Abstract
Introduction
Weight loss has been associated with higher physical activity (PA) levels and frequent dietary self-monitoring. Less is known about how PA self-monitoring affects adherence to PA goals, PA levels and weight change.
Methods
The SMART Trial is a clinical weight loss trial in which 210 overweight adults were randomized equally to one of three arms: 1) paper record (PR); 2) personal digital assistant with self-monitoring software (PDA); and 3) PDA with daily tailored feedback message (PDA+FB). PA self-monitoring and adherence to PA goals were based on entries in weekly submitted diaries. PA levels were measured via self-report by the past 6 month Modifiable Activity Questionnaire at baseline and 6 months.
Results
Data are presented on 189 participants with complete 6-month PA data [84% female, 77% White, mean age: 47.3 ± 8.8 years, mean BMI: 34.1 ± 4.5 kg/m2]. Median PA level was 7.96 MET-hr-wk−1 at baseline and 13.4 MET-hr-wk−1 at 6 months, with significant PA increases in all three arms. PDA+FB arm had a higher mean number of weekly self-monitoring entries than the PR arm (3.4 vs. 2.4; p=0.003) and were more likely to maintain high (i.e., 100%) adherence to PA goals over time than the PDA (p=0.02) or PR arms (p=0.0003). Both PA self-monitoring and adherence to PA goals were related to higher PA levels at 6 months. A higher mean rate of PA self-monitoring was associated with a greater percentage of weight decrease (rho=−0.49; p<0.0001) at 6 months.
Conclusions
PA self-monitoring and adherence to PA goals were more likely in participants in the PDA+FB arm and in turn predicted higher PA levels and weight loss.
Keywords: adherence, feedback, lifestyle change, technology
INTRODUCTION
Standard behavioral weight loss programs typically involve goals for reduced energy intake and increased energy expenditure (17). To facilitate individuals achieving these goals, behavioral strategies such as daily self-monitoring of dietary intake and physical activity (PA) are often used (31). Self-monitoring permits individuals to be aware of their current behaviors and to track their performance in comparison to study-specific goals (32) or current national guidelines for diet and PA (3).
The practice of self-monitoring healthy lifestyle behaviors has been found be to consistently associated with more weight loss (4, 7, 11, 12, 15). Although many intervention studies indicate that both participation in and self-monitoring of PA are part of the intervention, most studies examined only dietary self-monitoring in relation to meeting weight loss goals (4, 8, 16, 18). To the best of our knowledge, only two published studies specifically examined the effect of PA self-monitoring on weight loss (12, 15, 29). Carels and colleagues examined the role of weekly PA self-monitoring on PA levels and weight loss in 40 obese, sedentary men and women (15). They found that more frequent and consistent PA self-monitoring, measured by the number of weeks that paper PA diaries were completed, was associated with fewer difficulties related to exercise, greater weekly PA levels, and greater weight loss (15). However, Shay and colleagues (29) found that the total number of days adherent to PA self-monitoring was not associated with weight loss in a 12-week randomized clinical trial of 39 overweight or obese men and women that tested three self-monitoring methods: paper diary, web-based diary, and personal digital assistant (PDA)-based diary (29). Due to the contradictory findings and limited number of studies exploring this relationship, it is clear that more research in this area is needed.
Given that weight loss is associated with higher levels of PA and with more frequent dietary self-monitoring (1, 4, 32), it would seem intuitive that this monitoring PA would lead to more weight reduction. However, very little is known about whether self-monitoring PA is associated with weight change or how different self-monitoring methods may influence frequency of PA self-monitoring over time, adherence to PA goals or weight outcomes. Therefore, the purposes of the current study were to: 1) examine the frequency of PA self-monitoring behavior over 6 months with three self-monitoring methods - paper record (PR), PDA, or PDA with daily tailored feedback message (PDA+FB); and 2) determine the associations of PA self-monitoring with adherence to PA goals, PA levels and weight loss at 6 months in a behavioral weight loss trial.
METHODS
Study design and participants
The current study is a secondary data analysis of the SMART Trial: a single-center, randomized clinical trial of behavioral treatment for weight loss. The methods of the SMART Trial have been detailed elsewhere (13,14). Briefly, all participants received a 24-month standard behavioral weight loss treatment and were randomly assigned to use 1 of 3 self-monitoring tools: (1) PR, (2) PDA or (3) PDA+FB. Outcome data were collected at semi-annual assessments. This report focuses on the results from the baseline and 6-month assessments.
Participants were recruited from the community in three cohorts from 2006 to 2008. Eligible individuals were between 18 and 59 years of age and had a body mass index (BMI) between 27 and 43 kg/m2. We excluded individuals with conditions that required medical supervision of diet or PA and those who participated in a weight-loss program in the 6 months prior to recruitment or planned an extended vacation or relocation during the 24-month study period (13). The study protocol was approved by the University of Pittsburgh Institutional Review Board. All participants gave written informed consent after the study details were reviewed.
Intervention
All 3 treatment arms received different self-monitoring tools but the same standard behavioral intervention, which has been successfully used in multiple studies (9, 30, 31). The intervention included: daily self-monitoring of eating and PA behaviors, group sessions, daily dietary goals, and weekly PA goals.
Group Sessions
There were 16 weekly and 4 bi-weekly group sessions during the first 6 months. Sessions focused on nutritional and behavioral counseling and practical hands-on experiences to develop skills to implement a healthy lifestyle. Intervention details have been reported elsewhere (13).
Dietary and PA goals
Participants received a daily energy and fat gram goal based on their gender and baseline weight consistent with standard behavioral weight loss treatment (31). They were to gradually reach a weekly goal of 150 minutes of moderate intensity PA, which is concordant with national guidelines (3), by the sixth week.
Self-monitoring
Participants in the PR arm were given standard paper diaries and instructed to record all foods eaten, the calories and fat grams, as well as minutes of PA. Participants in the PDA and PDA+FB arms were provided with Palm Tungsten E2™ PDAs that was installed with self-monitoring software that tracked energy and fat consumption, displayed current intake related to daily goals, and provided easily accessed nutrition information (Dietmate Pro©) (5, 6) and CalculFit©, PICS, Reston, VA).
CalcuFit has five screens for data entry: cardiorespiratory, muscle strengthening, flexibility, pedometer steps, and water intake. On the cardiorespiratory screen, participants selected an aerobic activity from a drop-down menu, then entered the duration (in minutes) and perceived level of intensity. On the muscle strengthening screen, participants selected the muscle-strengthening exercise (e.g. lower body-calf rise) and then entered the amount of resistance and the number of repetitions. On the pedometer screen, participants were simply asked to enter the total number of steps taken during the day. Participants were not encouraged to enter data on the flexibility and water screens, but they could do so for their own information. Primary emphasis was placed on the cardiorespiratory data (i.e., minutes of physical activity) and these data were used for generating feedback. Participants were not required to enter strengthening or pedometer data. The CalcuFit program also allows individuals to graph their progress in any of the five areas.
Feedback
At each session, PR participants submitted their diaries to the interventionist and received new ones to use until the next session. Between sessions, the interventionist reviewed participants’ diaries and provided written feedback; the diaries with comments were returned to participants at the next session. The PDA and PDA+FB participants turned in their PDAs at the beginning of the session; the self-monitoring data were uploaded directly into the study database. As part of the upload process, the interventionists received printed reports that appeared similar to the standard paper records used by the PR arm, which were returned to the participants with written feedback at the next group session.
Participants in the PDA+FB arm had a custom software program installed on their PDAs with a feedback algorithm (Table 1) that provided messages tailored to their entries and provided positive reinforcement and guidance for goal attainment. The PA feedback messages were scheduled to be delivered every other day since participants were not expected to perform PA every day but were encouraged to be physically active on most days of the week (3). In order to generate the feedback message, the participants’ PA minutes for the current and previous days were compared to 42.8 minutes, or 2 times the average expected daily PA (i.e., the weekly goal of 150 minutes per week/7 days * 2). Participants received an automated message between 3 and 5 PM or 7 and 8 PM; the timing of the message was randomly selected per participant. Each message reflected one of three possible conditions: 1) no recorded PA; 2) the participant met or was likely to meet the goal (i.e., accumulated minutes were ≥ 34.25 minutes or 80% of the goal), 3) the participant did not meet or was not likely to meet the goal (i.e., accumulated minutes were ≤ 21.4 minutes or 50% of the goal). Participants were not able to respond directly to the feedback messages and research staff was not able to verify whether messages were read.
Table 1.
Feedback algorithm for PA goals with sample messages
| 3:00 PM–5:00 PM | 7:00 PM–8:00 PM | |
|---|---|---|
| No recording | No PA recorded for 2 consecutive days | |
| Don’t get disheartened, you still have time to meet your physical activity goals, Hint: Take a walk, it will pay off! | Must have been a very busy two days. Try to squeeze in at least a 10-minute walk tomorrow. | |
| Met PA goal | Average at least 80% of goal over 2 days | |
| So far so good! You are right on track with physical activity. | Super job on the physical activity. Try to repeat this tomorrow. | |
| Did not meet PA goal | Average less than 50% of goal over 2 days | |
| You are very close to meeting your physical goals. We are sure you can still do it today. | May be you can be more active for longer periods on your less hectic days. Try a few 10-minute bouts of exercise. | |
Measures
PA self-monitoring
Adherence to self monitoring PA was determined by the number of entries reporting any PA. Starting week 3, the number of days (0–7) on which the participant recorded any minutes of PA was summed across each week in the study. The average number of weekly entries over 18 sessions was also calculated. Week 3 was selected since participants PDA and PDA+FB arms did not learn how to use the Calcufit software until week 2 of the intervention.
Adherence to PA goals
PA adherence was calculated as accumulated minutes of PA (recorded by participant in PR or PDA) per week divided by PA goals at both baseline and 6 months (50 and 150 minutes, respectively, with instructions to increase PA gradually). Participants were then classified into one of four groups based on adherence level: high (100%), moderate (50–99%), low (<50%), and no self-monitoring data. For example, at six months a participant who recorded 160 minutes of weekly activity would have high (100%) adherence, whereas one with 60 minutes would have low (<50%) adherence.
Outcome measures
Our primary outcomes were change in body weight and PA levels from baseline to 6 months. We measured weight on a digital scale with the participant in light clothing and not wearing shoes. Reported PA was measured using a past 6 month version of the Modifiable Activity Questionnaire (MAQ), a reliable (24, 27) and valid (24, 27, 28) self-report questionnaire. This questionnaire assesses leisure and occupational activities; we analyzed the leisure activity estimates as the PA goal of the study was to increase leisure PA to meet recommendations (3). Study participants were asked if they participated in 39 common leisure physical activities, such as walking for exercise, at least 10 times over the previous 6 months. For each activity identified, participants were asked to identify which months over the previous 6 months they had participated in that specific activity and then report the average number of times (i.e., frequency) that activity was done during each of the previous 6 months. Finally, participants were asked to report the average number of minutes spent doing the specific activity each time (i.e., duration). Leisure PA levels were calculated as the product of the duration and frequency of each activity in hours per week (hr-wk−1), weighted by an estimate of the metabolic equivalent (MET) of that activity and summed for all activities performed (2). Leisure PA data were expressed as MET-hr-wk1.
Statistical analysis
Descriptive baseline characteristics of sample were presented as means ± SD or as percentages. The median (interquartile range) was presented for PA data due to non-normality and outliers. Wilcoxon rank-sum or two-sample t-tests were used to compare differences in baseline measurements of PA, weight, and BMI between the participants included in the current analysis and those excluded. The F-test from one-way analysis of variance (ANOVA) or the Kruskal-Wallis tests for continuous variables and chi-square test of independence for categorical variables were used to compare the baseline characteristics and the average number of entries over 6 months among the intervention arms.
PA self-monitoring was examined as a count variable (number of days =0–7). Mixed-effects Poisson regression modeling was conducted to assess the effect of intervention arms and time on adherence to PA self-monitoring. Adherence to PA goals was evaluated as a binary variable based on whether participants achieved the goal at each session (adherent: ≥100% of weekly goal, nonadherent: <100% of weekly goal). Mixed-effects logistic regression modeling was applied to examine the effect of intervention arms and time on adherence to PA goals. Both linear and nonlinear functions of time (e.g., square root and squared function of time) and the interaction between intervention arms and time were considered. We used the likelihood ratio test to compare nested models. If functions of time were not significant, they were eliminated to achieve more parsimonious models. Sensitivity analyses were conducted for outliers identified through graphical methods.
Spearman correlations were used to describe bivariate relationships among PA self-monitoring, PA levels at 6 months, change in PA levels from baseline to 6 months, and percent change in both body weight from baseline to 6 months for the entire sample and stratified by intervention arm. ANOVA was used to compare weight and PA levels among PA adherence groups (i.e., high (100%), moderate (50–99%), low (<50%), and no self-monitoring data) followed by Tukey post hoc analyses. PA data from the MAQ were logarithmically transformed for normality assumption. Statistical analyses were conducted using SAS version 9.1.3 (SAS Institute, Inc., Cary, NC). The significance level was set as P ≤ 0.05.
RESULTS
Participant characteristics
We included 189 participants for whom we had complete PA (i.e., MAQ) and weight data at 6 months in the current analysis. The 189 included participants are representative of the SMART population; those excluded from this analysis (n=21) do not differ from those included by any baseline characteristic, including weight, BMI or PA level. The proportion of excluded participants did not significantly differ among the three treatment groups (p=0.19). The majority were married, employed white females with a mean of educational attainment of 15.7 years (Table 2). The average BMI was in the mildly obese/class I obesity range and the participants were fairly sedentary with a median PA level of 7.96 MET-hour-wk−1. There were no differences in any of these baseline characteristics among the three treatment arms.
Table 2.
Baseline Sample Description (N=189)
| Characteristic | % (n) |
|---|---|
| Gender (Female) | 83.60 (158) |
| Race (White) | 77.25 (146) |
| Marital Status | |
| Currently married | 68.78 (130) |
| Never married | 14.29 (27) |
| Formerly married (divorced/ separated) | 16.93 (32) |
| Employment Status | |
| Employed full time | 82.54 (156) |
| Gross household income | |
| >$50,000 | 57.67 (109) |
| $30,000–$50,000 | 23.81 (45) |
| $10,000–$30,000 | 15.87 (30) |
| M ± SD | |
|---|---|
| Age (years) | 47.33±8.81 |
| Education (years) | 15.74±3.02 |
| BMI (kg/m2) | 34.06±4.54 |
| Weight (kg) | 93.84±15.43 |
| Physical Activity (MET-hr/week)* | 7.96 (2.63, 16.54) |
Physical activity data are presented as median (Q1, Q3).
PA self-monitoring and PA goals
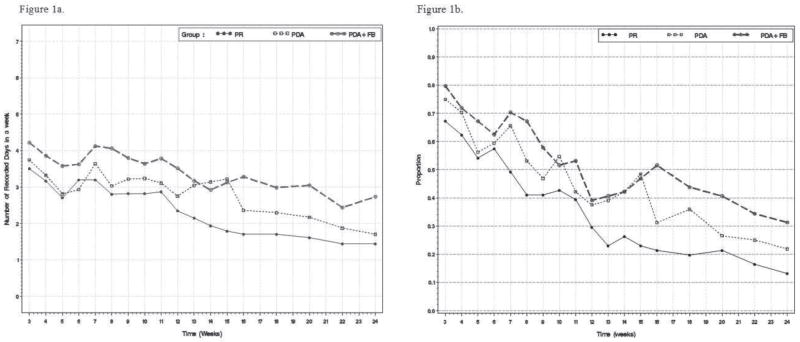
Figure 1a shows PA self-monitoring over time by intervention arm. PA self-monitoring was performed more frequently in the early weeks of the study, with self-monitoring declining significantly over time in all three arms. For example, in the total population only 41 (22%) turned in a diary with no PA self-monitoring at 6 weeks, whereas 100 (53%) turned in a diary with no PA self-monitoring at 24 weeks. However, the decline in PA self-monitoring over time was significantly lower in the PDA and PDA + FB arms when compared to the PR arm (both p <0.0001). Participants in the PDA+FB arm had a significantly greater average number of total weekly PA self-monitoring entries over the 18 sessions when compared to those in the PR arm (3.4 vs. 2.4, respectively; p=0.003). There were no significant differences in the mean number of weekly entries over the 18 sessions between the PDA+FB arm vs. PDA arm (3.4 vs. 2.9; p=0.09) or between the PDA arm vs. PR arm (2.9 vs. 2.4; p=0.12).
Figure 1.
Figure 1a. Mean days per week with physical activity self-monitoring, by treatment arm
Figure 1b. Proportion of participants meeting weekly physical activity goals, by treatment arm
Figure 1b shows the adherence to study PA goals over time by intervention arm; these findings mirror the PA self-monitoring data presented in Figure 1a. Participants in the PDA and PDA+FB arms were more likely to demonstrate high (i.e., 100%) adherence to weekly PA goals, although the adherence in all three arms declined over time. (Note that the weekly PA goal increased from 50 minutes to 150 minutes at 6 weeks.) The participants in the PDA + FB arm demonstrated the lowest decline in PA adherence over time, and this difference was significantly lower than the declines in both the PR (p=0.0003) and PDA (p=0.02) arms. The participants in the PDA arm did not differ in adherence to PA goals over time when compared to the PR arm (p=0.14). When outliers were omitted via sensitivity analysis, the results did not change, supporting the robustness of our findings.
Relationship of PA self-monitoring adherence and PA goal achievement to 6-month PA levels and weight change
Table 3 shows the associations among PA self-monitoring, PA levels, and weight loss at 6 months. A higher mean rate of PA self-monitoring over 18 sessions was associated with both higher PA levels at 6 months (rho=0.29; p<0.0001) and greater increases in PA levels over the first 6 months of the study (rho=0.20; p=0.0057). Similar trends were observed across all three intervention arms. Median 6-month PA (MET-hour-wk−1) was 22.4 (12.2, 37.5) for those with 100% PA adherence, compared with 11.7 (4.4, 16.4) for those with < 50% PA adherence and 9.1 (3.7, 24.5) for those who did not self-monitor PA (p <0.05).
Table 3.
Associations among PA Self-Monitoring, Physical Activity Levels and Change, and Weight Change at 6 Months
| MET-hours PA at 6 months | Change in MET-hours PA from baseline to 6 months | % Weight Change (6 months-baseline) | ||
|---|---|---|---|---|
| Mean Number of Weekly PA Self-Monitoring Episodes | All (N=189) | 0.29*** | 0.20** | −0.49*** |
| PR (n=61) | 0.27* | 0.16 | −0.53*** | |
| PDA (n=64) | 0.28* | 0.22 | −0.37** | |
| PDA+FB (n=64) | 0.29* | 0.15 | −0.56*** | |
|
| ||||
| MET-hours PA at 6 months | All (N=189) | 0.65*** | −0.27*** | |
| PR (n=61) | -- | 0.65*** | −0.10 | |
| PDA (n=64) | 0.75*** | −0.42*** | ||
| PDA+FB (n=64) | 0.55*** | −0.26* | ||
|
| ||||
| Change in MET-hours PA from baseline to 6 months | All (N=189) | −0.27*** | ||
| PR (n=61) | -- | -- | 0.13 | |
| PDA (n=64) | −0.41*** | |||
| PDA+FB (n=64) | −0.26* | |||
Statistics are Spearman correlation coefficients
p<0.05;
p<0.01;
p<0.001
Participants with greater PA self-monitoring also experienced a greater decrease in weight (rho=−0.47; p<0.0001) and a greater percentage of weight decrease (rho=−0.49; p<0.0001) at 6 months (Table 3). Higher PA levels and greater increases in PA levels were also associated with greater percentage of weight loss at six months (both rho=−0.27; both p=0.0002). Similar trends were seen across all three intervention arms, with the exception of the PR arm in which there did not seem to be a relationship between increase in PA levels and % weight change. Adherence to PA goals was also associated with weight loss. The group with high (i.e., 100%) adherence to PA goals experienced more weight loss at 6 months than those with < 50% PA adherence or no self-monitoring (p < 0.001).
DISCUSSION
We found that more frequent PA self-monitoring behavior and greater adherence to PA goals were associated with higher levels of PA and greater weight loss in a cohort of overweight adults in a clinical weight loss trial. Participants who were randomized to use a PDA with automated feedback messages for self-monitoring were more likely to perform PA self-monitoring than participants randomized to use a PR, with intermediate results in participants randomized to use a PDA without automated feedback messages. PA self-monitoring behavior declined over the course of the intervention in all three arms.
Very few studies have reported detailed information about PA self-monitoring behavior, and to our knowledge no other study has tested a self-monitoring method that gave participants feedback every other day on their progress towards a weekly PA goal (i.e., PDA+FB arm). Carels and colleagues (15) reported that consistent PA self-monitoring was associated with more minutes of exercise recorded in diaries and weight loss, but found no association with self-reported leisure PA. In this study, PA was estimated using the Paffenbarger Physical Activity Questionnaire (PPAQ). It is important to note that the PPAQ uses a past-year recall time frame (26), which does not match the total duration of the study (i.e., 6 months), as was done in the SMART trial. Asking participants to recall six months that were not part of the intervention period and averaging across 12 months could potentially have contributed to the non-significant findings. Further, the study by Carels et al. (15) included a relatively small number of participants and only examined more traditional paper diaries that were collected by research staff every 4–5 weeks with no reported feedback built into the protocol. In contrast, we gave all participants delayed written feedback and participants in the PDA+FB arm received feedback on PA behavior in “real time.”
In contrast, Shay and colleagues (29) randomly assigned participants to paper, PDA, and Web-based diaries and noted whether participants had been assigned to a “preferred” method of self-monitoring. Participants were given weekly feedback on diaries during the eight weekly intervention sessions. Although participants using their “preferred” method were more likely to perform PA self-monitoring, this behavior was not in turn related to increased weight loss. No information was reported about relationship of PA self-monitoring to PA levels. As was shown in the current study, participants in all three arms were less adherent to PA self-monitoring over time, with 29% adherence at weeks 7–12 compared to 60% adherence at weeks 1–6.
The current study adds to the small body of literature on PA self-monitoring by comparing three methods of PA self-monitoring and studying how adding feedback to a convenient PDA-based self-monitoring device may have impacted both self-monitoring behavior and PA and weight outcomes. Our results suggest that providing participants with incremental, “real time” feedback on progress toward a weekly PA goal may be more helpful in achieving that goal when compared to a more traditional form of feedback in which an interventionist comments on PA behavior two weeks after actual participation. Providing feedback in more immediate ways may help a participant adjust his or her PA behavior in time to make better progress toward a weekly goal. This is consistent with the theory of self-regulation, which is the theoretical underpinning of the self-monitoring and feedback strategy employed in the SMART trial intervention (21–23). The feedback message for PA served as a prompt to the participant that ‘someone’ was paying attention to what they were doing, which along with the message could have provided powerful reinforcement for performing PA and goal attainment. It is also possible that the feedback message itself served as a reminder to self-monitor PA. Unfortunately, we do not have data available about type of message received or the timing of PA self-monitoring in relation to message receipt to help us discern to what degree feedback message content influenced physical activity behavior.
The behavioral mechanism of PA self-monitoring could be similar to one hypothesized for dietary self-monitoring, in which a participant can gain self-awareness when goals are not being met and adjust subsequent caloric intake appropriately (12, 30). The goal of self-monitoring is that the person will learn to use the recorded information and compare performance to behavior goals and be able to self-reinforce for progress made, or to self-correct behaviors if that is indicated. However, not all individuals are able to make the connection between the recorded behavior and what is needed to achieve the intended goals (10). In this study, the provision of a feedback message may have facilitated individuals making that connection (25). There is likely also a strong association between the acts of dietary and PA self-monitoring. The 6-month results from the SMART trial show that adherence to dietary self-monitoring followed a pattern similar to adherence to PA self-monitoring (14).
While it is encouraging to see the positive associations between PA self-monitoring and PA and weight outcomes and the higher rates of self-monitoring in the PDA+FB arm, it is striking and concerning to see the decline in both PA self-monitoring and adherence to PA goals over the course of the study. Similar declines over time were observed in the Shay et al. study (29), as well as in our earlier studies (1). Similar findings of decreases in PA levels over time have been reported by other investigators (19, 20). The fact that declines were seen in all three arms of our study emphasize that neither technology (in the form of a PDA) nor feedback can entirely prevent some degree of non-adherence with self-monitoring and goal achievement. That being said, technology has advanced since the design of the SMART study and use of newer methods such as smart phones with wireless Internet connectivity could provide the opportunity for recording information and receiving feedback in “real time.” The feedback provided in the PDA+FB arm was also somewhat basic in that it provided automated messages that did not change as a participant advanced in the week or program. Over time, participants in the PDA+FB arm may have become desensitized to these messages and thus paid less attention to them. Clearly, further investigation is needed in exploring both the best technology to support PA self-monitoring as well as the underlying mechanisms that connect self-monitoring to sustained behavioral change.
The current study is unique in its evaluation of PA self-monitoring with the inclusion of a condition that provided frequent feedback messages in “real time”. Other strengths are a larger sample size than previous studies (15, 29) and a high retention rate, minimizing the potential bias of only gathering data only from those with high adherence to self-monitoring. Our study was limited by including fewer males and minorities, although the overrepresentation of women is fairly common in weight loss studies and the proportion of minorities in our study was about twice of that in the area in which participants were recruited. Further, PA was ascertained using a self-report measure. Thus, derived estimates may be affected by factors such as recall and social desirability bias. Even with our sophisticated self-monitoring methods, we were unable to determine whether days without any PA minutes recorded occurred because PA was not performed or not recorded. This supports additional work exploring the study objectives using direct measures of movement or a direct measure combined with self-report to maintain contextual information.
The current study demonstrates the potential importance of PA self-monitoring for lifestyle change and weight loss efforts. Based on our results, we believe that behavioral interventions should emphasize PA self-monitoring as a possible way to increase likelihood of success. Attention should be paid to providing persons with feedback on their performance, and further research is needed in mechanisms that can provide feedback in “real time” and compare immediate to delayed feedback. New technology may be able to play an important supporting role in PA self-monitoring, but regardless of the self-monitoring method consideration should be given to how to reengage persons in their attention to self-monitoring over time. The findings from the current study support follow-up studies that utilize an extended evaluation period in order to evaluate the impact of long-term PA self-monitoring on PA, weight loss, and weight maintenance.
Acknowledgments
Funding Disclosure: Dr. Conroy is supported by a career development award from NIH (K23 HL085405). The SMART Trial is supported by NIH Grant R01 DK071817 (PI: Burke).
Dr. Conroy is supported by a career development award from NIH (K23 HL085405). The SMART Trial is supported by NIH Grant R01 DK071817 (PI: Burke). The results of the current study do not constitute endorsement by ACSM.
References
- 1.Acharya SD, Elci OU, Sereika SM, et al. Adherence to a behavioral weight loss treatment program enhances weight loss and improvements in biomarkers. Journal of Patient Preference and Adherence. 2009;3:151–160. doi: 10.2147/ppa.s5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ainsworth B, Haskell WL, White MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32(suppl):S498–S504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 3.American College of Sports Medicine. Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30:975–991. doi: 10.1097/00005768-199806000-00032. [DOI] [PubMed] [Google Scholar]
- 4.Baker RC, Kirschenbaum DS. Self-monitoring may be necessary for successful weight control. Behavior Therapy. 1993;24:377–394. [Google Scholar]
- 5.Beasley J, Riley WT, Jean-Mary J. Accuracy of a PDA-based dietary assessment program. Nutrition Jun. 2005;21(6):672–677. doi: 10.1016/j.nut.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 6.Beasley JM, Riley WT, Davis A, Singh J. Evaluation of a PDA-based dietary assessment and intervention program: a randomized controlled trial. J Am Coll Nutr Apr. 2008;27(2):280–286. doi: 10.1080/07315724.2008.10719701. [DOI] [PubMed] [Google Scholar]
- 7.Boutelle KN, Kirschenbaum DS. Further support for consistent self-monitoring as a vital component of successful weight control. Obesity Research May. 1998;6(3):219–224. doi: 10.1002/j.1550-8528.1998.tb00340.x. [DOI] [PubMed] [Google Scholar]
- 8.Burke LE, Styn MA, Steenkiste AR, Music E, Warziski M, Choo J. A randomized clinical trial testing treatment preference and two dietary options in behavioral weight management: preliminary results of the impact of diet at 6 months--PREFER study. Obesity. 2006;14(11):2007–2017. doi: 10.1038/oby.2006.235. [DOI] [PubMed] [Google Scholar]
- 9.Burke LE, Warziski M, Styn MA, Music E, Hudson AG, Sereika SM. A randomized clinical trial of a standard versus vegetarian diet for weight loss: The impact of treatment preference. International Journal of Obesity. 2008;32:166–176. doi: 10.1038/sj.ijo.0803706. [DOI] [PubMed] [Google Scholar]
- 10.Burke LE, Swigart V, Warziski Turk M, Derro N, Ewing LJ. Experiences of self-monitoring: successes and struggles during treatment for weight loss. Qual Health Res. 2009 Jun;19(6):815–28. doi: 10.1177/1049732309335395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burke LE, Elci O, Wan J, et al. Self-Monitoring in Behavioral Weight Loss Treatment: SMART Trial Short-term Results. Obesity. 2009;17(Supplement 2):S273. [Google Scholar]
- 12.Burke LE, Wang J, Sevick MA. Self-monitoring in weight loss: a systematic review of the literature. Journal of the American Dietetic Association. 2010 doi: 10.1016/j.jada.2010.10.008. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burke LE, Styn MA, Glanz K, et al. SMART trial: A randomized clinical trial of self-monitoring in behavioral weight management - design and baseline findings. Contemporary Clinical Trials. 2009;30:540–551. doi: 10.1016/j.cct.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burke LE, Conroy MB, Sereika SM, et al. The Effect of Electronic Self-Monitoring on Weight Loss and Dietary Intake: A Randomized Behavioral Weight Loss Trial. Obesity (Silver Spring) 2010 Sep 16; doi: 10.1038/oby.2010.208. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carels RA, Darby LA, Rydin S, Douglass OM, Cacciapaglia HM, O’Brien WH. The relationship between self-monitoring, outcome expectancies, difficulties with eating and exercise, and physical activity and weight loss treatment outcomes. Ann Behav Med. 2005;30(3):182–190. doi: 10.1207/s15324796abm3003_2. [DOI] [PubMed] [Google Scholar]
- 16.Carels RA, Young KM, Coit C, Clayton AM, Spencer A, Hobbs M. Can following the caloric restriction recommendations from the Dietary Guidelines for Americans help individuals lose weight? Eating Behaviors Aug. 2008;9(3):328–335. doi: 10.1016/j.eatbeh.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Franz MJ, VanWormer JJ, Crain AL, et al. Weight-loss outcomes: a systematic review and meta-analysis of weight-loss clinical trials with a minimum 1-year follow-up. J Am Diet Assoc. 2007 Oct;107(10):1755–67. doi: 10.1016/j.jada.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 18.Hollis JF, Gullion CM, Stevens VJ, et al. Weight loss during the intensive intervention phase of the weight-loss maintenance trial. Am J Prev Med. 2008 Jul 8;35(2):118–126. doi: 10.1016/j.amepre.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jakicic JM, Winters C, Lang W, et al. Effects of intermittent exercise and use of home exercise equipment on adherence, weight loss, and fitness in overweight women: a randomized trial. JAMA. 1999;282(16):1554–1560. doi: 10.1001/jama.282.16.1554. [DOI] [PubMed] [Google Scholar]
- 20.Jakicic JM, Marcus BH, Gallagher KI, et al. Effect of exercise duration and intensity on weight loss in overweight, sedentary women: a randomized trial. JAMA. 2003;290(10):1323–1330. doi: 10.1001/jama.290.10.1323. [DOI] [PubMed] [Google Scholar]
- 21.Kanfer FH. The maintenance of behavior by self-generated stimuli and reinforcement. In: Jacobs A, Sachs LB, editors. The psychology of private events. New York: Academic Press; 1971. pp. 39–59. [Google Scholar]
- 22.Kanfer FH, Duerfeldt PH. Motivational Properties of Self-Reinforcement. Perceptual & Motor Skills. 1990;25(1):237–247. doi: 10.2466/pms.1967.25.1.237. [DOI] [PubMed] [Google Scholar]
- 23.Kanfer FH, Goldstein AP. Helping people change: A textbook of methods. 4. Elmsford: Pergamon Press, Inc; 1991. pp. 309–355. [Google Scholar]
- 24.Kriska AM, Knowler WC, LaPorte RE, et al. Development of questionnaire to examine relationship of physical activity and diabetes in Pima Indians. Diabetes Care Apr. 1990;13(4):401–411. doi: 10.2337/diacare.13.4.401. [DOI] [PubMed] [Google Scholar]
- 25.Miller WR, Rollnick S. Motivational interviewing: Preparing people for change. New York: Guilford Press; 2002. pp. 111–125. [Google Scholar]
- 26.Paffenbarger R, Wing RR, Hyde R. Physical activity as an index of heart attack risk in college alumni. AJE. 1978;108:161–175. doi: 10.1093/oxfordjournals.aje.a112608. [DOI] [PubMed] [Google Scholar]
- 27.Pereira MA, FitzerGerald SJ, Gregg EW, et al. A collection of Physical Activity Questionnaires for health-related research. Med Sci Sports Exerc Jun. 1997;29(6 Suppl):S1–205. [PubMed] [Google Scholar]
- 28.Schulz LOHI, Smith CJ, Kriska AM, Ravussin E. Energy intake and physical activity in Pima Indians: comparison with energy expenditure measured by doubly-labeled water. Obes Res. 1994;2:541–548. doi: 10.1002/j.1550-8528.1994.tb00103.x. [DOI] [PubMed] [Google Scholar]
- 29.Shay LE, Seibert D, Watts D, Sbrocco T, Pagliara C. Adherence and weight loss outcomes associated with food-exercise diary preference in a military weight management program. Eating Behaviors Dec. 2009;10(4):220–227. doi: 10.1016/j.eatbeh.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wadden TA, Crerand CE, Brock J. Behavioral treatment of obesity. Psychiatric Clinics of North America. 2005;28(1):151–170. doi: 10.1016/j.psc.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Wing RR. Behavioral approaches to the treatment of obesity. In: Bray GA, Bourchard C, James WPT, editors. Handbook of obesity: Clinical applications. 2. New York: Marcel Dekker; 2004. pp. 147–167. [Google Scholar]
- 32.Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutrition Jul. 2005;82(1 Suppl):222S–225S. doi: 10.1093/ajcn/82.1.222S. [DOI] [PubMed] [Google Scholar]