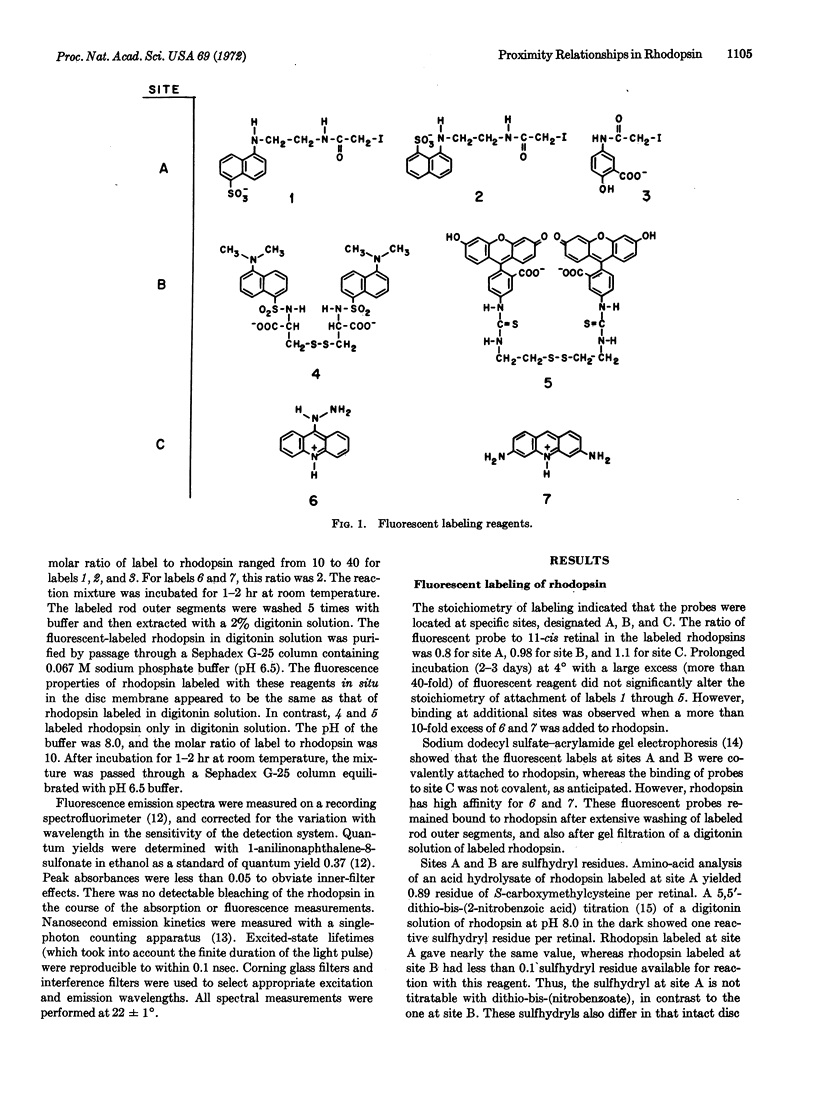
Abstract
Energy transfer was used as a spectroscopic ruler to deduce proximity relationships within bovine rhodopsin in digitonin solution. Rhodopsin was specifically labeled with fluorescent chromophores at three sites. Site A was alkylated by fluorescent derivatives of iodoacetamide. Site B was labeled by fluorescent disulfides, by a disulfide-sulfhydryl interchange reaction. Sites A and B are sulfhydryl residues. Acridine derivatives were tightly bound to site C by noncovalent interactions. The labeled rhodopsins retained their 500-nm absorption band and were regenerable after bleaching, suggesting that the fluorescent probes did not grossly perturb the conformation of the protein. A fluorescent chromophore at one of these sites served as the energy donor, while 11-cis retinal was the energy acceptor. The efficiency of singlet-singlet energy transfer was determined from the quantum yield and excited-state lifetime of the donor in the presence and absence of the acceptor. By Förster's theory, the apparent distances between 11-cis retinal and sites A, B, and C were calculated to be 75,55, and 48 Å, respectively. Energy transfer measurements on rhodopsin labeled at two of these sites gave these apparent distances: 35 Å for A to B, 32 Å for A to C, and 30 Å for B to C.
These energy transfer studies suggest that the rhodopsin molecule has a length of at least 75 Å. Thus, the rhodopsin molecule appears to be sufficiently long to traverse the disc membrane. Rhodopsin might act as a light-controlled gate.
Keywords: visual receptor, energy transfer, fluorescence spectroscopy, excited state, Förster theory
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beardsley K., Cantor C. R. Studies of transfer RNA tertiary structure by singlet-singlet energy transfer. Proc Natl Acad Sci U S A. 1970 Jan;65(1):39–46. doi: 10.1073/pnas.65.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Haugland R. P., Yguerabide J., Stryer L. Dependence of the kinetics of singlet-singlet energy transfer on spectral overlap. Proc Natl Acad Sci U S A. 1969 May;63(1):23–30. doi: 10.1073/pnas.63.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller J. Structure of visual pigments. I. Purification, molecular weight, and composition of bovine visual pigment500. Biochemistry. 1968 Aug;7(8):2906–2913. doi: 10.1021/bi00848a030. [DOI] [PubMed] [Google Scholar]
- Ka Luk C. Study of the nature of the metal-binding sites and estimate of the distance between the metal-binding sites in transferrin using trivalent lanthanide ions as fluorescent probes. Biochemistry. 1971 Jul 20;10(15):2838–2843. doi: 10.1021/bi00791a006. [DOI] [PubMed] [Google Scholar]
- LATT S. A., CHEUNG H. T., BLOUT E. R. ENERGY TRANSFER. A SYSTEM WITH RELATIVELY FIXED DONOR-ACCEPTOR SEPARATION. J Am Chem Soc. 1965 Mar 5;87:995–1003. doi: 10.1021/ja01083a011. [DOI] [PubMed] [Google Scholar]
- Latt S. A., Auld D. S., Valee B. L. Surveyor substrates: energy-transfer gauges of active center topography during catalysis. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1383–1389. doi: 10.1073/pnas.67.3.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MATTHEWS R. G., HUBBARD R., BROWN P. K., WALD G. TAUTOMERIC FORMS OF METARHODOPSIN. J Gen Physiol. 1963 Nov;47:215–240. doi: 10.1085/jgp.47.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NILSSON S. E. THE ULTRASTRUCTURE OF THE RECEPTOR OUTER SEGMENTS IN THE RETINA OF THE LEOPARD FROG (RANA PIPIENS). J Ultrastruct Res. 1965 Feb;12:207–231. doi: 10.1016/s0022-5320(65)80016-8. [DOI] [PubMed] [Google Scholar]
- Stryer L. Fluorescence spectroscopy of proteins. Science. 1968 Nov 1;162(3853):526–533. doi: 10.1126/science.162.3853.526. [DOI] [PubMed] [Google Scholar]
- Stryer L., Haugland R. P. Energy transfer: a spectroscopic ruler. Proc Natl Acad Sci U S A. 1967 Aug;58(2):719–726. doi: 10.1073/pnas.58.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stryer L. The interaction of a naphthalene dye with apomyoglobin and apohemoglobin. A fluorescent probe of non-polar binding sites. J Mol Biol. 1965 Sep;13(2):482–495. doi: 10.1016/s0022-2836(65)80111-5. [DOI] [PubMed] [Google Scholar]
- WALD G., BROWN P. K., GIBBONS I. R. The problem of visual excitation. J Opt Soc Am. 1963 Jan;53:20–35. doi: 10.1364/josa.53.000020. [DOI] [PubMed] [Google Scholar]
- Waggoner A. S., Stryer L. Induced optical activity of the metarhodopsins. Biochemistry. 1971 Aug 17;10(17):3250–3254. doi: 10.1021/bi00793a014. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yguerabide J., Epstein H. F., Stryer L. Segmental flexibility in an antibody molecule. J Mol Biol. 1970 Aug;51(3):573–590. doi: 10.1016/0022-2836(70)90009-4. [DOI] [PubMed] [Google Scholar]


