Abstract
We have previously prepared a prokaryotic expressed TNF-α mutant which exhibited a higher anti-tumor activity and a lower systemic toxicity compared with that of wild type TNF-α in both syngeneic murine tumor models and human tumor xenografts models. For its clinical use as an anti-tumor agent, we evaluate repeated dose toxicity, anaphylaxis, genetic toxicity, pharmacokinetic and metabolism in different animals according to the criteria for biological Investigational New Drug (IND) application. It was found to be safe at a dose of 4×106 IU/kg/day for 60 days after administration in rhesus monkeys but the TNF-α antibody level and liver toxicity needed to be monitored. No systemic anaphylaxis or genetic toxicity were found and the pharmacokinetic characteristics of the rmhTNF-α were suited for clinical use. Over 96.3% of rmhTNF-α could be reclaimed from the urine and feces in 24 hours after administration, which indicated the main excretion route. The results proved the characteristics of this rmhTNF-α satisfied clinical trial requirements. The related positive clinical trial results will be reported in future. This study of novel rmhTNF-α is of considerable importance, not only given the proven usefulness of TNF-α local application therapies under ILP (Isolated Limp Perfusion) and IHP (Isolated Hepatic Perfusion) conditions for selected indications, but also implicated for systemic application of TNF-α.
Keywords: Tumor necrosis factor-alpha, Mutation, Cancer therapy, Safety evaluation, Systemic toxicity, Pharmacokinetic
Introduction
Tumor necrosis factor alpha (TNF-α, cachectin) is a pleiotropic cytokine with a variety of biological activities, including cytotoxicity, immune cell proliferation, and mediation of inflammatory responses[1]. By means of receptor-dependent apoptosis or receptor-independent cytotoxic activity, TNF-α can inhibit a wide range of human and murine tumor cell lines and also tumor microvasculature endothelial cells in vivo and in vitro[2–6]. In 1975, Dr. Old’s research group isolated this cytokine as a serum factor, the remarkable ability of TNF-α, especially in combination with interferon, mediates hemorrhagic necrosis of certain tumors to an extent that is so far unmatched by any other cytokine[7–11]. Unfortunately, as TNF-α has also been implicated in various functions in immune and inflammatory responses, its clinical use for systemic treatment is limited by proinflammatory side effects, including fever, dose-limiting hypotension, hepatotoxicity, intravascular thrombosis, and hemorrhage. Clinical trials in cancer patients estimated that a TNF-α dose would be effective at only 5–25 times the maximum tolerated dose[12–15].
Various strategies, such as small molecule IAP (inhibitor of apoptosis) antagonists synergism, prodrugs and local application (isolated limb or hepatic perfusion), have been pursued to minimize the systemic toxicities of TNF-α, to increase TNF-α sensitivity and availability in tumor cells, and therefore to increase the therapeutic index[16–20]. A promising approach to achieve these aims is to design clinically applicable TNF-α mutants with low systemic toxicity and high efficiency and it has been of great interest[21–25]. Based on these works, we prepared a TNF-α mutant (recombinant mutated human TNF-α; rmhTNF-α) by deleting the first seven amino-acids at the N-terminal, replacing Pro 8, Ser 9 and Asp 10 with Arg 8, Lys 9 and Arg 10, and at the C terminal, Leu 157 with Phe 157. The potential clinical application is promising because of the much higher anti-tumor effects and at least 50 times higher LD50 (50% lethal dose) of this prokaryotic expressed rmhTNF-α than native TNF-α on several different syngeneic murine tumors and human tumor xenografts in nude mice[26].
For further study and potential clinical application, repeated dose toxicity, anaphylaxis, genetic toxicity, pharmacokinetic and metabolism of this mutant rmhTNF-α are investigated here in rodents and primates. The results in this paper and interrelated studies satisfied the requirements of later clinical trials. The studies of novel rmhTNF-α with low systemic toxicity and high efficiency is of considerable importance, not only given the proven usefulness of TNF-α local application therapies under ILP (Isolated Limp Perfusion) and IHP (Isolated Hepatic Perfusion) conditions for selected indications, but also implicated for systemic application of TNF-α.
Materials and Methods
Test article
The TNF-α mutant (recombinant mutated human TNF-α; rmhTNF-α) was prepared by protein engineering[26]. The rmhTNF-α was purified to 97.5% by high-performance liquid chromatography (HPLC) with a 0.1–1.2×109 IU/mg bioactivity estimated by standard procedures on the mouse fibroblast cell line L929, formulated with 3% mannitol, sterilized and lyophilized meeting regulatory requirements for pre-clinical studies.
Animals
ICR mice were 8–12 weeks old and weighed between 18–22 gm. Sprague-Dawley rats were approximately 6 weeks old and weighed about 220 gm. New Zealand white rabbits were 13–17 weeks old and weighed between 2.4 and 3.6 kg. Guinea pigs were 8–10 weeks old and with an average body weight of 260 gm. Rhesus monkeys, Macaca Speciosa Thibetanas Milne-Edwards, 3–5 years old and weighed between 4–6 kg. All of the rodents were purchased from Experimental Animal Center of Lanzhou Medical University (Lanzhou, China). The rhesus monkeys were purchased from Medical Science Experimental Animal institute of Sichuan (Chengdu, China).
The animals were maintained in an air-conditioned barrier-system animal room with an ambient temperature of 25±2°C, a relative humidity of 50±10%, and a 12 hrs on/off light cycle. All the animals were treated humanely, and the study protocols were in accordance with the Regulations of Good Laboratory Practice for nonclinical laboratory studies of drugs issued by the National Scientific and Technologic Committee of People’s Repubilc of China.
Repeat dose toxicity
10 female and 10 male rhesus monkeys were grouped to receive three doses of rmhTNF-α (2×105, 2×106 and 4×106 IU/kg/day for 60 days, i.m., which were 5, 50 and 100 times higher than the clinical dose for adults). TNF-α at 4×106 IU/kg/day and saline were used as controls. The general condition and behavior of all animals were checked daily. The following parameters were evaluated at day 7, 14, 30, and 60: general signs, food consumption, body weight, EKG, ophthalmology, hematology, hemostasis, clinical chemistry, urinalysis and anti-TNF antibody level. After 60 days, half of the experimental animals were sacrificed for necropsy. The remaining were raised for another 28 days for further observation and then underwent autopsy.
Local tolerance studies and Sensitization response
Local muscular irritation of rmhTNF-α was evaluated after a single i.m. dose in 4 New Zealand white rabbits (2 males and 2 females). The dose of 4×106 IU/kg rmhTNF-α (1 ml) was injected into the right thigh muscle of the rabbits, and saline as the negative control was injected into the corresponding muscle of the left leg. The animals were sacrificed for necropsy at 48 hrs after administration, and the muscles of injection sites were examined macroscopically and on histopathology.
Twelve guinea pigs received 2.5×105 IU/pig rmhTNF-α i.m. every two days for 3 times. As a challenge, 5×105 IU rmhTNF-α were administered intravenously 14 days later (n=6) or 21 days later (n=6). Saline and bovine serum albumin (BSA, 5 mg/pig/time, 10 mg/pig challenge) were used as blank and positive controls. Hypersensitivity responses, such as cough, dyspnea, spasm and even death, were monitored daily and recorded.
Genetic toxicity
Bacterial reverse mutation tests (Ames assay)
The mutagenic activity of rmhTNF-α was assessed in 4 Salmonella typhimurium mutant strains, TA97, TA98, TA100 and TA102. A serial dilution of rmhTNF-α were prepared and tested at five concentrations ranging from 5×105 IU, 2.5×106 IU, 5×106 IU, 2.5×107 IU, 5×107 IU, 5×108 IU for each plate. Negative (sterile water) and positive controls (2-aminofluorene, daunorubicin, MMS and 1, 8-dihydroxyanthraquinone) were run simultaneously with the test. Reverse mutation clones were counted and every sample was made in triplicate.
Bone marrow micronucleus assay
The activity of rmhTNF-α to induce bone marrow micronucleus was assessed in ICR strain mice. The first three groups (6 mice/group) received rmhTNF-α by intramuscular injection at the doses of 3.5×108, 0.7×108 to 0.14×108 IU/kg. Saline and cyclophosphamide (CPA, 30 mg/kg) were used as negative and positive controls. 48 hrs later, the animals were sacrificed and the thighbone marrow cells were analyzed following methanol fixing and Giemsa staining. The frequency of micronuclei was counted based on an examination of 1000 polychromatic erythrocytes (PCE) for every mouse.
Chromosome aberration assay
The potential of rmhTNF-α to induce chromosome aberrations was tested in cultured Chinese hamster lung (CHL) cells. rmhTNF-α ranging from 3125, 6250, 12500, 25000, 50000 IU/ml were applied to CHL cells 46 hrs. After colcemid arresting, methanol fixing and Giemsa staining, the percentage of CHL cells with chromosome aberrations was determined by examining 100 cells on every slide.
Teratogenicity test
3 doses of rmhTNF-α (2.5×108 IU/kg, 0.5×108 IU /kg and 0.1×108 IU /kg, i.m.) were injected into three cohorts of pregnant rats (20 animal/cohort) on the 6th and 15th day after of pregnancy. Normal saline and N, N′-methylenebis (1mg/kg) were used as negative and positive controls respectively. On the 19th day of pregnancy, exterior examination and skeleton examination of all fetuses were done after euthanasia.
Pharmacokinetic studies in mice with radioactive labeled rmhTNF-α
rmhTNF-α was labeled with iodine-125 and purified by S-200 exclusion chromatography (AKTA explorer; Amersham Pharmacia Biotech). [125I]-rmhTNF-α reaction product was determined to have high radiochemical purity (>95%) and specific radioactivity of 43 μCi/μg with 1×109 IU/mg bioactivity which was estimated by standard procedures on L929.
ICR mice were grouped to receive 3 doses of [125I]-rmhTNF-α (10, 20, and 40 μg/kg, i.m.). Every mouse received a single intraperitoneal dose of 0.2 ml NaI (100mg/ml) before administration. Another group received intravenous dose of [125I]-rmhTNF-α at concentrations of 10 μg/kg. Blood samples were collected from replicate animals (n=4, 2 males and 2 females) in each group at time points of 0.25, 0.5, 1, 2, 3, 4, 6, 8, 16 hrs after injection via the fossa orbitalis and the serum was divided. Each serum sample (10 μl) was applied to a SECS3000 chromatography column (PHenomenex, Torrance, CA, USA) and eluted with 0.05 M phosphate buffer, pH 6.9, at a flow rate of 1 ml/min in SP100 HPLC system (SIELC Technologies, Prospect Heights, IL, USA). The radioactivity of collected fractions within settled time was quantified in duplicate by LS 6500 liquid scintillation spectrophotometer (Beckman Instruments Inc., Fullerton, CA, USA). The [125I]-content of each sample was adjusted for sample volume and the concentration of prototype rmhTNF-α in each samples were determined according to the radioactivity-concentration standard curve.
Pharmacokinetic studies in rhesus monkeys with ELISA
4 rhesus (2 females and 2 males) monkeys received a single i.m. administration of rmhTNF-α (dissolved in PBS) at a dose of 40 μg/kg. Blood samples were collected at time points of 1, 2, 3, 4, 6, 8, 10 hrs post-dosing via the hind limb saphenous vein and the serum was divided.
Two TNF-α mouse-monoclonal antibodies against different epitopes (kindly given by the Immunology Department, the Fourth Military Medical University, Xi’an, China) were used as capture and detecting antibody. HRP (horse radish peroxidase) labeled anti-mouse IgG (Biotec, Dalian, China) and ABTS system (Sigma, USA) were used to develop the reaction with a Bio-Rad ELISA reader (450 nm). The concentrations of rmhTNF-α in serum samples were determined according to the absorbance and concerted standard curve.
Tissue distribution and metabolism studies
For tissue distribution and metabolism studies, ICR mice were housed in metabolic cage units with free access to food and water. Urine and feces were collected. Each mouse received [125I]-rmhTNF-α administration (10 μg/kg, i.m.) after a single intraperitoneal dosing of 0.2 ml NaI (100mg/ml, i.p.). At 0.5, 1, 2, 3 and 8 hrs post-dosing, animals (n = 6 per time point) were euthanized, and blood samples and selected tissues were collected. 100 mg of every selected tissues (brain, heart, kidneys, liver, spleen, lung, stomach, intestine, muscle, grease, ovaries, womb, testicles) was homogenized to yield serum (the actual weight was recorded if it did not reach 100 mg). The radioactivity of 20 μl of serum was quantified in duplicate by liquid scintillation spectrophotometer. The radioactivity of urine and feces collected over the following periods: 0–2, 2–4, 4–8, 8–12 and 12–24 hrs post-dosing (n=4, 2 males and 2 females) were quantified with same method. The corresponding quantity of prototype rmhTNF-α were decided by radioactivity and radioactivity-concentration standard curve.
Statistical analysis
The Student’s t -test was used to determine the significance between each experimental group according to DAS software developed by the Chinese Society of Pharmacology (Beijing, China). The difference was considered statistically significant when P<0.05.
Results
Repeated-dose toxicity study in monkey
As same as the former results[26], all monkeys in the TNF-α treatment group had severe edema in the perineum, abdomen and extremitas inferiors, however, only two monkeys in rmhTNF-α cohorts had minimal edema in perineum and eyelids. The edema of all animal disappeared after the 28 days of recovery. From the results of the hematological tests and clinical chemical analyses, the decline of erythrocyte, hemoglobin, leukocyte blood disk, albumin and creatinine plasma levels were found in TNF-α treatment group (P<0.05) but not in three rmhTNF-α groups. Neither rmhTNF-α at various doses nor TNF-α had negative effects on the monkeys’ blood pressure and heart rate. No abnormalities were observed in routine urinalysis of every cohort.
Either rmhTNF-α or TNF-α treatment animals showed high level of TNF-α antibody during the 15th through 30th day of administration (Fig.1). With the treatment ceasing, antibody response recovered without additional care. Inflammatory cell infiltration and mild atrophy at the center of the liver, mild colitis, enteritis, inflammation in pulmonary interstitial, mild gliocyte hyperplasia and striated muscle necrosis of injection site were found in TNF-α treatment animals’ autopsy. Only mild inflammatory cell infiltration at the center of the liver was found in rmhTNF-α cohorts.
Fig.1.
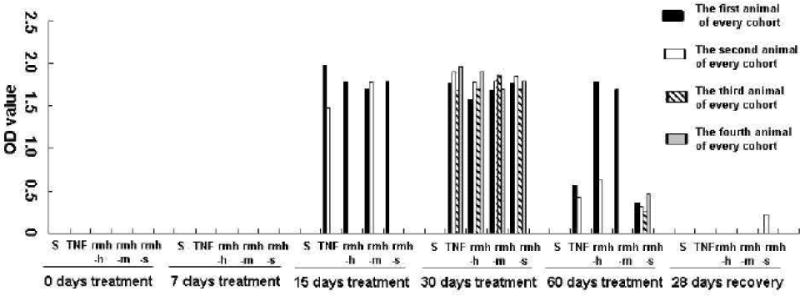
Changes of TNF-α antibody level in monkeys’ serum with rmhTNF-α or TNF-α treatments in repeated-dose toxicity study. Five cohorts of rhesus monkeys (2 females and 2 males for every cohorts) were treated with saline (S), TNF-α at 4×106 IU/kg/day (TNF), rmhTNF-α at 4×106 IU/kg/day (rmh-h), rmhTNF-α at 2×106 IU/kg/day (rmh-m) or rmhTNF-α at 2×105 IU/kg/day (rmh-s) for 60 days respectively. The serum TNF-α antibody elicited by treatments were tested using Sandwich ELISA assay before initial dose, after 7, 15, 30, 60 days treatments and 28 days recovery period. Either TNF-α or rmhTNF-α (at every dose) aroused immunoreaction after 30 days treatments. The peak levels of the rmhTNF-α and TNF-α antibody in animal serum appeared during the 15th through 30th day of administration. The antibody level decreased with the process of treatments. After 28 days recovery, only very low antibody level could be detected in one monkey in rmh-s cohort.
Local tolerance studies and Sensitization response
There were no hyperemia, hydropsia, necrosis and treatment-related clinical or histopathology findings at injection site in rabbits. There were no symptoms of allergic response in the guinea pigs receiving systemic administration of rmhTNF-α. The positive control, BSA, caused cough, spasm, tic, shock and even death in guinea pigs.
Genetic toxicity
The results of rmhTNF-α did not show any significant difference from that of saline in ames test (Table 1), bone marrow micronulecus assay (Fig. 2) or chromosome aberration assay (Fig. 3), indicating no genetic toxicity were found in vitro. In the teratogenicity tests, no fetal malformations such as umbilical hernia, tail absence, limb inversion, limb eversion which appeared in the methylenebis treated cohort were found. There were also no abnormalities seen in the skeletal development of these fetuses compared to the 39.4% malformation rate for methylenebis treatment (P<0.01).
Table 1.
Reverse mutation clones quantities of S. typhimurium strains treated with rmhTNF-α or mutagens (Ames assay) (*P<0.01).
| Reagent
|
rmhTNF-α (×106 IU/plate) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Strains | Saline | 0.5 | 2.5 | 5 | 25 | 50 | 500 | Mutagen | |
| −S9 | 130±12 | 120±3 | 110±30 | 101±17 | 107±21 | 127±28 | 129±13 | 3079(NF)* | |
| TA97 | +S9 | 149±4 | 139±9 | 138±5 | 131±13 | 130±7 | 139±9 | 157±15 | 3972(2-AF)* |
| −S9 | 32±2 | 29±4 | 33±0 | 32±2 | 30±3 | 30±2 | 32±5 | 1438(DNR)* | |
| TA98 | +S9 | 35±2 | 35±7 | 34±2 | 33±4 | 37±2 | 38±4 | 32±2 | 3062(2-AF)* |
| −S9 | 164±7 | 158±11 | 160±16 | 139±5 | 157±14 | 185±42 | 185±42 | 3357(MMS)* | |
| TA100 | +S9 | 189±6 | 170±3 | 183±5 | 167±6 | 175±17 | 177±13 | 164±3 | 2377(2-AF)* |
| −S9 | 218±24 | 258±12 | 273±5 | 280±11 | 264±11 | 266±9 | 290±0 | 3141(NF)* | |
| TA102 | +S9 | 295±11 | 291±7 | 279±2 | 283±12 | 290±8 | 281±15 | 261±0 | 1738(HDT)* |
S9: metabolic activation, a co-factor supplemented liver post-mitochondrial fraction from Aroclor 1254 induced rats. NF (2,4,7,-trinitrofluorenone) 0.2μg /plate; 2-AF(aflatoxin) 50μg /plate; DNR(daunorubicin) 10μg /plate; MMS (methyl methanesulfonate) 3μg /plate; HDT (hydroxyanthraquinone) 80μg /plate.
Fig.2.
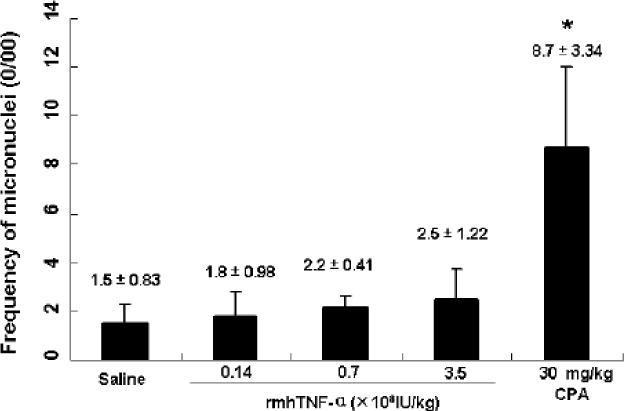
Frequency of micronuclei in mouse polychromatic erythrocytes after rmhTNF-α treatments (0/00). Activity of rmhTNF-α to induce bone marrow micronucleus was assessed in ICR mice. Three groups (6 mice/group) received 3.5×108, 0.7×108 to 0.14×108 IU/kg i.m. administrations of rmhTNF-α respectively. Saline and cyclophosphamide (CPA, 30 mg/kg) were used as negative and positive controls. 48 hrs later, the animals were sacrificed and the thighbone marrow cells were analyzed following methanol fixing and Giemsa staining. The frequency of micronuclei was counted based on an examination of 1000 polychromatic erythrocytes (PCE) for every mouse. The results did not show any significant difference between rmhTNF-α and saline cohorts (P>0.05) which indicated no micronuclei inducing toxicity for rmhTNF-α in mice.
Fig.3.
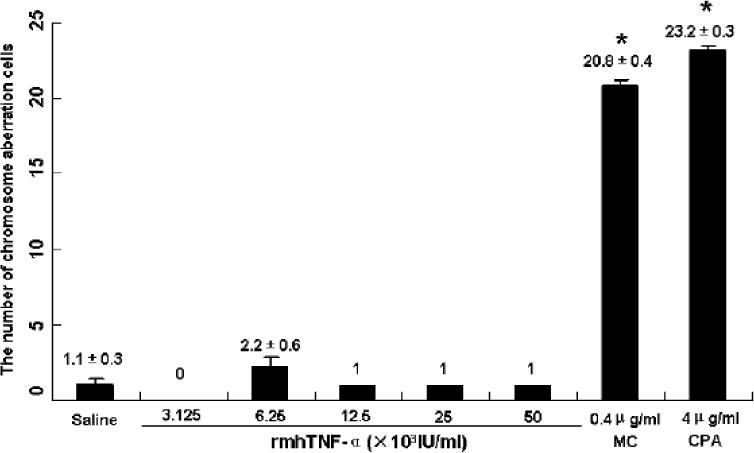
Percentage of Chinese hamster lung (CHL) cells with chromosome aberrations induced by rmhTNF-α (*P<0.01). MC: mitomycin C, CPA: Cyclophosphamide. The highest doses of rmhTNF-α, the doses of MC and CPA used in this experiment were 50% LD50 (the median lethal dose) of every reagent against CHL cells. There were no significant differences between rmhTNF-α groups and saline. MC and CPA used as positive controls had over twenty times toxicities than rmhTNF-α and saline.
RmhTNF-α Serum pharmacokinetics in mice
Concentration-versus-time datas of i.m. administrations, as derived from radioactivity measurements, were fitted to a one-compartment pharmacokinetic model. Datas of i.v. administration was fitted to a two-compartment pharmacokinetic model. According to the concentration change with time (Fig. 4), the rmhTNF-α could rapidly enter into circulation and the maximum serum concentration of rmhTNF-α could be reached in 0.5 hr. Pharmacokinetic parameter estimates, as shown in Table 2, were derived using WinNonLin 5.0.1 (Pharsight Corporation, Cary, NC). Systemic clearance (CL) and area under serum concentration-time curve (AUC) of i.m. administration show marked differences relative to i.v. Compared with intravenous administration, the absolute bioavailability of intramuscular administration of rmhTNF was 0.211.
Fig.4.
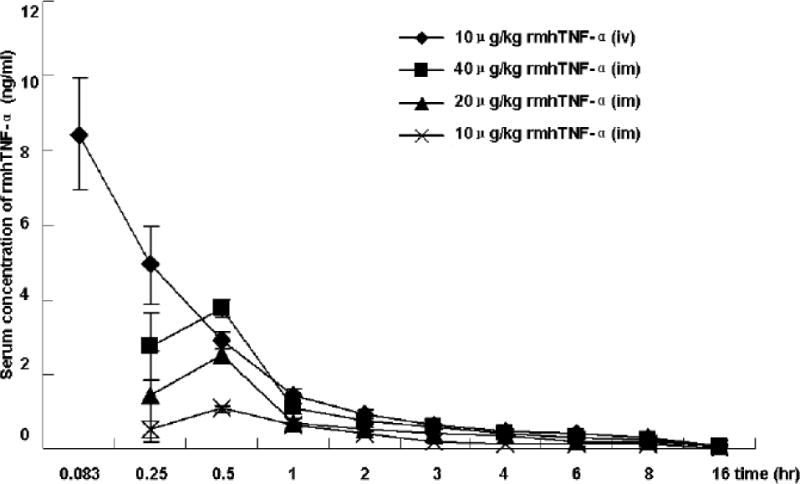
Serum concentration time-course of three intramuscular doses (10, 20, and 40 μg/kg) or one intravenous dose (10 μg/kg) of [125I]-rmhTNF-α administration in mice. Serum from replicate animals (n=4) in each group were collected at time points of 0.25, 0.5, 1, 2, 3, 4, 6, 8, 16 hrs post-dosing. The radioactivity of serum samples (after HPLC purification) were quantified in duplicate by liquid scintillation spectrophotometer. The concentration of prototype rmhTNF-α in each samples were determined according to the radioactivity-concentration standard curve. pharmacokinetic profiles characterized by rapid tissue distribution and the maximum serum concentration of rmhTNF-α could be reached in 0.5 hr in case of intramuscular doses.
Table 2.
Serum pharmacokinetic parameters of [125I]-rmhTNF-α following three intramuscular doses (10, 20, and 40 μg/kg) or one intravenous dose (10μg/kg) injection in mice.
| α | ß | t1/2α | t1/2 ß | Kα | t1/2 Kα | Tpeak | Vd | Cmax | CL | AUC | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (hr−1) | (hr−1) | (hr) | (hr) | (hr−1) | (hr) | (hr) | (L/kg) | (ng/ml) | (L.hr−1.kg−1) | (ng.hr/ml) | |
| 10μg/kg (iv) | 3.79 | 0.26 | 0.18 | 2.10 | – | – | – | 0.91 | – | 1.18 | 8.46 |
| 10μg/kg (im) | – | 0.72 | – | 0.98 | 3.31 | 0.21 | 0.59 | 7.72 | 0.84 | 5.56 | 1.79 |
| 20μg/kg (im) | – | 1.01 | – | 0.69 | 4.89 | 0.14 | 0.41 | 7.04 | 1.88 | 7.07 | 2.83 |
| 40μg/kg (im) | – | 1.16 | – | 0.60 | 6.47 | 0.11 | 0.32 | 8.73 | 3.14 | 10.14 | 3.94 |
RmhTNF-α Serum pharmacokinetics in Rhesus monkeys
The time for rmhTNF-α to reach its maximum serum concentration after administration differed greatly among monkeys. The average time was 1.7 hour. The maximum serum concentrations of the animals ranged from 575 to 1120 pg/ml which also varied greatly (Fig. 5). The elimination of rmhTNF-α in rhesus monkeys was also evaluated with the one-compartment model. The average half life in circulation was 1.9 hour (Table 3).
Fig.5.
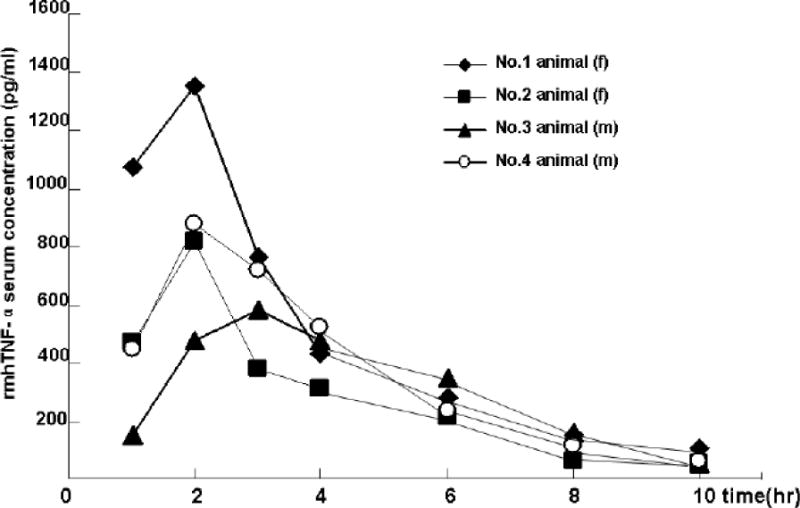
Serum concentration time-course of rmhTNF-α (40μg/kg, i.m.) in rhesus monkey. 4 rhesus (2 females and 2 males) monkeys received a single i.m. administration of rmhTNF-α (dissolved in PBS) at a dose of 40 ug/kg. Blood samples were collected at time points of 1, 2, 3, 4, 6, 8, 10 hrs post-dosing via the hind limb saphenous vein and the serum was divided. The concentrations of rmhTNF-α in serum samples were determined by ELISA. The time for rmhTNF-α to reach its maximum serum concentration after administration differed greatly among monkeys. The average time was 1.7 hrs. The maximum serum concentrations of the animals ranged from 575 to 1120 pg/ml which also varied greatly (Supplement Figure). The elimination of rmhTNF-α in rhesus monkeys was also evaluated with the one-compartment model. The average half life in circulation was 1.9 hrs.
Table 3.
Serum pharmacokinetics parameters of rmhTNF-α (40μg/kg) in rhesus monkey tested with sandwich ELISA.
| t1/2 Kα (hr) |
Tpeak (hr) |
Vd (L/kg) |
Cmax (pg/ml) |
CL (L.hr−1.kg−1) |
AUC (pg.hr/ml) |
|
|---|---|---|---|---|---|---|
| The first animal (f) | 2.35 | 0.99 | 27 | 1119 | 8 | 5082 |
| The second animal (f) |
2.02 | 1.34 | 46 | 744 | 16 | 2536 |
| The third animal (m) |
1.42 | 2.69 | 28 | 575 | 13 | 2967 |
| The fourth animal (m) |
1.90 | 1.84 | 30 | 895 | 11 | 3612 |
| X±s | 1.92±0.39 | 1.72±0.74 | 32.75±8.92 | 833±231 | 12±2.92 | 3549±1113 |
Tissue distribution and metabolism studies
According to the tissue distribution results (Fig. 6), rmhTNF-α could enter different tissues rapidly in half an hour after intramuscular administration. Except in serum, the drug concentration in the kidney was the highest compared to other tissues. The drug concentration was lowest in the brain. The stomach and intestine also showed rmhTNF-α distribution. The rmhTNF-α was mainly renally excreted and 86.8% of the prototype reagent could be reclaimed in urine 24 hrs after administration (Table 4). With 9.5% rmhTNF-α reclaimed in feces, the total amount could reach 96.3% in 24 hrs, which indicates the metabolism route of the rmhTNF-α.
Fig.6.
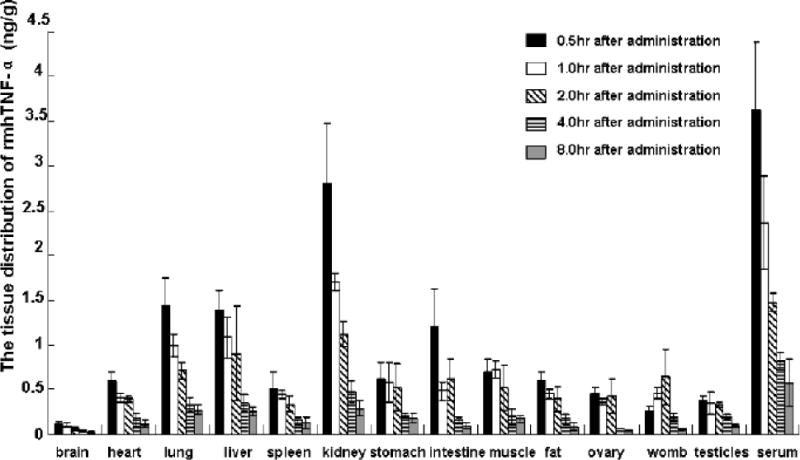
Tissue distribution profiles following one intramuscular doses of [125I]-rmhTNF-α (10μg/kg) in mice. Blood and selected tissues (100 mg for every kind)were collected at 0.5, 1, 2, 3 and 8 hrs post-dosing (n = 6 per time point). The radioactivity of 20 μl serum of every selected tissues (yielded by homogenizing) was quantified in duplicate by liquid scintillation spectrophotometer. The corresponding quantity of prototype rmhTNF-α in tissue were decided by radioactivity and radioactivity-concentration standard curve. Except in serum, the drug concentration in the kidney was the highest compared to other tissues. The drug concentration was lowest in the brain. There were also comparatively high distribution in lung, liver, and intestine.
Table 4.
Metabolism profile of one intramuscular doses of [125I]-rmhTNF-α (10μg/kg) in mice.
| Sample period (post-dose) |
Amount (ng/mouse) |
Urine Accumulated amount (ng/mouse) |
Excretory Rate (%) |
Amount (ng/mouse) |
Feces Accumulated amount (ng/mouse) |
Excretory Rate (%) |
|---|---|---|---|---|---|---|
| 0–2hr | 107.1±6.9 | 107.1±6.9 | 53.55 | 7.1±3.0 | 7.1±3.0 | 3.55 |
| 2–4hr | 35.1±2.1 | 142.2±4.5 | 71.10 | 6.9±1.4 | 14.0±2.2 | 7.00 |
| 4–8hr | 22.8±6.7 | 165.0±5.2 | 82.50 | 2.9±2.2 | 16.9±2.2 | 8.45 |
| 8–12hr | 3.2±1.2 | 168.2±4.2 | 84.10 | 0.7±0.1 | 17.6±1.7 | 8.80 |
| 12–24hr | 5.4±2.6 | 173.6±3.9 | 86.80 | 1.4±0.3 | 19.0±1.4 | 9.50 |
Discussion
Designing a clinically relevant TNF-α mutant with low systemic toxicity and high anti-tumor activity has been of intense pharmacological interest in the past two decades[20–25]. Human TNF-α, which binds to the murine TNF receptor 55 (TNF-R55, 55kDa) but not to the murine TNF receptor 75 (TNF-R75, 75kDa) exhibits retained antitumor activity and reduced systemic toxicity in mice compared to that of murine TNF-α, which binds to both murine TNF receptors[27,28]. Based on these results, many TNF-α mutants that selectively bind to TNF-R55 have been designed[22,29]. Nevertheless, TNF-R55, apart from its death domain which indicates the apoptosis, also can induce NF-kappa B activation especially in the presence of high concentrations of the TNF Receptor Associated Factor 2 (TRAF 2), as such activating the expression of pro-inflammatory genes which indicates its great side effect profile for treatment[30,31]. At the same time, NF-kappa B activation can impair the TNF-α induced apoptosis in cancer cells[32]. A TNF-α mutant that preferentially binds to TNF-R55 developed serious systemic toxicity in baboons and several observations that TNF-R75 induce the expressing cells apoptosis independent of TNF-R55 suggests that the role of TNF receptors in the TNF-α treatment is multiple and needs to be investigated further[5,29,33,34].
As the complicated functions of TNF receptors and even the receptor-independent cytotoxic activity of TNF-α by ion channels formation[35], the design of our rmhTNF-α is not based on receptor selective binding ability but on the fact that the increased basicity on the N-terminal can significantly increase the cytotoxicity of the TNF-α on tumor cells and previous studies about TNF-α mutants[25,36,37]. The N- and C-terminus were all reconstructed by protein engineering, the resulting rmhTNF-α had a clearly higher anti-tumor effect than TNF-α on mouse S180 sarcoma, H22 liver carcinoma, B16 melanoma xenografts in mice[26]. This rmhTNF-α has no preferential TNF-R55 binding selection. Between the rmhTNF-α resistant and sensitive tumor cell lines, there was no difference in TNF-R55 expression levels but a significant difference was seen in the activation of NF-kappa B[38]. We hypothesized that the mechanism of action of rmhTNF-α has no relationship with the binding ability of TNF-R55, but it may be correlated with the signaling pathway of NF-kappa B. This hypothesis is currently being studied.
For translating this rmhTNF-α into the clinic, we conducted pre-clinical safety studies in this paper. In repeated-dose toxicity study, the edema and liver toxicity induced by rmhTNF-α in monkeys were much lower than wild type TNF-α, which was consistent with former results. The lower edema induction of rmhTNF-α maybe correlated to the mutation enhancing the function of lectin-like domain of TNF-α. This could activate sodium transport in alveolar epithelial cells, peritoneal macrophages and microvascular endothelial cells, thus activated edema reabsorption in situ[39]. Although the clinical chemical analyses showed the liver toxicity only in the TNF-α treatment cohort but not in the rmhTNF-α cohorts, a little inflammatory cell infiltration was found at the center of liver after necropsy. Liver toxicity should still be noted during clinical use.
Induced antibody development is an inevitable problem in the development of biological products and there are no effective methods for control. Every dose of rmhTNF-α or TNF-α could induce high levels of TNF-α antibodies in monkey after 30 days of treatments. The development of antibodies will decrease the efficacy of the reagents and may elicit anaphylaxis in hosts. Fortunately, the antibody levels decreased with treatment. It could not be detected in some animals after 60 days of administration. These results support continued monitoring of TNF antibody levels during the treatment and cessation should be considered if the antibody levels are too high.
The pharmacokinetics of rmhTNF-α was studied in mice or monkeys. The maximum serum concentration of rmhTNF-α could be reached in 0.5 hr after intramuscular administration and the half-life was about 1 hr in mice which was not lower than i.v. administration. These two times were all prolonged to 1.7 hr and 1.9 hr in monkey, which indicated the longer functional time of rmhTNF-α in primate animals. The bio-distribution of rmhTNF-α accorded with normal biological products, and the clearance route is mainly renal. In both mice and monkey, over 95% of rmhTNF-α could be reclaimed from the urine and feces in 24 hrs after administration, which indicated the main excretion route.
After the pre-clinical study of rmhTNF-α satisfied clinical trial requirements, we conducted phase 1, phase 2 clinical tolerance and efficiency studies in five hospitals in China approved by Ministry of Health of the People’s Republic of China and State Food and Drug Administration. At the single doses ranging from 2.5×105 to 4×106 IU/m2/day, rmhTNF-α could be well tolerated with mild side effects, such as fever, fatigue, muscle soreness and anorexia. Compared with the maximum TNF-α dose (1×106 IU/m2/day) recommended by previous references, the tolerance dose of rmhTNF-α was 4 times higher than TNF-α in tumor patients. The overall response rate of rmhTNF-α and chemotherapy combination in lung cancer patients is 48.71% (CR+PR, p<0.01). We will report these results in future.
In summary, this prokaryotic expressed recombinant mutated human TNF-α (rmhTNF-α) has lower toxicity and higher efficacy in anti-tumor treatments than TNF-α. The pre-clinical animal studies promised it’s clinical trial worth and safety. In addition, TNF-α exerts anti-tumor effects by inducing apoptosis in not only tumor cells but also in endothelial cells in the microvasculature of tumors. To increase the therapeutic index of the rmhTNF-α, we coupled integrin αvß3 targeted RGD4C peptide to the N-terminal of rmhTNF-α. This RGD4C-rmhTNF-α fusion protein has tumor-specific delivery ability[40]. This paper can also give us more insight into the development of the RGD4C-rmhTNF-α or other TNF-α mutants as anti-cancer reagents.
Acknowledgments
We thank Prof. Qibing Mei (Division of Pharmacology, Fourth Military Medical University, Xi’an, China) for the instruction of pharmacokinetics studies. We are also grateful to Prof. Rei Han, Dr. Zhaodi Fu and others at Mataria Medical Institute of Chinese Academy of Medicine for animal experiments. This work is supported by Changjiang scholars and innovative Research Team (PCSIRT) programs in China.
References
- 1.Locksley RM, Killeen N, Lenardo MJ. The TNF and TNF receptor superfamilies: integrating mammalian biology. Cell. 2001;104:487–501. doi: 10.1016/s0092-8674(01)00237-9. [DOI] [PubMed] [Google Scholar]
- 2.Tartaglia LA, Ayres TM, Wong GH, Goeddel DV. A novel domain within the 55 kd TNF receptor signals cell death. Cell. 1993;74:845–53. doi: 10.1016/0092-8674(93)90464-2. [DOI] [PubMed] [Google Scholar]
- 3.Wallach D, Varfolomeev EE, Malinin NL, Goltsev YV, Kovalenko AV, Boldin MP. Tumor necrosis factor receptor and Fas signaling mechanisms. Annu Rev Immunol. 1999;17:331–67. doi: 10.1146/annurev.immunol.17.1.331. [DOI] [PubMed] [Google Scholar]
- 4.Chang MP, Wisnieski BJ. Comparison of the intoxication pathways of tumor necrosis factor and diphtheria toxin. Infect Immun. 1990;58:2644–50. doi: 10.1128/iai.58.8.2644-2650.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lucas R, Garcia I, Donati YR, Hribar M, Mandriota SJ, Giroud C, et al. Both TNF receptors are required for direct TNF-mediated cytotoxicity in microvascular endothelial cells. Eur J Immunol. 1998;28:3577–86. doi: 10.1002/(SICI)1521-4141(199811)28:11<3577::AID-IMMU3577>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 6.Slowik MR, Min W, Ardito T, Karsan A, Kashgarian M, Pober JS. Evidence that tumor necrosis factor triggers apoptosis in human endothelial cells by interleukin-1 -converting enzyme-like protease-dependent and -independent pathways. Lab Invest. 1997;77:257–67. [PubMed] [Google Scholar]
- 7.Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci USA. 1975;72:3666–70. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asher A, Mule JJ, Reichert CM, Shiloni E, Rosenberg SA. Studies on the anti-tumor efficacy of systemically administered recombinant tumor necrosis factor against several murine tumors in vivo. J Immunol. 1987;138:963–74. [PubMed] [Google Scholar]
- 9.Creasey AA, Reynolds MT, Laird W. Cures and partial regression of murine and human tumors by recombinant human tumor necrosis factor. Cancer Res. 1986;46:5687–90. [PubMed] [Google Scholar]
- 10.Sugarman BJ, Aqqarwal BB, Hass PE, Figari IS, Palladino MA, Jr, Shepard HM. Recombinant human tumor necrosis factor-alpha: effects on proliferation of normal and transformed cells in vitro. Science. 1985;230:943–45. doi: 10.1126/science.3933111. [DOI] [PubMed] [Google Scholar]
- 11.Fiers W. Tumor necrosis factor, Characterization at the molecular, cellular and in vivo level. FEBS Lett. 1991;285:199–212. doi: 10.1016/0014-5793(91)80803-b. [DOI] [PubMed] [Google Scholar]
- 12.Abbruzzese JL, Levin B, Ajani JA, Faintuch JS, Saks S, Patt YZ, et al. Phase I trial of recombinanthumang-interferon and recombinant human tumor necrosis factor in patients with advanced gastrointestinal cancer. Cancer Res. 1989;49:4057–61. [PubMed] [Google Scholar]
- 13.Blick M, Sherwin SA, Rosenblum M, Gutterman J. Phase I study of recombinant tumor necrosis factor in cancer patients. Cancer Res. 1987;47:2986–89. [PubMed] [Google Scholar]
- 14.Kilbourn RG, Gross SS, Jubran A, Adams J, Griffith OW, Levi R, et al. NG-methyl-L-arginine inhibits tumor necrosis factor-induced hypotension: implications for the involvement of nitric oxide. Proc Natl Acad Sci USA. 1990;87:3629–32. doi: 10.1073/pnas.87.9.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van der Poll T, van Deventer SJ, Hack CE, Wolbink GJ, Aarden LA, Büller HR, et al. Effects on leukocytes after injection of tumor necrosis factor into healthy humans. Blood. 1992;79:693–8. [PubMed] [Google Scholar]
- 16.Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, et al. IAP antagonists induce autoubiquitination of c-IAPs, NF-kappaB activation, and TNF-dependent apoptosis. Cell. 2007;131:669–81. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 17.Gerspach J, Németh J, Münkel S, Wajant H, Pfizenmaier K. Target-selective activation of a TNF prodrug by urokinase-type plasminogen activator (uPA) mediated proteolytic processing at the cell surface. Cancer Immunol Immunother. 2006;55:1590–600. doi: 10.1007/s00262-006-0162-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cherix S, Speiser M, Matter M, Raffoul W, Liénard D, Theumann N, et al. Isolated limb perfusion with tumor necrosis factor and melphalan for non-resectable soft tissue sarcomas: long-erm results on efficacy and limb salvage in a seleted group of patie. J Surg Oncol. 2008;98:148–55. doi: 10.1002/jso.21081. [DOI] [PubMed] [Google Scholar]
- 19.Bellavance EC, Alexander HR., Jr TNF-based isolated hepatic perfusion. Front Biosci. 2009;14:1771–84. doi: 10.2741/3339. [DOI] [PubMed] [Google Scholar]
- 20.Wang H, Chen K, Cai W, Li Z, He L, Kashefi A, et al. Integrin-targeted imaging and therapy with RGD4C-TNF fusion protein. Mol Cancer Ther. 2008;7:1044–53. doi: 10.1158/1535-7163.MCT-07-2084. [DOI] [PubMed] [Google Scholar]
- 21.Nakamura S, Kato A, Masegi T, Fukuoka M, Kitai K, Ogawa H, et al. A novel recombinant tumor necrosis factor-a mutant with increased anti-tumor activity and lower toxicity. Int J Cancer. 1991;48:744–8. doi: 10.1002/ijc.2910480519. [DOI] [PubMed] [Google Scholar]
- 22.Van Ostade X, Vandenabeele P, Everaerdt B. Human TNF mutants with selective activity on the p55 receptor. Nature. 1993;361:266–9. doi: 10.1038/361266a0. [DOI] [PubMed] [Google Scholar]
- 23.Shin NK, Lee I, Chang SG, Shin HC. A novel tumor necrosis factor-alpha mutant with significantly enhanced cytotoxicity and receptor binding affinity. Biochem Mol Biol Int. 1998;44:1075–82. doi: 10.1080/15216549800202142. [DOI] [PubMed] [Google Scholar]
- 24.Kuroda K, Miyata K, Fujita F, Koike M, Fujita M, Nomura M, et al. Human tumor necrosis factor-alpha mutant RGD-V29 (F4614) shows potent antitumor activity and reduced toxicity against human tumor xenografted nude mice. Cancer Lett. 2000;159:33–41. doi: 10.1016/s0304-3835(00)00529-2. [DOI] [PubMed] [Google Scholar]
- 25.Creasey AA, Doyle LV, Reynolds MT, Jung T, Lin LS, Vitt CR. Biological effects of recombinant human tumor necrosis factor and its novel muteins on tumor and normal cell lines. Cancer Res. 1987;47:145–9. [PubMed] [Google Scholar]
- 26.Yan Z, Zhao N, Wang Z, Li B, Bao C, Shi J, et al. A mutated human tumor necrosis factor-alpha improves the therapeutic index in vitro and in vivo. Cytotherapy. 2006;8:415–23. doi: 10.1080/14653240600845278. [DOI] [PubMed] [Google Scholar]
- 27.Tartaglia LA, Weber RF, Figari IS, Reynolds C, Palladino MA, Jr, Goeddel DV. The two different receptors for tumor necrosis factor mediate distinct cellular responses. Proc Natl Acad Sci USA. 1991;88:9292–6. doi: 10.1073/pnas.88.20.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barbara JA, Smith WB, Gamble JR, Van Ostade X, Vandenabeele P, Tavernier J, et al. Dissociation of TNF-alpha cytotoxic and proinflammatory activities by p55 receptor- and p75 receptor-selective TNF-alpha mutants. EMBO J. 1994;13:843–50. doi: 10.1002/j.1460-2075.1994.tb06327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Zee KJ, Stackpole SA, Montegut WJ, Rogy MA, Calvano SE, Hsu KC, et al. A human tumor necrosis factor (TNF) alpha mutant that binds exclusively to the p55 TNF receptor produces toxicity in the baboon. J Exp Med. 1994;179:1185–91. doi: 10.1084/jem.179.4.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, Yang Y, Ashwell JD. TNF-RII and c-IAP1 mediate ubiquitination and degradation of TRAF2. Nature. 2002;416:345–7. doi: 10.1038/416345a. [DOI] [PubMed] [Google Scholar]
- 31.Bouwmeester T, Bauch A, Ruffner H, Angrand PO, Bergamini G, Croughton K, et al. A physical and functional map of the human TNF-alpha/NF-kappa B signal transduction pathway. Nat Cell Biol. 2004;6:97–105. doi: 10.1038/ncb1086. [DOI] [PubMed] [Google Scholar]
- 32.Van Antwerp DJ, Martin SJ, Kafri T, Green DR, Verma IM. Suppression of TNF-alpha-induced apoptosis by NF-kappaB. Science. 1996;274:787–9. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 33.Heller RA, Song K, Fan N, Chang DJ. The p70 tumor necrosis factor receptor mediates cytotoxicity. Cell. 1992;70:47–56. doi: 10.1016/0092-8674(92)90532-h. [DOI] [PubMed] [Google Scholar]
- 34.Depuydt B, Van Loo G, Vandenabeele P, Declercq W. Induction of apoptosis by TNF receptor 2 in a T-cell hybridoma is FADD dependent and blocked by caspase-8 inhibitors. J Cell Sci. 2005;118:497–504. doi: 10.1242/jcs.01640. [DOI] [PubMed] [Google Scholar]
- 35.Baldwin RL, Mirzabekov T, Kagan BL, Wisnieski BJ. Conformation and ion channel activity of lymphotoxin at neutral and low pH. J Immunol. 1995;154:790–98. [PubMed] [Google Scholar]
- 36.Soma G, Kitahara N, Tsuji Y, Kato M, Oshima H, Gatanaga T, et al. Improvement of cytotoxicity of tumor necrosis factor (TNF) by increase in basicity of its N-terminal region. Biochem Biophys Res Commun. 1987;148:629–35. doi: 10.1016/0006-291x(87)90923-5. [DOI] [PubMed] [Google Scholar]
- 37.Kamijo R, Takeda K, Nagumo M, Konno K, Hasegawa A, Inaka K, et al. Induction of differentiation of human monoblastic and myeloblastic leukemia cell lines by TNF muteins. Biochem Biophys Res Commun. 1989;160:820–7. doi: 10.1016/0006-291x(89)92507-2. [DOI] [PubMed] [Google Scholar]
- 38.Meng JR, Zhao N, Yan Z, Li YS, Wang WY, Chang YM, et al. Expression of TNFRs in tumor cells and effect of novel rmhTNF on tumor cell growth. Chin J Cell Mol Immunol Chinese. 2002;18:10–3. [Google Scholar]
- 39.Elia N, Tapponnier M, Matthay MA, Hamacher J, Pache JC, Brundler MA, et al. Functional identification of the alveolar edema reabsorption activity of murine tumor necrosis factor-alpha. Am J Respir Crit Care Med. 2003;168:1043–50. doi: 10.1164/rccm.200206-618OC. [DOI] [PubMed] [Google Scholar]
- 40.Wang H, Yan Z, Shi J, Han W, Zhang Y. Expression, purification, and characterization of a neovasculature targeted rmhTNF-alpha in Escherichia coli. Protein Exp Purif. 2006;45:60–5. doi: 10.1016/j.pep.2005.05.009. [DOI] [PubMed] [Google Scholar]


