Abstract
Objective
To describe patterns of weight loss and regain and their effect on the pro-inflammatory cytokines IL-6 and TNF-α, and anti-inflammatory cytokines adiponectin and IL-10 during a 24-month weight loss trial.
Materials/Methods
Participants were obese adults (N = 66) who lost and regained ≥10 lbs during a 24-month clinical trial of behavioral weight loss treatment. Measurements of cytokines and weight were conducted at baseline, 6, 12, 18, and 24 months. Linear mixed modeling was used to determine percent change in weight and cytokines from baseline.
Results
The sample was predominantly female (80.3%) and White (86.4%), with a mean age of 48.4 ± 7.3 years and mean BMI of 34.5 ± 4.4 kg/m2. At baseline, men had higher waist circumference, body weight, and energy intake, and lower percent body fat and adiponectin. The largest decrease in weight was observed at 6 months with a mean 11% decrease (p < .0001). A significant gender-by-weight change interaction on percent change in adiponectin was observed [b(se) = 0.9 (0.2), p = .0003], with men having a larger increase in adiponectin with weight loss compared to women. There was a significant effect of weight gain over time with increases in IL-6 [b(se) = 0.9 (0.3), p = .001].
Conclusions
Overall, weight loss was significantly associated with improvements in adiponectin and IL-6. Those improvements remained at 24 months, following weight regain. The association between weight change and adiponectin was different between genders. Implementing strategies that support sustained weight loss can help prevent a state of chronic systemic inflammation and its associated adverse effects.
Keywords: Cytokines, behavioral weight loss, weight regain
INTRODUCTION
Weight maintenance after intentional weight loss is difficult to achieve [1–3]. Individuals may develop a pattern of repeated weight loss and regain, which can lead to the development of diabetes [4] and cardiovascular disease [5]. However, there are inconsistencies in the literature regarding the effect of the cyclical pattern of weight loss and regain on an individual’s health.
Adipose tissue is an active endocrine organ that produces and secretes pro-inflammatory cytokines (e.g., IL-6 and TNF-α,) and anti-inflammatory cytokines (e.g., adiponectin and IL-10). There is evidence that IL-6 and TNF-α are elevated in obese individuals and decrease with weight loss [7, 8]. In contrast, adiponectin and IL-10 are diminished in obese individuals and tend to increase with weight loss [9, 10]. Thus, obesity could be viewed as a chronic inflammatory state, characterized by a dysregulation in pro- and anti-inflammatory cytokines.
There is limited literature on long-term weight cycling and cytokine expression. The purpose of our study was to describe the effect of weight cycling on multiple cytokines in a subset of participants who lost and regained at least 10 lbs during the 24-month SMART Trial, a behavioral weight loss study. This prospective study examined the association between changes in weight and changes in cytokines from baseline to 6, 12, 18, and 24 months. To the best of our knowledge, this is the first such report.
SUBJECTS AND METHODS
Participant Characteristics
A sub-group of 66 SMART Trial (NCT00277771, [11]) participants who lost and regained ≥10 lbs at any time in the 24-month study were included in the analysis. Individuals were included if they were 18–59 years of age and had a BMI between 27 and 43 kg/m2. All eligibility criteria were published elsewhere [11]. Participants provided written informed consent; the study was approved by the University of Pittsburgh Institutional Review Board.
Measures
A medical history form and a sociodemographic data questionnaire were completed at baseline. Energy intake was measured in kilocalories per day (kcal/day) by two unannounced 24-hour dietary recalls, conducted on one leisure day and one work day. A blood sample was obtained for total adiponectin, IL-10, IL-6, and TNF-α at baseline, 6, 12, 18, and 24 months.
Anthropometric measurements
Height was measured with a wall-mounted stadiometer and recorded in centimeters. Weight and percent body fat were measured following an overnight fast using a Tanita Scale and Body Fat Analyzer (Tanita Corporation of America, Inc., Arlington Heights, IL) while participants wore light clothing and stood erect with bare feet on the scale’s footpads.
Cytokine measurements
TNF-α was measured by Luminex technology multiplex ELISA (Linco Research, Inc.; St. Charles, MO). The minimum detectable level (MDL) was 0.14 pg/mL, with an assay range of 0.64–10,000 pg/mL. The intra- and inter-assay CVs were 1.4–7.9% and <21%, respectively. Quantitative sandwich enzyme immunoassay technique was used to measure IL-6 (Quantiglo Human IL-6 Immunoassay; R&D Systems, Minneapolis), with the MDL ranging from 0.05–0.35 pg/mL and an assay range of 0.48–1,500 pg/mL. The intra- and inter-assay CVs were 3.0–5.8% and 6.3–9.6%, respectively.
IL-10 was measured by quantitative sandwich enzyme immunoassay technique (Quantikine HS Human IL-10 Immunoassay; R&D Systems, Minneapolis). The MDL was <0.5 pg/mL, with an assay range of 0–50 pg/mL. The intra- and inter-assay CVs were 6.6–8.5% and 8.1–15.6%, respectively. Finally, the ELISA technique was used for the adiponectin assay (Quantikine Human Adiponectin/Acrp30 Immunoassay; R&D Systems; Minneapolis), with a MDL of 0.25 ng/mL and intra- and inter-assay CVs of 2.5–4.7% and 5.8–6.9%, respectively All cytokine assays were performed at the Laboratory for Clinical Biochemistry Research at the University of Vermont.
Data Analysis
Summary statistics were reported as mean (SD) and frequency count (%). All non-normal weight and cytokine variables were log-transformed. Independent sample t-tests were used to compare baseline means between women and men. Spearman correlations were used to test the associations between baseline body weight and cytokines. Percent change from baseline to 6, 12, 18 and 24 months were used in the analyses of weight and cytokines. Percent change from baseline was calculated as [(follow-up – baseline)/ baseline) x 100]. Linear mixed modeling was applied to assess the effects of weight change since baseline on change in cytokines from baseline, adjusting for time, age, race/ethnicity, gender, baseline body weight, baseline energy intake, baseline cytokine concentration, percent change in energy intake, and percent change in weight. To determine whether associations between percent change in weight and percent change in cytokines varied by gender, an interaction between gender and percent weight change was included in the models. In each cytokine model, a random intercept was used for each participant, and an unstructured covariance structure was assumed. P-values from the F-tests were reported. In all analyses, the Bonferroni approach was used to correct for multiple testing (four tests: adjusted p=.0125). Analyses of the data were conducted using SAS version 9.2 (SAS Institute, Cary, NC) and IBM SPSS Statistics version 21.0 (IBM Corporation, Armonk, NY).
RESULTS
Baseline characteristics for women and men are displayed in the Table. Participants’ mean age was 48.4 years. Compared to women, men had significantly higher weight, waist circumference, energy intake, and adiponectin, but significantly lower percent body fat.
Table.
Baseline characteristics of the participants
| Characteristic | Women (n=53) | Men (n=13) | Total (N=66) |
|---|---|---|---|
| Age (years) | 47.6 (7.3) | 51.5 (7.2) | 48.4 (7.4) |
| Weight (kg)* | 92.8 (13.7) | 111.9 (14.3) | 96.5 (15.7) |
| BMI (kg/m2) | 34.3 (4.3) | 35.4 (4.6) | 34.5 (4.4) |
| Waist (cm)* | 105.1 (12.3) | 119.3 (11.4) | 107.9 (13.3) |
| Energy intake (kcal/day)* | 2029 (480) | 2781 (696) | 2177 (604) |
| Body Fat Composition (%)* | 44.0 (4.5) | 33.9 (5.3) | 42.0 (6.1) |
| Adiponectin (μg/mL)* | 18.2 (9.5) | 11.6 (5.2) | 16.9 (9.2) |
| IL-10 (pg/mL) | 8.8 (4.3) | 8.7 (3.6) | 8.8 (4.1) |
| IL-6 (pg/mL) | 2.5 (1.4) | 2.8 (1.7) | 2.6 (1.5) |
| TNF-α (pg/mL) | 5.0 (4.8) | 5.5 (1.7) | 5.1 (4.3) |
Mean (SD)
kg=kilograms; kg/m2=kilograms per square meters; cm=centimeters; kcal/day=kilocalories per day; MET-h/wk=metabolic equivalent of task-hours per week; μg/mL=micrograms per deciliter; pg/mL=picograms per deciliter
p<.05 women vs. men from t-tests
Associations between baseline weight and baseline cytokines
Weight was significantly correlated with adiponectin (r = −.31, p=.01), IL-6 (r = .41, p=.001), and TNF-α (r = .43, p<.0001), but there was no significant correlation between IL-10 and weight (r = .05, p=.68). There were significant correlations between cytokines, including adiponectin and IL-6 (r = −.33, p=.01), adiponectin and TNF-α (r = −.31, p=.01), and IL-6 and TNF-α (r = .25, p=.04). There were no significant associations between IL-10 and the other cytokines.
Description of percent change in weight and cytokines by time
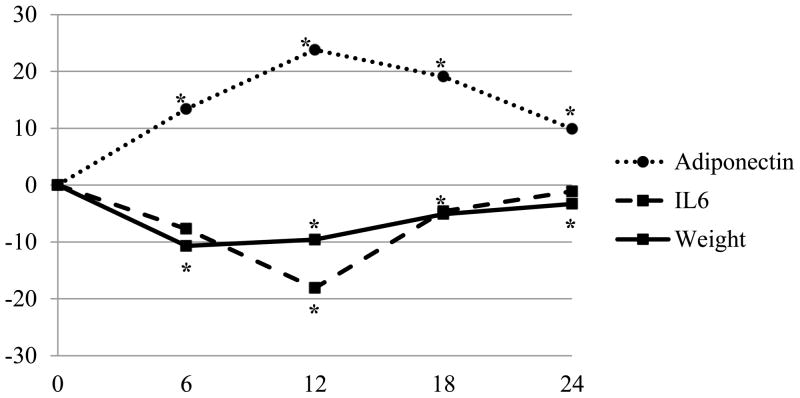
Since only adiponectin and IL-6 had significant values, they are displayed in the Figure. The largest decrease in weight was observed at 6 months (-11%, p<.0001), with evidence of regain at 12, 18, and 24 months. Concurrent with baseline to 6-month weight loss, adiponectin increased by 13.4% (p<.0001). From baseline to 12 months, adiponectin significantly increased by 23.8% (p<.0001), while IL-6 significantly decreased from baseline (-18.1%, p=.0003). Baseline to 18-month changes were significant only for adiponectin (19.1%, p=.0001); at 24 months, adiponectin was significantly higher than at baseline (9.9%, p=.004) but was lower than the 18-month values.
Figure.
Description of percent change in weight, adiponectin, and IL-6 over time (N=66)
Least squares means
*p<.05 at for adiponectin at 6, 12, 18, and 24 months; for IL-6 at 12 months; and for weight at 6, 12, 18 and 24 months
p-values from F-tests in model with time only
The associations between percent change in weight and percent changes in cytokines
The associations between percent change in weight and in cytokines reveal that, controlling for covariates, a significant interaction between gender and percent change in weight on percent change in adiponectin over time was detected [b (se) = 0.9 (0.2), p = .0003], with men having a higher percent increase in adiponectin with weight loss, compared to women with the same amount of weight loss. For example, if men had an overall weight loss of 20%, they increased their adiponectin concentration by about 20%; however, women with the same amount of weight loss only increased their adiponectin concentration by about 10%. The opposite was true for weight regain; compared to women, men had greater decreases in adiponectin with the same amount of weight regain. Change in weight was significantly associated with change in IL-6 [b (se) = 0.9 (0.3), p = .001], and marginally associated with change in IL-10 [b (se) = 0.3 (0.2), p = .07]. There was no significant association between weight changes and TNF-α changes [b (se) = −0.1 (0.1), p = .22].
DISCUSSION
We observed improvements in cytokines after weight loss, and despite participants regaining most of their lost weight by 24 months, improvements in cytokines persisted. Moreover, we demonstrated that decreases in weight predicted an increase in adiponectin and a decrease in IL-6. We also showed that the association between weight change and change in adiponectin was different for men and women. Although there were significant associations between weight and adiponectin, IL-6, TNF-α at baseline, only adiponectin was significantly associated with weight loss, and an 11% weight loss led to a significant increase in adiponectin. Because adiponectin is exclusively released from adipose tissue, it may be more sensitive to the hypertrophic changes in adipocytes. Conversely, IL-6, TNF-α, and IL-10 originate from a variety of sources, which can affect serum expression of cytokines during weight changes. Although the weight-loss associated increases in adiponectin have been well-described [12–16], there are inconsistencies regarding changes in the other cytokines with weight loss. Sofer et al. found IL-6 significantly decreased at 6 months of weight loss treatment but did not find any significant changes in TNF-α [17]. In contrast, Pakiz et al. found that TNF-α significantly decreased with weight loss with no significant changes in IL-6 [18]. Methodological differences among these studies may have contributed to the variability of the results.
We observed continued improvements in cytokines from the 6-month point, demonstrating that the improved cytokine concentration occurring with weight loss can be sustained despite weight regain. This suggests a delayed effect of weight regain on cytokine concentration. At 24 months, adiponectin declined and IL-6 increased from the point of greatest weight loss, suggesting that regain attenuates the effects of weight loss on some cytokines. Our findings that improvements in cytokines persist despite weight regain support similar findings reported by others [12, 19]. However, Erez et al. found a positive association between adiponectin and weight loss but no significant association between adiponectin and weight regain [16].
Baseline correlation did not reveal significant associations between IL-10 and adiposity measures or other cytokines. There is evidence that IL-10 is reduced in obese states [20]. However, the evidence on IL-10 and weight change is limited, making this association unclear. We noticed that men had greater increases in adiponectin with weight loss than women, despite both groups having the same amount of weight loss. We also noticed that men had greater decreases in adiponectin with regain than women, despite both groups having the same amount of regain. This suggests that weight loss studies should target men, as they may have great health benefits from weight loss.
The only study similar to ours was conducted by Lien et al. who described changes following a 6-month behavioral weight loss intervention followed by weight regain [19]. They did not observe a significant change in adiponectin levels concurrent with the weight loss, nor did they observe any significant changes in IL-6 during weight loss or regain. However, they observed an increase in adiponectin from baseline to post-weight regain, also showing the sustained improvement in adiponectin despite weight regain [19].
Bluher et al. examined cytokines in participants enrolled in a study of Mediterranean diet vs. low carbohydrate diet [12] and demonstrated an increase in adiponectin with weight loss; however, there was a continued increase in adiponectin levels at 24 months. There are several differences between Bluher et al.’s and the current study. First, Bluher et al. included participants with type 2 diabetes, those with varying weight change patterns (e.g. maintained, lost or regained weight), and did not restrict energy intake whereas our study recommended a restricted calorie and 25% fat eating plan. Additionally, women represented 14% of their sample while 80% of our participants were women. Lastly, Bluher et al. used a trend analysis that precluded them from determining whether the association between weight change and change in cytokines was statistically significant [12]. It is possible that failing to control for part of the sample that maintained their weight may have contributed to the observed increase in adiponectin at 24 months in Bluher et al.’s study. Limiting the sample to only participants who lost and regained weight precludes the occurrence of bias that may occur when including individuals who maintained the weight loss.
Regarding study limitations, we did not have a control group with which to compare our results. Although we found significant associations between changes in weight and changes in cytokines, we cannot make any causal inference. We included healthy individuals within specific ages and BMIs, thus our results cannot be generalized to dissimilar populations. Our study also has several strengths. We studied a unique population of individuals who lost and regained ≥10 lbs and also incorporated a longitudinal study design with data collected at five time points over two years. In summary, changes in weight were associated with changes in adiponectin and IL-6. The improvement in these cytokines persisted even with nearly total weight regain, suggesting that weight loss can have protective effects despite weight regain. Because the majority of individuals who lose weight experience weight regain, it is important to develop strategies to prevent regain to reduce the duration of exposure to an inflammatory state, thus reducing the risk of developing chronic diseases such as diabetes and atherosclerosis.
Acknowledgments
This work was supported by NIH grants R01-DK071817, R01-DK071817-04S1,R01-DK071817-05S1, and K24-NR010742; also the Data Management Core of the Center for Research in Chronic Disorders at the School of Nursing (P30-NR03924), the General Clinical Research Center (NCRR-GCRC 5M01-RR000056) and the Clinical Translational Research Center (NCRR/CTSAUL1 RR024153) at the University of Pittsburgh.
Abbreviations
- BMI
body mass index
- kg
kilograms
- kg/m2
kilograms per square meters
- lbs
pounds
- cm
centimeters
- IL-6
interleukin-6
- IL-10
interleukin-10
- TNF-α
tumor necrosis factor-alpha
- SMART
Self-Monitoring And Recording using Technology
- mg/dL
milligrams per deciliter
- μg/mL
micrograms per milliliter
- pg/mL
picograms per milliliter
- MDL
minimum detectable level
- SD
standard deviation
- SE
standard error
- kcal/day
kilocalories per day
Footnotes
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
EJA analyzed the data and drafted the manuscript. MMB provided statistical consultation on the manuscript and reviewed the manuscript. MAS, LHK, RWE, and LEB all provided consultation on the content of the manuscript and reviewed the manuscript. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wing RR. Behavioral approaches to the treatment of obesity. In: Bray GA, Bourchard C, James WPT, editors. Handbook of obesity: Clinical applications. New York: Marcel Dekker; 2004. pp. 147–67. [Google Scholar]
- 2.Stevens VL, Jacobs EJ, Sun J, et al. Weight cycling and mortality in a large prospective us study. Am J Epidemiol. 2012;175(8):785–92. doi: 10.1093/aje/kwr378. [DOI] [PubMed] [Google Scholar]
- 3.Kraschnewski JL, Boan J, Esposito J, et al. Long-term weight loss maintenance in the united states. Int J Obes (Lond) 2010;34(11):1644–54. doi: 10.1038/ijo.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Waring ME, Eaton CB, Lasater TM, et al. Incident diabetes in relation to weight patterns during middle age. Am J Epidemiol. 2010;171(5):550–6. doi: 10.1093/aje/kwp433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Graci S, Izzo G, Savino S, et al. Weight cycling and cardiovascular risk factors in obesity. Int J Obes Relat Metab Disord. 2004;28(1):65–71. doi: 10.1038/sj.ijo.0802537. [DOI] [PubMed] [Google Scholar]
- 6.Thompson HJ, McTiernan A. Weight cycling and cancer: Weighing the evidence of intermittent caloric restriction and cancer risk. Cancer Prev Res (Phila) 2011;4(11):1736–42. doi: 10.1158/1940-6207.CAPR-11-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moschen AR, Molnar C, Geiger S, et al. Anti-inflammatory effects of excessive weight loss: Potent suppression of adipose interleukin 6 and tumour necrosis factor alpha expression. Gut. 2010;59(9):1259–64. doi: 10.1136/gut.2010.214577. [DOI] [PubMed] [Google Scholar]
- 8.Infanger D, Baldinger R, Branson R, et al. Effect of significant intermediate-term weight loss on serum leptin levels and body composition in severely obese subjects. Obes Surg. 2003;13(6):879–88. doi: 10.1381/096089203322618704. [DOI] [PubMed] [Google Scholar]
- 9.Hung J, McQuillan BM, Thompson PL, et al. Circulating adiponectin levels associate with inflammatory markers, insulin resistance and metabolic syndrome independent of obesity. Int J Obes (Lond) 2008;32(5):772–9. doi: 10.1038/sj.ijo.0803793. [DOI] [PubMed] [Google Scholar]
- 10.Jung SH, Park HS, Kim KS, et al. Effect of weight loss on some serum cytokines in human obesity: Increase in il-10 after weight loss. J Nutr Biochem. 2008;19(6):371–5. doi: 10.1016/j.jnutbio.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 11.Burke LE, Styn MA, Glanz K, et al. Smart trial: A randomized clinical trial of self-monitoring in behavioral weight management-design and baseline findings. Contemp Clin Trials. 2009;30(6):540–51. doi: 10.1016/j.cct.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bluher M, Rudich A, Kloting N, et al. Two patterns of adipokine and other biomarker dynamics in a long-term weight loss intervention. Diabetes Care. 2012;35(2):342–9. doi: 10.2337/dc11-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Summer SS, Brehm BJ, Benoit SC, et al. Adiponectin changes in relation to the macronutrient composition of a weight-loss diet. Obesity (Silver Spring) 2011;19(11):2198–204. doi: 10.1038/oby.2011.60. [DOI] [PubMed] [Google Scholar]
- 14.Ata SM, Vaishnav U, Puglisi M, et al. Macronutrient composition and increased physical activity modulate plasma adipokines and appetite hormones during a weight loss intervention. J Womens Health (Larchmt) 2010;19(1):139–45. doi: 10.1089/jwh.2009.1472. [DOI] [PubMed] [Google Scholar]
- 15.Lazzer S, Vermorel M, Montaurier C, et al. Changes in adipocyte hormones and lipid oxidation associated with weight loss and regain in severely obese adolescents. Int J Obes (Lond) 2005;29(10):1184–91. doi: 10.1038/sj.ijo.0802977. [DOI] [PubMed] [Google Scholar]
- 16.Erez G, Tirosh A, Rudich A, et al. Phenotypic and genetic variation in leptin as determinants of weight regain. Int J Obes (Lond) 2011;35(6):785–92. doi: 10.1038/ijo.2010.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sofer S, Eliraz A, Kaplan S, et al. Greater weight loss and hormonal changes after 6 months diet with carbohydrates eaten mostly at dinner. Obesity (Silver Spring) 2011;19(10):2006–14. doi: 10.1038/oby.2011.48. [DOI] [PubMed] [Google Scholar]
- 18.Pakiz B, Flatt SW, Bardwell WA, et al. Effects of a weight loss intervention on body mass, fitness, and inflammatory biomarkers in overweight or obese breast cancer survivors. Int J Behav Med. 2011;18(4):333–41. doi: 10.1007/s12529-010-9079-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lien LF, Haqq AM, Arlotto M, et al. The stedman project: Biophysical, biochemical and metabolic effects of a behavioral weight loss intervention during weight loss, maintenance, and regain. OMICS. 2009;13(1):21–35. doi: 10.1089/omi.2008.0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Manigrasso MR, Ferroni P, Santilli F, et al. Association between circulating adiponectin and interleukin-10 levels in android obesity: Effects of weight loss. J Clin Endocrinol Metab. 2005;90(10):5876–9. doi: 10.1210/jc.2005-0281. [DOI] [PubMed] [Google Scholar]