Abstract
We examined the relationship of apathy with neurocognitive performance, age, disease markers, and functional disability in 61-HIV-infected individuals. Apathy was assessed with the Apathy Evaluation Scale and was significantly associated with highest HIV plasma level, functional disability, and neurocognitive performance. individuals with higher apathy levels demonstrated a stronger association between age and processing speed performance. Our findings suggest that apathy is related to poor neuropsychological functioning, HIV plasma levels, and increased functional disability in individuals with HIV Additionally, to our knowledge, this is the first study to demonstrate an interactive effect of age and apathy on neuropsychological performance in HIV.
Keywords: HIV, Aging, Apathy, Cognitive performance, Functional disability
Apathy, typically defined as lack of motivation not attributable to diminished level of consciousness, cognitive impairment, or emotional distress (Marin, 1991), is a known neuropsychiatric sequela of central nervous system (CNS) disease. Apathy has been observed in a variety of psychiatric and neurologic disorders, including schizophrenia, stroke, Parkinson's disease, Huntington's disease, and various dementias (Caeiro, Ferro, & Figueira, 2012; Kuzis et al., 1999; Marin, 1991; Marin, Firinciogullari, & Biedrzycki, 1994; Ott, Noto, & Fogel, 1996; Starkstein & Brockman, 2011; Starkstein et al., 1992). It is especially prominent in disorders where frontal lobes and subcortical structures are affected, and it is hypothesized to be due to damage to these brain regions (Cummings, 1993; Marin, 1991; Marin, Firinciogullari, & Biedrzycki, 1993). Marin and his colleagues, who frrst proposed apathy as a distinct syndrome in 1991 and have been prime contributors to apathy research, define apathy as a cluster of symptoms reflecting lack of motivation in cognitive, emotional, and motoric domains that is not due to another cause, such as mood disturbance, intellectual capacity, or attention (Marin, 1990, 1991; Marin et al., 1993).
Although apathy and depression may appear superficially similar, the definition of apathy as a construct assumes that it can occur independently. While depression is conceptualized as a disturbance of mood, apathy is defined by reduction in spontaneous initiation of cognitive, emotional, and motoric activities, with no requirement that it be accompanied by dysphoric emotion or a loss of interest. Moreover, there is no requirement that the reduction in initiation of activity be accompanied by change in appetite, change in sleep, a subjective feeling of fatigue, feelings of worthlessness, or suicidal thoughts or impulses. In order to discriminate between these two similar constructs, Marin et al. (1994) studied patients with stroke, Alzheimer's disease, and depression and found that the relationship between depression and apathy varied among diagnostic groups (Marin et al. , 1994). Additionally, Starkstein et al. (1992) found that Parkinson's patients with higher ratings of apathy evidenced different neurocognitive deficits than patients with high depression scores, and that apathy, unlike depression, was associated with advanced age, disease severity, and overaJJ cognitive impairment.
Given HIV's known viral damage to frontal-subcortical brain regions, it is not surprising that apathy is a common neuropsychiatric symptom among HIV-positive individuals (Castellon, Hinkin, Wood, & Yarema, 1998; Paul, Flanigan, et al., 2005). In a small cohort of HIV-infected patients, Tate found that 26% of patients reported clinically significant apathy (Tate et al., 2003). Previous studies have demonstrated significant correlations between severity of cognitive impairment and increased apathy ratings, suggesting the possibility that both may result from viral damage to shared subcortical systems (Castellon, Hinkin, & Myers, 2000; Castellon et al. , 1998; Cole et al., 2007; Paul, Brickman, et al.., 2005; Paul, Flanigan, et al., 2005). While this hypothesis is supported by both neuropsychological and neuroimaging research, not all studies have reported significant relationships between cognitive impairment and apathy in individuals with HIV (Rabkin et al., 2000).
Castellon et al. (1998) reported increased apathy to be strongly correlated with poor performance on a measure of working memory in a small cohort of HIV-positive patients with varying levels of disease severity. Castellon et al. (2000) also reported strong associations between ratings of apathy and performance on tasks of divided attention and response inhibition. More recently, Paul, Flanigan, et al. (2005) found that apathy was significantly related to per1ormance on measures of learning efficiency and cognitive flexibility, providing further evidence that both apathy and neuropsychological impairment may evolve from viral damage to frontostriatal systems (Castellon et al. , 2000; Castellon et al. , 1998). However, Rabkin et al. (2000) did not find an association between ratings of apathy and neurocognitive performance in a cohort of HIV-positive men and women. One possible explanation for their lack of findings is that apathy is only associated with certain cognitive domains–namely, working memory (Alexander, Crutcher, & DeLong, 1990; Cummings, 1993), which is one of several cognitive functions not assessed in the study. This highlights the need for further investigation of the association of apathy with a broad range of cognitive domains, including: working memory, processing speed, divided attention, response inhibition, learning efficiency, and cognitive flexibility.
In addition, very little is known about the relationship between apathy and aging in individuals with HIV. Research in both healthy and diseased populations has shown that rates and severity of apathy increase with age (Brodaty, Altendorf, Withall, & Sachdev, 2010; Brodaty et al., 2005; Starkstein & Brockman, 2011; Starkstein, Jorge, Mizrahi, & Robinson, 2006). For example, in individuals with Alzheimer's disease, Parkinson's disease, and stroke, apathy is associated with advanced age (Brodaty et al. , 2005; Jonsson et al., 2010; Starkstein, Bolduc, Preziosi, & Robinson, 1989; Starkstein, Jorge, & Mizrahi, 2006; Starkstein et al., 1992; Zahodne et al., 2011). However, to date, no studies have looked at the relationship between apathy and age in the HIV-positive population. This is of particular interest as individuals with HIV survive into older age ranges. At the present time, adults over 50 account for more than 24% of the people Jiving with HIV in the US, and this number is expected to increase to 50% by 2015 (Centers for Disease Control and Prevention, CDC, 2007).
There is also little known about the relationship between apathy and HIV disease severity, and findings linking apathy with disease markers have been equivocal (Castellon et al., 2000; Castellon et al., 1998; Paul, Flanigan, et al., 2005; Rabkin et al., 2000). Individuals' current CD4 (cluster of differentiation 4) cell count has consistently been unrelated to apathy and neurocognitive performance (Castellon et al., 2000; Castellon et al., 1998; Paul, Flanigan, et al., 2005; Rabkin et al., 2000). However, Castellon et al. (1998) reported apathy to be related to diagnosis of acquired immunodeficiency syndrome (AIDS), and Paul, Flanigan, et al. (2005) found apathy to be related to disease duration. On the other hand, Rabkin et al. (2000) did not find associations between apathy and any HIV disease markers. CD4 cell count and HIV viral plasma load fluctuate acutely, and, therefore, current levels may not be optimal markers of disease-related CNS dysfunction. ln contrast, CD4 nadir (i.e., the lowest recorded CD4 count during the course of the illness) and highest recorded HIV RNA plasma level may be more useful indices of disease impact. However, these disease markers have yet to be studied in relation to apathy.
In addition to its role as a potential marker of HJV-related CNS dysfunction, behavioral apathy has been associated with occupational and social dysfunction, as well as poor treatment compliance and general health (Mayo, Fellows, Scott, Cameron, & Wood-Dauphinee, 2009; Velligan, Ritch, Sui, DiCocco, & Huntzinger, 2002). For example, among patients with stroke, Alzheimer's disease, Parkinson's disease, Huntington's disease, and schizophrenia, apathy is correlated with impairment in activities of daily living (ADL), diminished quality of life, increased burden to care-givers, decreased general health, and poor treatment compliance (Chase, 2011). Among individuals with HJV, one study (Kamat et at., 2012) found higher levels of self-reported apathy to be associated with greater impairment in functioning, and two studies have reported a relationship between apathy and poor medication adherence (Barclay et al., 2007; Rabkin et al., 2000). While this preliminary evidence suggests that apathy is related to functional deficits in HIV-infected individuals, Tate et al. (2003) found no association between apathy and quality of life in HIV-positive individuals; thus further research is necessary.
Although research has provided preliminary evidence that apathy is associated with neuro-cognitive performance in HIV-positive individuals (Castellon et al., 2000; Castellon et al., 1998; Paul, Flanigan,et al., 2005), much remains unknown about this relationship with respect to age and disease characteristics. In the current study, apathy's relationship with neurocognitive performance, age, HIV disease characteristics, and functional disability was examined in a cohort of HIV-infected patients across age groups, using a comprehensive neuropsychological battery. Consistent with previous findings, it was hypothesized that apathy would be associated with neurocognitive deficits and functional disability. Additionally, the authors' wished to explore the interaction of apathy and the aging process on neuropsychological functioning in HIV-positive individuals. It was predicted that apathy would moderate the effect of age on neurocoginitive performance, such that the relationship between age and neurocognitive performance would be stronger in individuals with higher levels of apathy.
METHOD
Participants
This study examined data from 116 HIV-positive individuals recruited from the Center for Positive Living/I.D. Clinic at the Montefiore AIDS Center, Bronx, NY, between November 2011 and April 2012. Participants were recruited to provide representation in 10-year age bins from age 20 years to 70 years and above. Eligibility requirements included age 18 and older and diagnosis of HIV Diagnosis was confirmed by review of medical records, HJV-related blood investigations, and medication records, by two clinical research assistants who were blinded to performance on the study predictor and outcome measures. Patients with a Mini Mental State Examination (MMSE) score below 25, which is the recommended cutoff for normal cognition (Crum, Anthony, Bassett, & Folstein, 1993), were excluded from participation. Patients were also excluded if they possessed factors likely to impact cognitive performance, such as history of bead injury, neurologic disorder, developmental disability, or self-reported current use of cocaine, heroin, andjor other intravenous drugs. Patients were not excluded if they reported a history of substance abuse or if they reported clinically elevated depression. Approximately 23.3% (n = 27) of participants reported a history of intravenous drug use, and l5SYo (n = 18) bad a diagnosis of comer-bid hepatitis C virus (HCV) according to medical records. The mean MMSE score was 27.6 (1 .5).
Procedure
Each participant provided written, informed consent following study protocols approved by the local institutional review board. Eligible participants completed demographic questionnaires and self-report measures of functional disability, apathy, and mood. A trained research assistant administered a battery of neuropsychological tests, described below. All measures were administered, scored, and double-scored according to standardized procedures. The entire protocol lasted approximately two hours, and participants were given breaks to minimize possible effects of fatigue.
Neuropsychiatric measures
Apathy
Apathy was measured with Marin's Apathy Evaluation Scale- Self (AES) (Marin, 1991). The psychometric properties of this scale have been well established with internal consistency reliability ranging from .86 to .94 and test- retest reliability ranging from .76 to .94 (Marin, Biedrqcki, & Firinciogullari, 1991; Marin et al., 1993, 1994). The AES is a brief self-report measure of apathy consisting of 18 items related to motivation, self-injtiation, and drive over the past four weeks. It has been shown to be psychometrically robust for assessing apathy in healthy individuals as well as medical patients (Clarke et al., 2011) and has been used previously with an HIV-positive population (Castellon et al., 2000; Paul, Flanigan, et al., 2005; Rabkin et al., 2000). Participants responded to their degree of agreement with each item using a 4-point Likert scale, where J = not at all and 4 = extremely. Scores range from J 8 to 72, with higher scores reJlecting greater apathy. Mean AES score in a normative healthy sample is 24.4 (4.5), with a suggested cutoff criterion of 34 (Kant, Duffy, & Pivovarnik, I 998). The dependent measure in the current study was total score.
Mood
Mood was assessed with the 21-item Beck Depression lnventory-II (BDI-II), a self-report rating scale that queries presence and prominence of cognitive, affective, and somatic symptoms of depression over the past two weeks (Beck, 1987). Scores on each item range from zero (symptom absent) to three (presence of symptom is pronounced), yielding a possible total score of zero to 63. BDI-II cognitive-affective and somatjc sub-scores were calculated according to the manual (Beck, 1987) because positively endorsed somatic items may be related to physical illness in medically ill populations. This method is commonly used by other HIV investigators (Castellon et al., 2006; Castellon et al., 1998; Law et al., 1995; Law et al., 1994).
Neurocognitive measures
The neuropsychological measures used in this study included the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS; Randolph, Tierney, Mohr, & Chase, 1998); the Trail Making Test, Parts A and B (Halstead, 1947); the Stroop Color Word Interference Test (Golden, 1978; Stroop, 1935); the Controlled Oral Word Association Test (COWAT), phonemic (“F,” “A,” “S”) and semantic (animals) Huency (Benton & Hamsher 1989; Spreen & Strauss, 1998); the 15-item short version of the revised Boston Naming Test (BNT; Goodglass & Kaplan, 2000; Kaplan, Goodglass, & Weintraub, 1983); the Digit Symbol Coding subtest of the Wechsler Adult Intelligence Scale-Third Edition (WAJS-III; Wechsler, 1997); the Purdue Pegboard test (Tiffin & Asher, 1948); and grip strength using a band dynamometer (Halstead, 1947; Reitan, 1955). The RBANS was used as a brief general neuropsychological screening battery. The other aforementioned tests were selected to represent measures of attention, information processing speed, language functioning, working memory, executive functioning, and the motor speed domains. Many of these tests have been used by previous researchers as part of standard HIV cognitive assessment batteries (Paul, Flanigan, et al., 2005; Rabkin et al., 2000; Valcour, Paul, Neuhaus, & Shikuma, 2011). Raw scores on all of the neuropsychological tests except for the RBANS were used as continuous measures. For the RBANS, scaled total and index scores were used.
Functional disability measures
Late-life function and disability instrument
The Late-Life Function and Disability Instrument (LL-FDI) is a measure of instrumental activity of daily living, personal role, and social role functioning (Haley et al., 2002). The LLFDI assesses both functioning and disability. The functioning component is a 32-item measure of difficulty in performing physical functional tasks (Haley et al., 2002). It contains three subscales: 7 items for upper extremity (arms and hands), 14 items for basic lower extremity (standing, stooping, and walking), and 11 items for advanced lower extremity (physical activity and end urance). Participants choose degree of difficulty with regard to performing each task on a 5-point Likert scale, where 5 represents no difficulty, and 1 indicates inability to perform the task. Total score, which was computed by summing the three subscales, served as the dependent measure.
The disability component of the LL-FDI is a 16-item measure of difficulty in executing activities requisite to social roles at home and in the community. This scale measures the frequency with which individuals perform these activities (Jette et al., 2002). Questions are phrased: “To what extent do you feel limited in ... ?” and “How often do you .. . ?” Participants responded on a 5-point Likert scale. Total score, which was computed by summing the items, served as the dependent variable. Higher scores indicated less limitation and more frequent engagement in the activities.
HIV-related medical measures
Two independent researchers trained and certified in Lhe hospital's electronic records system extracted and confirmed the following measures from participants' medical records: date of HIV diagnosis, presence of AIDS diabrnosis, current CD4 cell count, CD4 nadir, current HIV RNA plasma level, and highest recorded JIV RNA plasma leveL Disease duration, measured as months since HIV diagnosis, was also calculated for each participant.
Statistical analyses
The Statistical Package for the Social Sciences (SPSS), Version 17.0 (2009) was used for all data analyses. HIV RNA plasma levels were expressed in log10 units because of the great range, from less than 400 copies (“undetectable”) to several million. Pearson correlations were used to examine the association between apathy and the 1ollowing variables: demographic characteristics, performance on individual neuropsychological tests, disease markers, and functional disability measures. Because of the multiple measures and comparisons entailed, we used p < .01 to denote statistical significance. Relationships found significant at the bivariate level were further examined using multiple regression analysis.
To assess apathy's association with neuro-cognitive functioning, multiple regression analysis was conducted using neurocognitive measures as the dependent variables and AES score as the independent variable. Age, gender, years of education, ethnicity, history of intravenous drug use, and comorbid hepatitis C infection (HCV) were entered as covariates to control for their potential confounding effects on neurocognitive performance. Age was excluded as a covariate from the regressions containing RBANS measures as dependent variables. BDI-II cognitive- affective subscore was also entered to control for the overlap between apathy and depression. Hierarchical regression analysis was conducted using a two-way product term between apathy and age to examine the interactive effect of age and apathy on neurocognitive performance. Simple slopes tests (Aiken & West, 1991) were used to interpret the significant interactions.
To assess the association between apathy and HIV disease markers, simultaneous linear regression analysis was conducted using the disease markers as dependent variables and AES score as the independent variable. Age was entered as a covariate to control for its potential confounding effect on disease status, and BDI-II cognitive-affective sub-score was entered to control for the overlap between apathy and depression. Finally, simultaneous linear regression analysis was conducted using the functional disability measures as dependent variables and AES score as tbe independent variable. Age, disease duration, nadir CD4 count, and highest HIV RNA plasma level were entered as covariates because of their potential confounding effects on functional disability. BDI-II cognitive-affective subscorc was also entered to control for the overlap between apathy and depression.
RESULTS
Demographic and medical characteristics for the study participants are presented in Table 1. Participants' ages ranged from 22 to 79 years, and 63.8% (n = 74) of participants were 50 years or older. Of the 116 participants, 34.5% (n = 40) were 60 years or older, 17.2% (n = 20) were 65 years or older, and 7.8% (n = 9) were 70 years or older. When the sample was split according to age (younger = less than 50 years; older = 50 years and older), the two groups did not sigoiJicantly differ on years of education (t = −0.672, p = .502), gender (t = 0.657, p = .512), or apathy (t = 1.51 3, p = .132). The younger and older participants also did not significantly differ on any of the disease markers (nadir CD4: t = −0.330, p = .742; highest HIV RNA plasma: t = −0.058, p = .954, disease duration: t = −1.132. p = .259). Interestingly, the two groups did display significantly different levels of depression, such that older participants scored higher on the BDI- II total score (t = 3.228, p = .001) and cognitive- afrective subscore (t = 4.037, p < .001). Participants' education level ranged from 5 to 19 years, and 54% (n = 63) attained at least a high-school diploma. Females made up 66.4% of the sample. Approximately 51.7% (n = 60) of participants had a diagnosis of AIDS at the time of assessment, and 90.5% were on antiretroviral therapy. Mean disease duration, measured as years since initial diagnosis, was 12.2 (1.3) years. Approximately 72.4% (n = 84) of participants had undetectable viral loads indicating successful viral suppression. Mean neuropsychological test scores for the current sample are presented in Table 2.
TABLE 1.
Participants’ demographic and medical characteristics
| Total sample (n = 116) |
||
|---|---|---|
| Characteristic | Mean | SD |
| Age (years) | 51.9 | 14.48 |
| Education (years) | 11.7 | 2.36 |
| Gender (% females) | 66.4 | |
| Race (%) | ||
| Black | 55.2 | |
| White | 32.8 | |
| Other | 12.1 | |
| Ethnicity (%) | ||
| Hispanic/Latino | 40.5 | |
| Not Hispanic/Latino | 59.5 | |
| Time since HIV diagnosis (years) | 12.23 | 1.31 |
| CD4 cell count, most recent | 558.47 | 409.25 |
| CD4 cell count, nadir | 246.58 | 228.23 |
| Log10 HIV RNA plasma level, most recent | 1.18 | 1.72 |
| Log10 HIV RNA plasma Level, highest | 4.1 | 1.68 |
| AIDS diagnosis (%) | 51.7 | |
| Antiretroviral therapy (%) | 90.5 | |
TABLE 2.
Neuropsychological test score means, standard deviations, and ranges
| Total sample (n = 116) |
||
|---|---|---|
| Measure | Mean (SD) | Range |
| RBANS | ||
| Immediate Memory Index | 69.96 (14.55) | 44–106 |
| Visuospatial/Constructional Index | 73.94 (15.00) | 50–126 |
| Language Index | 86.29 (12.37) | 47–108 |
| Attention Index | 81.18 (16.55) | 43–118 |
| Delayed Memory Index | 72.10 (18.43) | 40–117 |
| Total Scale | 70.20 (12.41) | 40–99 |
| Trails A | 51.44 (25.01) | 16–170 |
| Trails B | 153.77 (67.58) | 33–240 |
| Phonemic Fluency (FAS) | 30.61 (10.64) | 5–58 |
| Semantic Fluency (Animals) | 14.07 (4.56) | 2–28 |
| Boston Naming Test | 10.77 (2.61) | 3–15 |
| Coding | 43.35 (17.34) | 8–87 |
| Stroop | ||
| Word | 79.06 (14.60) | 35–100 |
| Color | 54.56 (13.70) | 15–86 |
| Color-Word | 25.52 (10.54) | 6–57 |
| Purdue | ||
| Dominant | 11.40 (2.50) | 5–17 |
| Nondominant | 10.83 (2.55) | 4–16.5 |
| Both | 8.38 (2.21) | 2.5–13.5 |
| Grip Strength | 57.50 (21.10) | 13–117 |
Notes. RBANS = Repeatable Battery for the Assessment of Neuropsychological Status.
The mean apathy score in the current sample was 30.1 (7.76), and 31% (n = 36) of participants reported clinically significant apathy according to the recommended cutoff (Kant et al., 1998). The mean total BOT- H score in the current sample, 14.6 (10.6), indicated mild levels of depression for the group, overall. The mean cognitive-affective and somatic subscales were 7.9 (7.4) and 6.8 (4.4), respectively. Correlations between apathy, depression measures, and demographic characteristics are presented in Table 3. Apathy score significantly correlated with total BDI-II score as well as both cognitive- affective and somatic subscales. Participants with lower levels of education displayed significantly higher levels of apathy. Unlike depression, apathy was not correlated with age or gender in the current sample.
TABLE 3.
Correlations between apathy, depression, and demographic characteristics
| Variable | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| 1. Apathy Evaluation Scale | ||||||
| 2. BDI–II Total | .64** | |||||
| 3. BDI–II Cog–Aff | .57** | .95** | ||||
| 4. BDI–II Somatic | .61** | .83** | .64** | |||
| 5. Age | –.07 | –.16** | –.21** | –.02 | ||
| 6. Gender | .08 | .14** | .09 | .19** | –.06 | |
| 7. Years of education | –.23** | –.10 | –.13* | .00 | –.07 | .00 |
Notes. BDI–II = Beck Depression Inventory–II; Cog–Aff = Cognitive–Affective. Gender: male = 0, female = 1.
p = .01 to p < .05 (two-tailed)
p < .01 (two-tailed).
Apathy and neurocognitive performance
Correlations between apathy, depression measures, and neurocognitive performance are presented in Table 4. Apathy score significantly correlated with raw scores on the following neurocognitive measures: RBANS Total Scale and all index scores, Trails A, Phonemic Fluency, Coding, and Stroop Word reading time. Higher levels of apathy were associated with poorer performance. BDI-II scores were significantly correlated with performance on the RBANS Total Scale and Attention Index. Results from the multiple regression models are presented in Table 5 and indicated that when controlling for age, gender, years of education, ethnicity, history of intravenous drug use, comorbid HCV, and BDI-II cognitive-affective subscore, apathy remained a significant predictor of performance on RBANS VisuospatialfConstruction Index, RBANS Total Scale, Trail Making Test Part A, Phonemic Fluency, WAIS-III Coding, and Stroop Word Naming. Participants with higher levels of apathy displayed poorer performance.
TABLE 4.
Bivariate correlations between neurocognitive variables and neuropsychiatric measures
| Neuropsychiatric measures |
||||
|---|---|---|---|---|
| Measure | Apathy | BDI Total | BDI Cog–Aff | BDI Som |
| RBANS | ||||
| Immediate Memory Index | –.17** | –.07 | –.08 | .03 |
| Visuospatial/Constructional Index | –.29** | –.12 | –.10 | –.15* |
| Language Index | –.15* | –.09 | –.08 | –.06 |
| Attention Index | –.21** | –.19** | –.19** | –.13* |
| Delayed Memory Index | –.18** | –.11 | –.07 | –.13* |
| Total Scale | –.30** | –.17** | –.15* | –.13* |
| Trails A | .24** | .02 | .04 | .13* |
| Trails B | .15* | .02 | –.04 | .07 |
| Phonemic Fluency (FAS) | –.18** | –.05 | .03 | .03 |
| Semantic Fluency (Animals) | –.08 | –.01 | –.04 | –.01 |
| Coding | –.36** | –.09 | .01 | –.08 |
| BNT | –0.10 | –.10 | –.08 | –0.10 |
| Stroop | ||||
| Word | –.26** | –.01 | .04 | –.08 |
| Color | –.11 | –.03 | –.01 | .00 |
| Color-Word | –.07 | –.07 | –.02 | –.12 |
| Purdue | ||||
| Dominant | –.13 | .01 | .01 | –.17 |
| Nondominant | –.10 | .04 | .07 | –.17 |
| Both | –.07 | .03 | .10 | –.19* |
| Grip Strength | –.01 | –.03 | .03 | –.10 |
Notes. RBANS = Repeatable Battery for the Assessment of Neuropsychological Status; BDI = Beck Depression Inventory; Cog–Aff = Cognitive–Affective; Som = Somatic.
p = .01 to p < .05 (two-tailed).
p < .01 (two-tailed).
TABLE 5.
Regression results for predicting neurocognitive performance
| Apathy Evaluation Scale |
||||
|---|---|---|---|---|
| Neurocognitive measure | B | SE | R2 | Cohen's f2 |
| RBANS | ||||
| Immediate Memory Index | –0.15 | 0.15 | .10 | 0.11 |
| Visuospatial/Constructional Index | –0.29** | 0.14 | .23 | 0.30 |
| Language Index | –0.14 | 0.12 | .15 | 0.18 |
| Attention Index | –0.08 | 0.16 | .14 | 0.16 |
| Delayed Memory Index | –0.20* | 0.19 | .11 | 0.12 |
| Total Scale | –0.25** | 0.12 | .23 | 0.30 |
| Trails A | 0.21** | 0.22 | .32 | 0.47 |
| Phonemic Fluency (FAS) | –0.21** | 0.10 | .14 | 0.16 |
| Coding | –0.28** | 0.14 | .43 | 0.75 |
| Stroop Word Naming | –0.33** | 0.14 | .30 | 0.43 |
Notes. RBANS = Repeatable Battery for the Assessment of Neuropsychological Status.
p = .01 to p > .05 (two-tailed).
p < .01 (two-tailed).
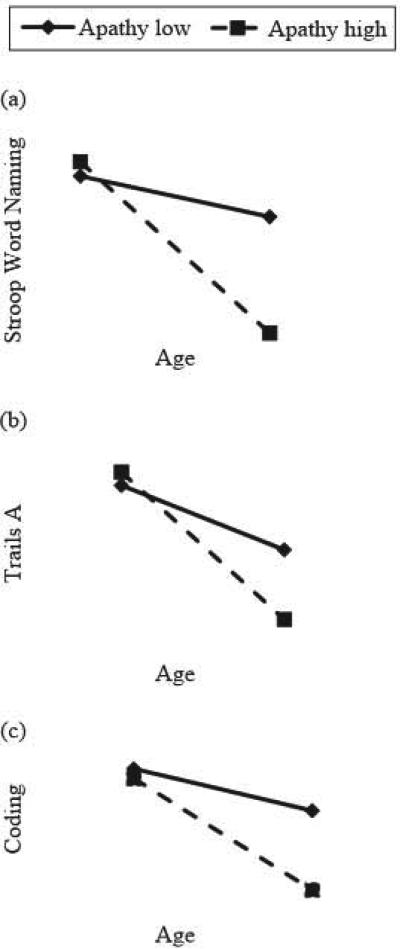
Hierarchical multiple regression indicated that the Apathy × Age interaction was significant in predicting performance on Trail Making Test Part A (R2 = .303, β = −.774, p = .008), Stroop Word Naming (R2 = .287, β = −.848, p = .005), and WAIS- III Coding (R2 = .278, β = −.778, p = .009). These interactions are visually depicted in Figure I (a, b, and c) with high/low values of apathy as plus/minus one standard deviation, respectively, from the sample mean (see Aiken & West, 1991; Cohen, Cohen, West, & Aiken, 2003). Examination of simple slopes revealed that the negative relationship between age and neurocognitive performance was stronger for participants with higher levels of apathy (Trail Making Test Part A: β = −.565, p = .008; Stroop Word Naming: β = −.772, p < .001; Coding: β = −.568, p = .009), than for those with lower apathy levels (Trail Making Test Part A: β = −.543, p = .008; Stroop Word Naming: β = −.738, p = .001; Coding: β = −.546, p = .009).
Figure 1.
Significant interactions between age and apathy in predicting Stroop Word Naming, Trails A, and Wechsler Adult Intelligence Scale-Third Edition (WAIS-III) Coding performance. High and low values of apathy represented as 1 standard deviation above and below the mean of the current sample. Age represents the minimum and maximum values in the current sample.
Apathy and HIV disease characteristics
Correlations between apathy, depression measures, and HIV disease markers are presented in Table 6. In terms of HIV disease characteristics, apathy was only Significantly associated with highest level of HIV RNA plasma (r = .181 , p = .006). Apathy remained a significant predictor of highest level of HJV RNA plasma after controlling for age and BDI-II cognitive-affective subscore (R2 = .201 , β = .212, p = .001). Among the depression scores, only the BDI-II somatic subscore and recent CD4 cell count were significantly correlated (r = .187, p = .007) .
TABLE 6.
Correlations between neuropsychiatric measures, disease markers, and functional disability
| Measure | AES | BDI–Total | BDI Cog–Aft | BDI Som |
|---|---|---|---|---|
| Disease marker | ||||
| AIDS diagnosis | -.11 | -.01 | -.08 | .03 |
| Disease duration | .05 | .04 | .01 | .11 |
| Recent log10 HIV RNA plasma level | .05 | .01 | .03 | -.06 |
| Highest log10 HIV RNA plasma level | .18** | .08 | .09 | .06 |
| Recent CD4 cell count | .07 | 0.1 | .07 | .19** |
| Nadir CD4 cell count | -.06 | -.02 | -.04 | .03 |
| Functional disability | ||||
| LL-FDI: Functioning | -.33** | -.31** | -.20** | -.41** |
| LL-FDI: Disability | -.49** | -.50** | -.44** | -.52** |
Notes. BDI = Beck Depression Inventory; Cog–Aff = Cognitive–Affective; Som = Somatic; LL-FDI = Late-Life Function and Disability Instrument. AIDS diagnosis: 0 = no, 1 = yes.
*p = .01 to p < .05 (two-tailed).
p < .01 (two-tailed).
Apathy and functional disability
Correlations between apathy, depression measures, and functional disability measures are presented in Table 6. Apathy was significantly associated with ADL functioning (r = −.327, p < .001) and disabj}jty (r = −.493, p < .001) on the bivariate level, such that higher levels of apathy were associated with greater physical difficulty and more limitation in daily activities. BDI-II total score as well as the cognitive- affective and somatic subscores were also significantly correlated with ADL functioning and disability on the bivariate level. Apathy continued to be a significant predictor of ADL functioning (R2 = .2444, β = −.356, p < .001) and ADL disability (R2 = .358, β = −.422, p < .001), while controJling tor age, disease duration, nadir CD4 count, highest HJV RNA plasma level, and BDI-II cognitive affective subscore.
DISCUSSION
Apathy, a known neuropsychiatric symptom of diseases aJJecting frontal, subcortical, and related brain structures, has been extensively studied in numerous CNS diseases. However, the nature of apathy in HIV remains unclear. The current study demonstrated associations between apathy and neuropsychological performance in a cohort of HIV-positive patients. Additionally, apathy was found to magnify the negative effect of age on processing speed performance. Though apatihy was not found to be associated with overall disease duration or AIDS diagnosis, as demonstrated by previous reports (Castellon et al., 2000; Paul, Flanigan, et al., 2005), a relationship between apathy and highest level of HIV RNA plasma was observed. Finally, apathy predicted greater functional disability in individuals with HIV, which highlights the deleterious real-world corollaries of apathy in this population. Together, these results shed light on the role of apathy in HIV and its relationship with cognitive, clinical, and disease correlates.
The results of this study confirm the association between apathy and neurocognitive performance in HIV-infected individuals, specifically on attention and information processing speed tasks (Castellon et al., 2000; Castellon et al., 1998; Paul, Flanigan, et al., 2005). Interestingly, apathy was tound to be associated with poor performance on a broad range of neuropsychological tasks and not exclusively to performance in cognitive domains controlled by frontal-subcortical structures. Notably, apathy was not associated with language impairment (e.g., Boston Naming Test, RBANS Language Index), problems in manual dexterity (e.g., Grip Strength, Purdue Pegboard Test), specific measures of executive functioning (e.g., Semantic Fluency, Trail Making Test Part B, Stroop Color-Word), or memory problems (e.g., RBANS memory scales). In fact, apathy was associated with poor performance on measures with little cognitive specificity, such as Trail Making Part A, WAIS-III Coding, Stroop Word Naming, Phonemic Fluency, and RBANS Total Score. These gross cognitive measures tend to be sensitive to almost any cognitive or neurological problem and have very limited specificity. Furthermore, these tasks draw upon many cognitive processes at relatively low levels of complexity and are not indicative of any specific or localized frontal-subcortical damage.
However, it is possible that participants' generalized and nonspecific neuropsychological decrements are due to apathy's negative impact on processing speed. Notably, all of the tests associated with apathy level were timed tasks. Apathy's generalized reduction in mental speed is not surprising given the current study's conceptualization and operationalization of apathy as a generalized depletion in initiation and motivation. It is also consistent with the prefrontal cortex's role in the ability to sustain high levels or cognitive output and mental effort (Alexander et al., 1990; Cummings, 1993; Tekin & Cummings, 2002). Thus, it is possible that the observed associations between apathy and neurocognitive performance are due to HIV-related damage to shared frontal-subcortical structures. However, the current study did not directly asses this relationship, and further examination is necessary utilizing neurophysiological and neuroimaging techniques.
The current study also sought to explore the interaction of apathy and the aging process on neuropsychological functioning. A significant interaction between age and apathy on cognitive performance was observed. This interaction was only seen in regard to performance on measures of processing speed, which is not surprising given that slowed processing is a well-established consequence of aging and is one of the cognitive domains most closely linked to apathy in individuals with HIV (Castellon et al., 2000; Castellon et al., 1998; Paul, Flanigan, et al., 2005). Processing speed performance is sensitive to almost any cognitive injury, and the present findings suggest that the double assault of age and apathy in HIV-positive individuals magnifies their individual negative effects. To our knowledge, this is the first report of an interaction between age and apathy on neuropsychological performance.
In addition to understanding the specific cognitive impairments involved in HIV-related apathy, it is equally important to identify disease markers that may serve as prognostic indicators. Previous research linking degree of apathy with specific disease characteristics has thus far been equivocal. In the current study, highest recorded level of HIV RNA plasma significantly predicted apathy score, while nadir CD4 count, disease duration, and AIDS diagnosis were not associated. Unlike previous studies on apathy and HIV (Castellon et al., 2000; Castellon et al., 1998; Paul, Flanigan, et al., 2005; Rabkin et al., 2000), the current study examined a wide range or disease markers. In fact, to our knowledge, this is the first study to examine nadir CD4 count and highest HIV RNA plasma level in relation to apathy.
The present study's findings suggest that highest level of HIV RNA plasma may serve as a valuable disease correlate of HIV-associated CNS disruption. This is not surprising given that HIV RNA plasma measures the amount of virus in the body (Cysique & Brew, 2009), and lower plasma levels have been associated with better performance on neuropsychological tests (McCutchan et al., 2007). That highest HIV RNA plasma level, and not current level, was associated with apathy in the current sample is most likely due to the fact that 91% of the participants were on antiretroviral therapy at the time of assessment and evidenced successful viral suppression. This is consistent with recent neuroirnaging research that has shown HIV-related cortical atrophy to be related to cumulative disease history as opposed to current disease status (Kallianpur et al., 2012; Thompson et al., 2005). Furthermore, neuronal damage occurs even after successful suppression of viral plasma, due to reservoirs of HIV-inlected monocytes that accumulate in perivascular spaces in the brain and initiate inflammatory processes (Kallianpur et al., 2002). Thus, history of uncontrolled viremia, perhaps even in the context of current viral suppression, may put individuals at greater risk for CNS disruption and concomitant apathy. It is important to note that although this is a novel and interesting finding, the effect size for the observed relationship between apathy and highest HIV RNA plasma did not meet traditional levels of interpretation for medium efiect (e.g., Cohen). As this is the first study to empirically examine highest level of HIV RNA plasma in relation to apathy, further examination in an independent sample is warranted.
Finally, the relationship between apathy and functional disability was examined among individuals with HIV. Individuals with higher levels of apathy reported greater difficulty performing physical functional tasks, more limitation in activities requisite to social roles at home and in the community, and less frequent engagement in social and communal activities. This is consistent with research on patients with stroke, Alzheimer's disease, Parkinson's disease, H untington's disease, and schizophrenia, as weiJ as Kamat et al.'s (2012) study on individ uals with HIV These findings highlight the potential harmful real-world correlates of apathy and stress the importance of accurate detection and efficacious treatment of apathy in this population. As HIV survival rates continue to increase, research on the long-term neuropsychiatric effects of the disease becomes more crucial. Additional research, particularly neuroimaging studies, is necessary to clarify the relationship between apathy, cognition, and the pre-frontal cortex, as well as the synergistic relationship of apathy and age on processing speed in HIV-infected individuals.
Acknowledgments
The authors would like to thank the research participants who enrolled in this study, all of the research staff who helped with data collection (especially Deena Peyser and Eugene Dolce), and the community I-I IV care providers who referred many of their patients to our study.
This research was supported by funding from the National Institute of Allergy and Infectious Diseases (grant number P30AI051519-09s].
REFERENCES
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Sage Publications; Thousand Oaks, CA: 1991. [Google Scholar]
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: Parallel substrates for motor, oculomotor, “pre-frontal” and “limbic” functions. Progress in Brain Research. 1990;85:119–146. [PubMed] [Google Scholar]
- Barclay TR, Hinkin CH, Castellon SA, Mason KI, Reinhard MJ, Marion SD, Durvasula RS. Age-associated predictors of medication adherence in HIV-positive adults: Health beliefs, self-efficacy, and neurocognitive status. Health Psychology. 2007;26(1):40–49. doi: 10.1037/0278-6133.26.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT. Beck Depression Inventory manual. Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]
- Benton AL, Hamsher K. Multilingual Aphasia Examination. AJA Associates; Iowa City, IA: 1989. [Google Scholar]
- Brodaty H, Altendorf A, Withall A, Sachdev P. Do people become more apathetic as they grow older? A longitudinal study in healthy individuals. International Psychogeriatrics. 2010;22(3):426–436. doi: 10.1017/S1041610209991335. [DOI] [PubMed] [Google Scholar]
- Brodaty H, Sachdev PS, Withall A, Altendorf A, Valenzuela MJ, Lorentz L. Frequency and clinical, neuropsychological and neuroimaging correlates of apathy following stroke—the Sydney Stroke Study. Psychological Medicine: A Journal of Research in Psychiatry and the Allied Sciences. 2005;35(12):1707–1716. doi: 10.1017/S0033291705006173. [DOI] [PubMed] [Google Scholar]
- Caeiro L, Ferro JM, Figueira ML. Apathy in acute stroke patients. European Journal of Neurology. 2012;19(2):291–297. doi: 10.1111/j.1468-1331.2011.03508.x. [DOI] [PubMed] [Google Scholar]
- Castellon SA, Hardy DJ, Hinkin CH, Satz P, Stenquist PK, Van Gorp WG, Moore L. Components of depression in HIV-1 infection: Their differential relationship to neurocognitive performance. Journal of Clinical and Experimental Neuropsychology. 2006;28(3):420–437. doi: 10.1080/13803390590935444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellon SA, Hinkin CH, Myers HF. Neuropsychiatric disturbance is associated with executive dysfunction in HIV-1 infection. Journal of the International Neuropsychological Society: JINS. 2000;6(3):336–347. doi: 10.1017/s1355617700633088. [DOI] [PubMed] [Google Scholar]
- Castellon SA, Hinkin CH, Wood S, Yarema KT. Apathy, depression, and cognitive performance in HIV-1 infection. The Journal of Neuropsychiatry and Clinical Neurosciences. 1998;10(3):320–329. doi: 10.1176/jnp.10.3.320. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, CDC . HIV/AIDS Surveillance Report. United States Department of Health and Human Services; Atlanta, GA: 2007. [Google Scholar]
- Chase TN. Apathy in neuropsychiatric disease: Diagnosis, pathophysiology, and treatment. Neurotoxicity Research. 2011;19(2):266–278. doi: 10.1007/s12640-010-9196-9. [DOI] [PubMed] [Google Scholar]
- Clarke DE, Ko JY, Kuhl EA, van Reekum R, Salvador R, Marin RS. Are the available apathy measures reliable and valid? A review of the psychometric evidence. Journal of Psychosomatic Research. 2011;70(1):73–97. doi: 10.1016/j.jpsychores.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J, Cohen P, West SG, Aiken LS. Applied multiple regression/correlation analysis for the behavioral sciences. 3rd ed. Lawrence Erlbaum Associates; Mahwah, NJ: 2003. [Google Scholar]
- Cole MA, Castellon SA, Perkins AC, Ureno OS, Robinet MB, Reinhard MJ, Hinkin CH. Relationship between psychiatric status and frontal-subcortical systems in HIV infected individuals. Journal of the International Neuropsychological Society. 2007;13(3):549–554. doi: 10.1017/S135561770707066X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crum RM, Anthony JC, Bassett SS, Folstein MF. Population based norms for the Mini-Mental State Examination by age and education level. Journal of the American Medical Association. 1993;269(18):2386–2391. [PubMed] [Google Scholar]
- Cummings JL. Frontal-subcortical circuits and human behavior. Archives of Neurology. 1993;50:873–880. doi: 10.1001/archneur.1993.00540080076020. [DOI] [PubMed] [Google Scholar]
- Cysique LA, Brew BJ. Neuropsychological functioning and antiretrovira1 treatment in HIV/AIDS: A review. Neuropsychology Review. 2009;19(2):169–185. doi: 10.1007/s11065-009-9092-3. [DOI] [PubMed] [Google Scholar]
- Golden JC. Stroop Color and Word Test. Stoelting Co; Chicago, IL: 1978. [Google Scholar]
- Goodglass H, Kaplan E. Boston Naming Test. Lippincott Williams & Wilkins; Philadelphia, PA: 2000. [Google Scholar]
- Haley SM, Jette AM, Coster WJ, Kooyoomjian JT, Levenson S, Heeren T, Ashba J. Late Life Function and Disability Instrument: II. Development and evaluation of the function component. The Journals of Gerontology: Series A: Biological Sciences and Medical Sciences. 2002;57A(4):M217–M222. doi: 10.1093/gerona/57.4.m217. [DOI] [PubMed] [Google Scholar]
- Halstead WC. Brain and intelligence. Chicago University Press; Chicago, IL: 1947. [Google Scholar]
- Heaton RK, Grant I, Butter N, White DA, Kirson D, Atkinson JH, Ellis RJ. The HNRC 500: Neuropsychology of HIV infection at different disease stages. Journal of the International Neuropsychological Society. 1995;1:231–251. doi: 10.1017/s1355617700000230. [DOI] [PubMed] [Google Scholar]
- Jette AM, Haley SM, Coster WJ, Kooyoomjian JT, Levenson S, Heeren T, Ashba J. Late Life Function and Disability Instrument: I. Development and evaluation of the disability component. The Journals of Gerontology: Series A: Biological Sciences and Medical Sciences. 2002;57A(4):M209–M216. doi: 10.1093/gerona/57.4.m209. [DOI] [PubMed] [Google Scholar]
- Jonsson M, Edman Å, Lind K, Rolstad S, Sjögren M, Wallin A. Apathy is a prominent neuropsychiatric feature of radiological white-matter changes in patients with dementia. International Journal of Geriatric Psychiatry. 2010;25(6):588–595. doi: 10.1002/gps.2379. [DOI] [PubMed] [Google Scholar]
- Kallianpur KJ, Kirk GR, Sailasuta N, Valcour V, Shiramizu B, Nakamoto BK, Shikuma C. Regional cortical thinning associated with detectable levels of HIV DNA. Cerebral Cortex. 2012;22(9):2065–2075. doi: 10.1093/cercor/bhr285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat R, Woods SP, Marcotte TD, Ellis RJ, Grant I. Implications of apathy for everyday functioning outcomes in persons living with HIV infection. Archives of Clinical Neuropsychology. 2012;27(5):520–531. doi: 10.1093/arclin/acs055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant R, Duffy JD, Pivovarnik A. Prevalence of apathy following head injury. Brain Injury. 1998;12:87–92. doi: 10.1080/026990598122908. [DOI] [PubMed] [Google Scholar]
- Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. 2nd ed. Lea & Febiger; Philadelphia, PA: 1983. [Google Scholar]
- Kuzis G, Sabe L, Tiberti C, Merello M, Leiguarda R, Starkstein SE. Neuropsychological correlates of apathy and depression in patients with dementia. Neurology. 1999;52:1403–1407. doi: 10.1212/wnl.52.7.1403. [DOI] [PubMed] [Google Scholar]
- Law WA, Mapou RL, Roller TL, Martin A, Nannis ED, Temoshok LR. Reaction time slowing in HIV-1-infected individuals: Role of the preparatory interval. Jourrnal of Clinical and Experimental Neuropsychology. 1995;17(1):122–133. doi: 10.1080/13803399508406587. [DOI] [PubMed] [Google Scholar]
- Law WA, Martin A, Mapou RL, Roller TL, Salazar AM, Temoshok LR, Rundell JR. Working memory in individuals with HIV infection. Jourrnal of Clinical and Experimental Neuropsychology. 1994;16(2):173–182. doi: 10.1080/01688639408402628. [DOI] [PubMed] [Google Scholar]
- Marin RS. Differential diagnosis and classification of apathy. American Journal of Psychiatry. 1990;147:22–30. doi: 10.1176/ajp.147.1.22. [DOI] [PubMed] [Google Scholar]
- Marin RS. Apathy: A neuropsychiatric syndrome. Journal of Neuropsychiatry & Clinical Neurosciences. 1991;3:243–254. doi: 10.1176/jnp.3.3.243. [DOI] [PubMed] [Google Scholar]
- Marin RS, Biedrzycki RC, Firinciogullari S. Reliability and validity of the Apathy Evaluation Scale. Psychiatry Research. 1991;38:143–162. doi: 10.1016/0165-1781(91)90040-v. [DOI] [PubMed] [Google Scholar]
- Marin RS, Firinciogullari S, Biedrzycki RC. The sources of convergence between measures of apathy and depression. Journal of Affective Disorders. 1993;28(2):117–124. doi: 10.1016/0165-0327(93)90040-q. [DOI] [PubMed] [Google Scholar]
- Marin RS, Firinciogullari S, Biedrzycki RC. Group differences in the relationship between apathy and depression. Journal of Nervous & Mental Disease. 1994;182:235–239. doi: 10.1097/00005053-199404000-00008. [DOI] [PubMed] [Google Scholar]
- Mayo NE, Fellows LK, Scott SC, Cameron J, Wood-Dauphinee S. A longitudinal view of apathy and its impact after stroke. Stroke. 2009;40(10):3299–3307. doi: 10.1161/STROKEAHA.109.554410. [DOI] [PubMed] [Google Scholar]
- McCutchan JA, Wu JW, Robertson K, Koletar SL, Ellis RJ, Cohn S, Williams PL. HIV suppression by HAART preserves cognitive function in advanced, immune-reconstituted AIDS patients. AIDS. 2007;21(9):1109–1117. doi: 10.1097/QAD.0b013e3280ef6acd. [DOI] [PubMed] [Google Scholar]
- Ott BR, Noto RB, Fogel BS. Apathy and loss of insight in Alzheimer's disease: A SPECT imaging study. Journal of Neuropsychiatry and Clinical Neuroscience. 1996;8(1):41–46. doi: 10.1176/jnp.8.1.41. [DOI] [PubMed] [Google Scholar]
- Paul RH, Brickman AM, Navia B, Hinkin C, Malloy PF, Jefferson AL, Flanigan TP. Apathy is associated with volume of the nucleus accumbens in patients with HIV. Journal of Neuropsychiatry and Clinical Neurosciences. 2005;17:167–171. doi: 10.1176/appi.neuropsych.17.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul R, Flanigan TP, Tashima K, Cohen R, Lawrence J, Alt E, Hinkin C. Apathy correlates with cognitive function but not CD4 status in patients with human immunodeficiency virus. Journal of Neuropsychiatry and Clinical Neurosciences. 2005;17:114–118. doi: 10.1176/jnp.17.1.114. [DOI] [PubMed] [Google Scholar]
- Rahkin JG, Ferrando SJ, van Gorp W, Ricppi R, McElhiney M, Sewell M. Relationship among apathy, depression, and cognitive impairment in HIV/AIDS. Journal of Neuropsychiatry and Clinical Neurosciences. 2000;12:451–457. doi: 10.1176/jnp.12.4.451. [DOI] [PubMed] [Google Scholar]
- Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): Preliminary clinical validity. Journal of Clinical and Experimental Neuropsychology. 1998;20(3):310–319. doi: 10.1076/jcen.20.3.310.823. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Investigation of the validity of Halstead's measures of biological intelligence. JAMA Archives of Neurology and Psychiatry. 1955;73(1):28–35. doi: 10.1001/archneurpsyc.1955.02330070030005. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A compendium of neuropsychological tests. 2nd ed. Oxford University Press; New York, NY: 1998. [Google Scholar]
- Starkstein SE, Bolduc PL, Preziosi TJ, Robinson RG. Cognitive impairments in different stages of Parkinson's disease. The Journal of Neuropsychiatry and Clinical Neurosciences. 1989;1(3):243–248. doi: 10.1176/jnp.1.3.243. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Brockman S. Apathy and Parkinson's disease. Current Treatment Options in Neurology. 2011;13(3):267–273. doi: 10.1007/s11940-011-0118-9. [DOI] [PubMed] [Google Scholar]
- Starkstein SE, Jorge R, Mizrahi R. The prevalence, clinical correlates and treatment of apathy in Alzheimer's disease. The European Journal of Psychiatry. 2006;20(2):96–106. [Google Scholar]
- Starkstein SE, Jorge R, Mizrahi R, Robinson RG. A prospective longitudinal study of apathy in Alzheimer's disease. Journal of Neurology, Neurosurgery & Psychiatry. 2006;77(1):8–11. doi: 10.1136/jnnp.2005.069575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkstein SE, Mayberg HS, Preziosi TJ, Andrezejewski P, Leiguarda R, Robinson RG. Reliability, validity, and clinical correlates of apathy in Parkinson's disease. Journal of Neuropsychiatry & Clinical Neurosciences. 1992;4:134–139. doi: 10.1176/jnp.4.2.134. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Tate D, Paul RH, Flanigan TP, Tashima K, Nash J, Adair C, Cohen RA. The impact of apathy and depression on quality of life in patients infected with HIV. AIDS Patient Care and STDs. 2003;17(3):115–120. doi: 10.1089/108729103763807936. [DOI] [PubMed] [Google Scholar]
- Tekin S, Cummings JL. Frontal-subcortical neuronal circuits and clinical neuropsychiatry: An update. Journal of Psychosomatic Research. 2002;53(2):647–654. doi: 10.1016/s0022-3999(02)00428-2. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Dutton RA, Hayashi KM, Toga AW, Lopez OL, Aizenstein HJ, Becker JT. Thinning of the cerebral cortex visualized in HIV/AIDS reflects CD4+ T lymphocyte decline. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15647–15652. doi: 10.1073/pnas.0502548102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiffin J, Asher EJ. The Purdue pegboard: Norms and studies of reliability and validity. Journal of Applied Psychology. 1948;32(3):234–247. doi: 10.1037/h0061266. [DOI] [PubMed] [Google Scholar]
- Valcour V, Paul R, Neuhaus J, Shikuma C. The effects of age and HIV on neuropsychological performance. Journal of the International Neuropsychological Society. 2011;17(1):190–195. doi: 10.1017/S1355617710001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velligan DI, Ritch JL, Sui D, DiCocco M, Huntzinger CD. Frontal Systems Behavior Scale in schizophrenia: Relationships with psychiatric symptomatology, cognition and adaptive function. Psychiatry Research. 2002;113(3):227–236. doi: 10.1016/s0165-1781(02)00264-0. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale III. The Psychological Corporation; San Antonio, TX: 1997. [Google Scholar]
- Zahodne LB, Marsiske M, Okun MS, Rodriguez RL, Malaty I, Bowers D. Mood and motor trajectories in Parkinson's disease: Multivariate latent growth curve modeling. Neuropsychology. 2011;26(1):71–80. doi: 10.1037/a0025119. [DOI] [PMC free article] [PubMed] [Google Scholar]