall S. Stafford, MD, PhD
Leslie A. Pruitt, PhD
Kathy Berra, MSN, NP-C, FAAN
Veronica Yank, MD
Tufts University, Boston, MA
Roger A. Fielding, PhD – Field Center Principal Investigator
Miriam E. Nelson, PhD – Field Center Co-Investigator
Sara C. Folta, PhD – Field Center Co-Investigator
Edward M. Phillips, MD
Christine K. Liu, MD
Erica C. McDavitt, MS
Kieran F. Reid, PhD, MPH
Won S. Kim, BS
Vince E. Beard, BS
University of Florida, Gainesville, FL
Todd M. Manini, PhD – Field Center Principal Investigator
Marco Pahor, MD – Field Center Co-Investigator
Stephen D. Anton, PhD
Susan Nayfield, MD
Thomas W. Buford, PhD
Michael Marsiske, PhD
Bhanuprasad D. Sandesara, MD
Jeffrey D. Knaggs, BS
Megan S. Lorow, BS
William C. Marena, MT, CCRC
Irina Korytov, MD
Holly L. Morris, MSN, RN, CCRC (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Margo Fitch, PT (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Floris F. Singletary, MS, CCC-SLP (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Jackie Causer, BSH, RN (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
Katie A. Radcliff, MA (Brooks Rehabilitation Clinical Research Center, Jacksonville, FL)
University of Pittsburgh, Pittsburgh, PA
Anne B. Newman, MD, MPH – Field Center Principal Investigator
Stephanie A. Studenski, MD, MPH – Field Center Co-Investigator
Bret H. Goodpaster, PhD
Nancy W. Glynn, PhD
Oscar Lopez, MD
Neelesh K. Nadkarni, MD, PhD
Kathy Williams, RN, BSEd, MHSA
Mark A. Newman, PhD
George Grove, MS
Janet T. Bonk, MPH, RN
Jennifer Rush, MPH
Piera Kost, BA (deceased)
Diane G. Ives, MPH
Wake Forest University, Winston Salem, NC
Stephen B. Kritchevsky, Ph.D. – Field Center Principal Investigator
Anthony P. Marsh, PhD – Field Center Co-Investigator
Tina E. Brinkley, PhD
Jamehl S. Demons, MD
Kaycee M. Sink, MD, MAS
Kimberly Kennedy, BA, CCRC
Rachel Shertzer-Skinner, MA, CCRC
Abbie Wrights, MS
Rose Fries, RN, CCRC
Deborah Barr, MA, RHEd, CHES
Yale University, New Haven, CT
Thomas M. Gill, MD – Field Center Principal Investigator
Robert S. Axtell, PhD, FACSM – Field Center Co-Investigator (Southern Connecticut State University, Exercise Science Department)
Susan S. Kashaf, MD, MPH (VA Connecticut Healthcare System)
Nathalie de Rekeneire, MD, MS
Joanne M. McGloin, MDiv, MS, MBA
Karen C. Wu, RN
Denise M. Shepard, RN, MBA
Barbara Fennelly, MA, RN
Lynne P. Iannone, MS, CCRP
Raeleen Mautner, PhD
Theresa Sweeney Barnett, MS, APRN
Sean N. Halpin, MA
Matthew J. Brennan, MA
Julie A. Bugaj, MS
Maria A. Zenoni, MS
Bridget M. Mignosa, AS
Cognition Coordinating Center, Wake Forest University, Winston Salem, NC
Jeff Williamson, MD, MHS – Center Principal Investigator
Kaycee M Sink, MD, MAS – Center Co-Investigator
Hugh C. Hendrie, MB, ChB, DSc (Indiana University)
Stephen R. Rapp, PhD
Joe Verghese, MB, BS (Albert Einstein College of Medicine of Yeshiva University)
Nancy Woolard
Mark Espeland, PhD
Janine Jennings, PhD
Electrocardiogram Reading Center, University of Florida, Gainesville, FL
Carl J. Pepine MD, MACC
Mario Ariet, PhD
Eileen Handberg, PhD, ARNP
Daniel Deluca, BS
James Hill, MD, MS, FACC
Anita Szady, MD
Spirometry Reading Center, Yale University, New Haven, CT
Geoffrey L. Chupp, MD
Gail M. Flynn, RCP, CRFT
Thomas M. Gill, MD
John L. Hankinson, PhD (Hankinson Consulting, Inc.)
Carlos A. Vaz Fragoso, MD
Cost Effectiveness Analysis Center
Erik J. Groessl, PhD (University of California, San Diego and VA San Diego Healthcare System)
Robert M. Kaplan, PhD (Office of Behavioral and Social Sciences Research, National Institutes of Health)
Footnotes
Dr. Mike Miller had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Data analyses were conducted by Dr. Mike Miller, Dr. Walter Ambrosius and Dr. Mark Espeland.
Reference List
- 1.The Older Population: 2010 Census Briefs. Washington, DC: U.S. Department of Commerce; 2012. [Google Scholar]
- 2.Katz S, Branch LG, Branson MH, Papsidero JA, Beck JC, Greer DS. Active life expectancy. N Engl J Med. 1983;309:1218–1224. doi: 10.1056/NEJM198311173092005. [DOI] [PubMed] [Google Scholar]
- 3.Branch LG, Guralnik JM, Foley DJ, et al. Active life expectancy for 10,000 Caucasian men and women in three communities. J Gerontol. 1991;46:M145–50. doi: 10.1093/geronj/46.4.m145. [DOI] [PubMed] [Google Scholar]
- 4.Lonergan ET, Krevans JR. A national agenda for research on aging. N Engl J Med. 1991;324:1825–1828. doi: 10.1056/NEJM199106203242527. [DOI] [PubMed] [Google Scholar]
- 5.Guralnik JM, LaCroix AZ, Abbott RD, et al. Maintaining mobility in late life. I. Demographic characteristics and chronic conditions. Am J Epidemiol. 1993;137:845–857. doi: 10.1093/oxfordjournals.aje.a116746. [DOI] [PubMed] [Google Scholar]
- 6.Branch LG, Jette AM. A prospective study of long-term care institutionalization among the aged. Am J Public Health. 1982;72:1373–1379. doi: 10.2105/ajph.72.12.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corti MC, Guralnik JM, Salive ME, Sorkin JD. Serum albumin level and physical disability as predictors of mortality in older persons. JAMA. 1994;272:1036–1042. [PubMed] [Google Scholar]
- 8.Khokhar SR, Stern Y, Bell K, et al. Persistent mobility deficit in the absence of deficits in activities of daily living: a risk factor for mortality. J Am Geriatr Soc. 2001;49:1539–1543. doi: 10.1046/j.1532-5415.2001.4911251.x. [DOI] [PubMed] [Google Scholar]
- 9.Hirvensalo M, Rantanen T, Heikkinen E. Mobility difficulties and physical activity as predictors of mortality and loss of independence in the community-living older population. J Am Geriatr Soc. 2000;48:493–498. doi: 10.1111/j.1532-5415.2000.tb04994.x. [DOI] [PubMed] [Google Scholar]
- 10.Lampinen P, Heikkinen E. Reduced mobility and physical activity as predictors of depressive symptoms among community-dwelling older adults: an eight-year follow-up study. Aging Clin Exp Res. 2003;15:205–211. doi: 10.1007/BF03324501. [DOI] [PubMed] [Google Scholar]
- 11.Newman AB, Simonsick EM, Naydeck EM, et al. Association of long-distance corridor walk performance with mortality, cardiovascular disease, mobility limitation, and disability. JAMA. 2006;295:2018–2026. doi: 10.1001/jama.295.17.2018. [DOI] [PubMed] [Google Scholar]
- 12.Shumway-Cook A, Patla A, Stewart A, Ferrucci L, Ciol MA, Guralnik JM. Environmental components of mobility disability in community-living older persons. J Am Geriatr Soc. 2003;51:393–398. doi: 10.1046/j.1532-5415.2003.51114.x. [DOI] [PubMed] [Google Scholar]
- 13.Shumway-Cook A, Patla AE, Stewart A, Ferrucci L, Ciol MA, Guralnik JM. Environmental demands associated with community mobility in older adults with and without mobility disabilities. Phys Ther. 2002;82:670–681. [PubMed] [Google Scholar]
- 14.LIFE investigators. Pahor M, Blair SN, et al. Effects of a Physical Activity Intervention on Measures of Physical Performance: Results of the Lifestyle Interventions and Independence for Elders Pilot (LIFE-P) Study. J Gerontol A Biol Sci Med Sci. 2006;61:1157–1165. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- 15.Fielding RA, Rejeski WJ, Blair SN, et al. The Lifestyle Interventions and Independence for Elders (LIFE) Study: Design and Methods. J Gerontol A Biol Sci Med Sci. 2011;66:1226–1237. doi: 10.1093/gerona/glr123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48:314–318. [PubMed] [Google Scholar]
- 18.Marsh AP, Lovato LC, Glynn NW, et al. Lifestyle Interventions and Independence for Elders Study: Recruitment and Baseline Characteristics. J Gerontol A Biol Sci Med Sci. 2013;68:1549–1558. doi: 10.1093/gerona/glt064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borg G. Perceived exertion and pain scales. Champaign IL: Human Kinetics; 1988. [Google Scholar]
- 20.Andresen EM, Rothenberg BM, Kaplan RM. Performance of a self-administered mailed version of the Quality of Well-Being (QWB-SA) questionnaire among older adults. Med Care. 1998;36:1349–1360. doi: 10.1097/00005650-199809000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Stewart AL, Verboncoeur CJ, McLellan BY, et al. Physical activity outcomes of CHAMPS II: a physical activity promotion program for older adults. J Gerontol A Biol Sci Med Sci. 2001;56:M465–M470. doi: 10.1093/gerona/56.8.m465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rolland YM, Cesari M, Miller ME, Penninx BWJH, Atkinson H, Pahor M. Reliability of the 400-meter usual pace walk test as an assessment of mobility limitation in older adults. J Am Geriatr Soc. 2004;52:972–976. doi: 10.1111/j.1532-5415.2004.52267.x. [DOI] [PubMed] [Google Scholar]
- 23.Guralnik JM, Ferrucci L, Pieper CF, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55:M221–M231. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 24.Matthew CE. Calibration of accelerometer output for adults. Med Sci Sports Exerc. 2005;37:S512–S522. doi: 10.1249/01.mss.0000185659.11982.3d. [DOI] [PubMed] [Google Scholar]
- 25.Therneau TM, Grambsch PM. Modeling Survival Data, Extending the Cox Model. New York: Springer Science; 2000. [Google Scholar]
- 26.Van der Wal WM, Geskus RB. An R Package for Inverse Probability Weighting. J Stat Softw. 2011;43:1–23. [Google Scholar]
- 27.Messier SP, Miller GD, Morgan TP, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet and Activity Promotion Trial (ADAPT) Arthritis and Rheumatism. 2004;50:1501–1510. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 28.Ettinger WH, Burns R, Messier SP, et al. The Fitness Arthritis and Seniors Trial (FAST): a randomized trial comparing aerobic exercise and resistance exercise to a health education program on physical disability in older people with knee osteoarthritis. JAMA. 1997;277:25–31. [PubMed] [Google Scholar]
- 29.Berry MJ, Rejeski WJ, Adair NE, Ettinger WH, Zaccaro DJ, Sevick MA. A randomized, controlled trial comparing long-term and short-term exercise in patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2003;23:60–68. doi: 10.1097/00008483-200301000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Rejeski WJ, Axtell R, Fielding R, et al. Promoting physical activity for elders with compromised function: the lifestyle Interventions and Independence for elders (LIFE) study physical activity intervention. Clin Interv Aging. 2013;8:1119–1131. doi: 10.2147/CIA.S49737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Studenski S, Perera S, Patel K, et al. Gait speed and survival in older adults. JAMA. 2011;305:50–58. doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Physical Activity Guidelines Advisory Committee. Physical Activity Guidelines Advisory Committee Report, 2008. Washington, DC: U.S. Department of Health and Human Services; 2008. [Google Scholar]
- 33.Szanton SL, Allen JK, Seplaki CL, Bandeen-Roche K, Fried LP. Allostatic load and frailty in the women’s health and aging studies. Biol Res Nurs. 2009;10:248–256. doi: 10.1177/1099800408323452. [DOI] [PMC free article] [PubMed] [Google Scholar]