Abstract
Background
Steatotic livers are vulnerable to the deleterious effects of ischaemia–reperfusion injury (IRI) that occur after hepatic surgery. Ischaemic preconditioning (IPC) has been shown to abrogate the effects of IRI in patients undergoing hepatic surgery. Experimental studies have suggested that IPC may be beneficial in steatotic livers subjected to IRI.
Objective
The aim of this systematic review was to evaluate the effects of IPC on steatotic livers following hepatic IRI in experimental models.
Methods
An electronic search of the OVID Medline and EMBASE databases was performed to identify studies that reported clinically relevant outcomes in animal models of hepatic steatosis subjected to IPC and IRI.
Results
A total of 1093 articles were identified, of which 18 met the inclusion criteria. There was considerable heterogeneity in the type of animal model, and duration and type of IRI. Increased macrovesicular steatosis (> 30%) was associated with a poor outcome following IRI. Ischaemic preconditioning was found to be beneficial in > 30% steatotic livers and provided for decreased histological damage, improved liver function findings and increased survival.
Conclusions
Experimental evidence supports the use of IPC in steatotic livers undergoing IRI. These findings may be applicable to patients undergoing liver surgery. However, clinical studies are required to validate the efficacy of IPC in this setting.
Introduction
Ischaemia–reperfusion injury (IRI) is a phenomenon whereby prolonged ischaemia and subsequent re-oxygenation lead to a pathophysiological injury process.1 It is a major cause of liver damage during hepatic surgery2,3 and occurs most frequently during liver transplantation and resection. In liver resection, in-flow vascular occlusion (the Pringle manoeuvre4) is used to decrease blood loss intraoperatively, but may lead to warm IRI; conversely, in orthotopic liver transplantation (OLT) the liver is subjected to cold–rewarming IRI when the donor liver is reperfused in the recipient.3 Ischaemia–reperfusion injury is associated with numerous downstream sequelae and may ultimately result in liver failure.2 This is particularly true in steatotic livers, for which increased rates of graft failure have been described in recipients of grafts with moderate-to-severe (≥ 30%) hepatic steatosis.5,6 Complication rates post-liver resection have likewise been reported to be two- or three-fold higher in this patient group.7,8
A number of techniques have been developed to ameliorate the detrimental physiological effects of IRI. One such strategy is ischaemic preconditioning (IPC), which was first described in a renal9 and subsequently in a cardiovascular10 model. Both cohorts demonstrated that a brief initial phase of ischaemia followed by reperfusion (preconditioning) prior to a period of prolonged ischaemia led to decreased tissue damage and enhanced functional outcomes. Following this, the effects of IPC have been investigated in other organ systems to assess whether it confers similar benefits.1 The effects of IPC on the liver were first described in 1993 with reference to the demonstration in an animal model that occlusion of hepatic inflow for 5 min followed by 10 min of reperfusion led to improved survival and hepatic function following 90 min of ischaemia.11 These findings have subsequently been replicated in human clinical studies.12,13
Conversely, the impact of IPC in steatotic livers is less well elucidated, especially in the setting of OLT. The efficacy of IPC in steatotic livers subjected to IRI was first described in 200014 and the procedure has subsequently become an integral strategy for attenuating IRI in experimental steatotic livers.15,16 There is limited research to support the role of IPC clinically,17 and indeed recent Cochrane reviews concluded that there is no evidence to support or refute the use of IPC in liver resection or transplantation and that further studies are necessary.18,19
Despite continued interest in the potential benefits of IPC, there has been no comprehensive overview of evidence evaluating its impact on outcomes following IRI in experimental models of hepatic steatosis. Defining this relationship is of critical importance in the formation of a framework for the further clinical development of IPC. Therefore, the aims of this study were to systematically review the literature and to provide a succinct description of the impact of IPC on outcomes in experimental studies of steatotic livers subjected to IRI.
Materials and methods
A systematic electronic search was conducted through the OVID Medline and EMBASE databases from their inception to September 2012 according to Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) recommendations.20,21 A combination of keyword searches (.mp) and MeSH terms (/) was used as follows: (ischaemic preconditioning.mp OR ischemic preconditioning.mp OR Ischemic preconditioning/) AND (liver.mp OR hepatic.mp OR Liver/ OR steatosis.mp OR exp Fatty liver/). The search was limited to English-language articles. There were no database-stipulated limits to ‘animal’ experimentation as recommended by SYRCLE guidelines.20 This ensured that publications describing work in either human or animal models would not be overlooked.
Inclusion criteria required that studies had investigated the use of IPC in hepatic IRI in any animal model. Studies were excluded if they: (i) did not represent original research (systematic reviews, narrative reviews, commentaries and editorials); (ii) included subjects with non-alcoholic steatohepatitis (NASH) rather than simple steatosis (NASH was defined as steatosis with hepatocellular injury and inflammation without fibrosis22), or (iii) did not report clinically relevant outcomes [graft or recipient survival, histological findings or liver function test (LFT) results].
The primary reviewer (MJJC) executed the searches, using titles and abstracts to manually screen identified articles. Eligibility was determined using a standardized proforma and data were subsequently extracted to an excel spreadsheet. Discrepancies were adjudicated independently by the senior author (ASJRB). Duplicates were excluded. Publications with overlapping study populations were filtered to include only the text with the largest number of subjects. Information obtained included type of animal model, severity and type of steatosis, duration of IPC, duration and type of hepatic IRI (partial/total, warm/cold) and outcome (recipient survival, histology or LFT results). Basic descriptive statistics were used to summarize data for this systematic review. Tabulated depictions of information were used when appropriate to facilitate ease of interpretation. For studies with incomplete study details or outcome measures, the corresponding author was contacted via e-mail for additional data. If data were presented graphically, the authors were contacted for numerical values and if these were not available, data were measured using digital image analysis software (ImageJ; http://imagej.nih.gov/ij/). Results are shown as the mean ± standard error of the mean (SEM).
The methodological quality of the included studies was assessed using a 15-point rating system described by Wever et al.23 This was tabulated separately for warm ischaemia–reperfusion and cold preservation–reperfusion injury. If there was no discrepancy between the number of animals described in the methods section and the number stated in the figure legends or the results section, it was assumed that no animals had been excluded from analysis. The potential for publication bias was assessed by a search for asymmetry in funnel plots for the different outcome measures.
Results
A total of 503 and 590 articles were identified in the Medline and EMBASE databases, respectively. After the exclusion of duplicates, 1043 abstracts were screened and 18 full-text papers were acquired for further evaluation. No additional studies were identified from manual searches of the reference lists. All 18 studies met the inclusion criteria (Fig. 1) and were included in the analysis. Among the 18 studies, 13 examined the impact of IPC in warm IRI15,16,24–34 (Table S1, online) and the remaining five studies investigated the impact of IPC in cold IRI35–39 (Table S2, online). Experiments in all 18 studies had been carried out using male rodents and none had imposed any delay or interval between the IPC stimulus and the IRI episode.
Figure 1.
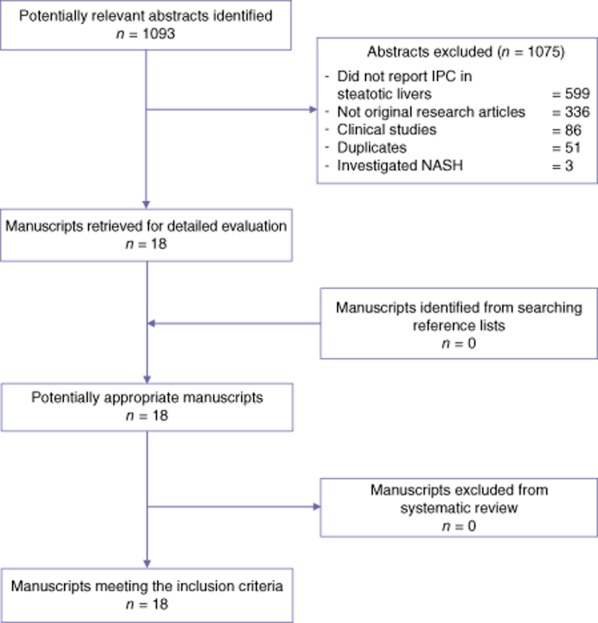
Quorum diagram. IPC, ischaemic preconditioning; NASH, non-alcoholic steatohepatitis
Warm IRI
Thirteen studies examined the effect of IPC on warm IRI in steatotic livers.15,16,24–34 The majority were performed in rats or mice (n = 12) and one had used a rabbit model. Eight studies used a genetic model of hepatic steatosis;15,24,27–30,32,33 four studies used dietary modifications so that steatosis was induced by either a choline-deficient diet (n = 2)31,34 or a high-cholesterol diet (n = 2).25,26 One study had used both a genetic model (ob/ob mouse) and choline-deficient diet-induced steatosis in C57 mice.16 Eleven of the 13 studies had included both sham (laparotomy only) and non-preconditioned steatotic livers as controls.15,24–33 The remaining two studies had used non-preconditioned steatotic livers as control livers.16,34
Moderate-to-severe (> 30%) steatosis was present in 12 of the 13 studies.15,16,24–31,33,34 One study reported mild (< 30%) steatosis.32 Five studies investigated a predominance of macrovesicular steatosis (MaS),24–26,32,33 five studies examined mixed hepatic steatosis,15,27–30 two studies investigated a predominance of microvesicular steatosis (MiS)31,34 and one study16 investigated both types of steatosis independently.
Eight studies used 5-min ischaemia and 10-min reperfusion (5 + 10) in their IPC protocol,24,31 three studies used 10-min ischaemia16,32,34 and 10-min reperfusion (10 + 10) and one study used 10-min ischaemia with 15-min reperfusion (10 + 15).33 One study investigated all three durations of IPC experiment and used non-preconditioned steatotic livers for comparison.15
All 13 studies used partial vascular occlusion to the median and left liver lobes to induce ischaemia to 70% of the liver. To assess the effect of IPC on survival rates, one study induced total hepatic ischaemia27 and another study performed partial vascular occlusion to 70% of the liver and resected the non-ischaemic lobes (30% of the liver) at the onset of reperfusion.15 The most common duration of warm ischaemia was 60 min (n = 7; range: 45–90 min). There was wide variation in the duration of reperfusion (from 30 min to 24 h); the most common duration was 24 h (seven of 13 studies). Studies that applied multiple reperfusion end-points used non-preconditioned steatotic livers as control material for each reperfusion end-point.15,16,24,28–30,34
Outcome measures included survival (n = 2) (Table 1), histological changes (n = 10) (Table 2) and LFT findings (n = 13) (Table 3). Assessment of histological injury based on a point-counting method15 was used in eight studies.15,24,27–30,32,33 Two studies assessed the percentage of hepatocellular necrosis as the histological end-point.16,34 Of the eight studies that described histological assessment utilizing a point-counting method, seven studies reported the percentage of Grade 3 necrosis within the livers15,24,27–30,33 and the remaining study reported the average histological injury score in each experimental group.32 In two of 10 studies, the histological assessment was blinded.32,33 Seven studies used a commercial enzymatic kit to measure serum LFTs15,24,25,27–30 and the remaining six studies used an automated biochemistry analyser.16,26,31–34
Table 1.
Survival outcomes in experimental models of warm ischaemia–reperfusion injury and hepatic steatosis with ischaemic preconditioning (IPC)
| Authors | Animal | Steatosis model | Steatosis, % | Type of steatosis | Duration of IPC, min | Duration of ischaemia, min (type of ischaemia) | Duration of reperfusion | Survival of preconditioned (non-preconditioned) steatotic livers |
|---|---|---|---|---|---|---|---|---|
| Serafin et al.27 | Rat | Genetic | 60–70% | Mixed | 5 + 10a | 60 (total) | ≤ 30 days | 70% (0%)b |
| Serafin et al.15 | Rat | Genetic | 60–70% | Mixed | 5 + 10a | 60 (total) | ≤ 30 days | 70% (0%)b |
Mixed, presence of both macrovesicular and microvesicular steatosis.
5 min total ischaemia + 10 min reperfusion.
P < 0.05 versus non-preconditioned steatotic livers.
Table 2.
Histological findings in experimental models of warm ischaemia–reperfusion injury and hepatic steatosis with ischaemic preconditioning (IPC)
| Authors | Animal | Steatosis model | Steatosis, % | Type of steatosis | Duration of IPC, min | Duration of ischaemia, min (type of ischaemia) | Duration of reperfusion | Results in preconditioned (non-preconditioned) steatotic livers |
|---|---|---|---|---|---|---|---|---|
| Steenks et al.32 | Rat | Genetic | < 30% | MaS | 10 + 10a | 45 (partial) | 60 min | Hepatic injury score: 1.6 ± 0.2 (1.3 ± 0.5) |
| Saidi et al.33 | Rat | Genetic | 30–60% | MaS | 10 + 15b | 75 (partial) | 180 min | Grade 3 necrosis: 15% (55%)d |
| Tacchini et al.24 | Rat | Genetic | 60–70% | MaS | 5 + 10c | 60 (partial) | 24 h | Grade 3 necrosis: 70 ± 10% (30 ± 5%)d |
| Serafin et al.27 | Rat | Genetic | 60–70% | Mixed | 5 + 10c | 60 (partial) | 360 min 24 h | Grade 3 necrosis: 10 ± 5% (35 ± 10%)d Grade 3 necrosis: 27.5 ± 5% (75 ± 5%)d |
| Casillas-Ramirez et al.28 | Rat | Genetic | 60–70% | Mixed | 5 + 10c | 60 (partial) | 24 h | Grade 3 necrosis: 30 ± 1% (74 ± 1%)d |
| Massip-Salcedo et al.29 | Rat | Genetic | 60–70% | Mixed | 5 + 10c | 60 (partial) | 24 h | Grade 3 necrosis: 28 ± 1% (74 ± 1%)d |
| Massip-Salcedo et al.30 | Rat | Genetic | 60–70% | Mixed | 5 + 10c | 60 (partial) | 360 min 24 h | Grade 3 necrosis: 12 ± 1% (35 ± 2%)d Grade 3 necrosis: 24 ± 1% (75 ± 2%)d |
| Serafin et al.15 | Rat | Genetic | 60–70% | Mixed | 5 + 10c | 60 (partial) | 24 h | Grade 3 necrosis: 30 ± 5% (74 ± 5%)d |
| Selzner et al.34 | Mouse | CDD | 70% | MiS | 10 + 10a | 75 (partial) | 24 h | Grade 3 necrosis: 35 ± 10% (70 ± 10%)d |
| Selzner et al.16 | Mouse | Genetic or CDD | 58% (genetic) 70% (CDD) | MaS MiS | 10 + 10a | 45 (partial) | 24 h | Grade 3 necrosis: 24 ± 3% (70 ± 8.5%)d Grade 3 necrosis: 6 ± 0.2% (26 ± 5%)d |
10 min total ischaemia + 10 min reperfusion.
5 min total ischaemia + 15 min reperfusion.
5 min total ischaemia + 10 min reperfusion.
P < 0.05 versus non-preconditioned steatotic livers (results shown as mean ± standard error of the mean).
CDD, choline-deficient diet; MaS, macrovesicular steatosis; MiS, microvesicular steatosis; Mixed, presence of both macrovesicular and microvesicular steatosis.
Table 3.
Liver function tests in experimental models of warm ischaemia–reperfusion injury and hepatic steatosis with to ischaemic preconditioning (IPC)
| Authors | Animal | Steatosis model | Steatosis, % | Type of steatosis | Duration of IPC, min | Duration of ischaemia, min (type of ischaemia) | Duration of reperfusion | Results in preconditioned (non-preconditioned) steatotic livers |
|---|---|---|---|---|---|---|---|---|
| Steenks et al.32 | Rat | Genetic | < 30% | MaS | 10 + 10a | 45 (partial) | 60 min | ALT: 4100 ± 1500 (2500 ± 200 U/l) AST: similar to ALT findings |
| Saidi et al.33 | Rat | Genetic | 30–60% | MaS | 10 + 15b | 75 (partial) | 180 min | AST: 3285 ± 122.3 (5436.3 ± 984.7 U/l)d |
| Koti et al.26 | Rat | HC | 30–60% | MaS | 5 + 10c | 45 (partial) | 120 min | ALT: 474.8 ± 122.3 (5436.3 ± 984.7 U/l)d AST: 630.8 ± 76.9 (3166.3 ± 379.6 U/l)d |
| Hafez et al.25 | Rabbit | HC | 40–60% | MaS | 5 + 10c | 60 (partial) | 420 min | ALT: 54 ± 14 (178 ± 34 IU/l)d AST: 199 ± 33 (406 ± 86 IU/l)d |
| Tacchini et al.24 | Rat | Genetic | 60–70% | MaS | 5 + 10c | 60 (partial) | 360 min 24 h | ALT: 675 ± 100 (400 ± 50 U/l)d ALT: 152 ± 51 (405 ± 101 U/l)d |
| Serafin et al.27 | Rat | Genetic | 60–70% | Mixed | 5 + 10c | 60 (partial) | 360 min | ALT: 205 ± 53 (1003 ± 152 U/l)d |
| Casillas-Ramirez et al28 | Rat | Genetic | 60–70% | Mixed | 5 + 10c | 60 (partial) | 24 h | ALT: 310 ± 49 (2708 ± 301 U/l)d |
| Massip-Salcedo et al.29 | Rat | Genetic | 60–70% | Mixed | 5 + 10c | 60 (partial) | 24 h | ALT: 408 ± 59 (2505 ± 310 U/l)d |
| Massip-Salcedo et al.30 | Rat | Genetic | 60–70% | Mixed | 5 + 10c | 60 (partial) | 360 min 24 h | ALT: 180 ± 21 (3502 ± 501 U/l)d ALT: 255 ± 110 (2502 ± 508 U/l)d |
| Serafin et al.15 | Rat | Genetic | 60–70% | Mixed | 5 + 10c 0 + 10a 10 + 15b |
60 (partial) | 24 h | ALT: 153 ± 23 (405 ± 75 U/l)d ALT: 370 ± 102 U/l ALT: 273 ± 25 U/ld |
| Rolo et al.31 | Rat | CDD | 70% | MiS2 | 5 + 10c | 90 (partial) | 720 min | ALT: 705 ± 18 (1801 ± 10 IU/l)d AST: 953 ± 102 (1802 ± 203 IU/l)d |
| Selzner et al.34 | Mouse | CDD | 70% | MiS | 10 + 10a | 75 (partial) | 240 min 24 h | AST: 8010 ± 1008 (15508 ± 501 U/l)d AST: 4008 (13510 ± 2050 U/l)d |
| Selzner et al.16 | Mouse | Genetic or CDD | 58% (genetic) 70% (CDD) | MaS MiS | 10 + 10a | 45 (partial) | 24 h | AST: 13620 ± 4692 (19940 ± 4471 U/l) AST: 3975 ± 869 (8025 ± 998 U/l)d |
10 min total ischaemia + 10 min reperfusion.
5 min total ischaemia + 15 min reperfusion.
5 min total ischaemia + 10 min reperfusion.
P < 0.05 versus non-preconditioned steatotic livers (results shown as mean ± standard error of the mean).
ALT, alanine aminotransferase; AST, aspartate aminotransferase; CDD, choline-deficient diet; HC, high cholesterol; MaS, macrovesicular steatosis; MiS, microvesicular steatosis; Mixed, presence of both macrovesicular and microvesicular steatosis.
The quality assessments of the included studies of warm IRI are shown in Table S3 (online). The average score reported was 9/15 (67 ± 7%), the lowest was 8/14 (57%) and the highest was 12/15 (80%). Only two studies reported the randomization of animals across treatment groups.30,32 There was no report of allocation concealment in either study. Of the 12 studies that reported histology outcome, only two (17%) stated that histological assessment was blinded.32,33 The other outcome measures were not blinded in any of the studies included. There were no reports of the exclusion of animals from analysis and only three of the 13 (23%) studies reported control of animal body temperature.25,26,33
Cold IRI
Five studies examined the effect of IPC and cold IRI in steatotic livers.35–39 All of these studies had been performed in a rodent genetic model of hepatic steatosis (Zucker fa/fa). Four studies36–39 used both sham (laparotomy only) and non-preconditioned steatotic livers and one used non-preconditioned steatotic livers as control livers.35 Four studies36–39 reported on moderate (30–60%) mixed steatosis and one study described minimal to no hepatic steatosis.35 Four studies used 5 + 10 IPC36–39 and one study used 10 + 10 IPC prior to cold ischaemia.35 Following cold ischaemia, livers were reperfused in vivo using an OLT model in all studies. The durations of cold ischaemia used were 240 min (n = 1) or 360 min (n = 4). The organs were flushed with University of Wisconsin (Madison, WI, USA) solution in all studies and stored for the duration of cold ischaemia at 4 °C on ice.
Outcome measures included survival (n = 2) (Table 4), histology (n = 4) (Table 5) and LFT findings (n = 4) (Table 6). All four of the studies that assessed histological injury used a point-counting method15 and none of the observers were blinded. Two of the studies reported only the severity of necrosis and two studies reported average histological injury scores. In all four studies, commercial enzymatic kits were used to measure liver function.
Table 4.
Survival outcome in experimental models of orthotopic liver transplantation and hepatic steatosis with ischaemic preconditioning (IPC)
| Authors | Animal | Steatosis model | Steatosis, % | Type of steatosis | Duration of IPC, min | Duration of ischaemia, min (perfusate) | Duration of reperfusion | Survival of preconditioned (non-preconditioned) steatotic livers |
|---|---|---|---|---|---|---|---|---|
| Niemann et al.35 | Rat | Genetic | < 10% | Minimal | 10 + 10a | 240 (UW) | 24 h | 87.5% (25%)c |
| Jimenez-Castro et al.36 | Rat | Genetic | 40–60% | Mixed | 5 + 10b | 360 (UW) | 14 days | 70% (30%)c |
10 min total ischaemia + 10 min reperfusion.
5 min total ischaemia + 10 min reperfusion.
P < 0.05 versus non-preconditioned steatotic livers.
Mixed, presence of both macrovesicular and microvesicular steatosis; UW, University of Wisconsin solution.
Table 5.
Histological findings in experimental models of orthotopic liver transplantation and hepatic steatosis with ischaemic preconditioning (IPC)
| Authors | Animal | Steatosis model | Steatosis, % | Type of steatosis | Duration of IPC, min | Duration of ischaemia, min (perfusate) | Duration of reperfusion | Results in preconditioned (non-preconditioned) steatotic livers |
|---|---|---|---|---|---|---|---|---|
| Jimenez-Castro et al.36 | Rat | Genetic | 40–60% | Mixed | 5 + 10a | 360 (UW) | 240 min | Hepatic injury score: 1.4 ± 0.2 (3.8 ± 0.1)b |
| Casillas-Ramirez et al.37 | Rat | Genetic | 40–60% | Mixed | 5 + 10a | 360 (UW) | 240 min | Hepatic injury score: 1.4 ± 0.2 (3.8 ± 0.1)b |
| Carrasco-Chaumel et al.38 | Rat | Genetic | 40–60% | Mixed | 5 + 10a | 360 (UW) | 240 min | Grade 3 necrosis: 25 ± 1% (65 ± 1%)b |
| Fernandez et al.39 | Rat | Genetic | 40–60% | Mixed | 5 + 10a | 360 (UW) | 240 min | ↓Severity of necrosis |
5 min total ischaemia + 10 min reperfusion.
P < 0.05 versus non-preconditioned steatotic livers (results shown as mean ± standard error of the mean).
Mixed, presence of both macrovesicular and microvesicular steatosis; UW, University of Wisconsin solution.
Table 6.
Liver function tests in experimental models of orthotopic liver transplantation and hepatic steatosis with ischaemic preconditioning (IPC)
| Authors | Animal | Steatosis model | Steatosis, % | Type of steatosis | Duration of IPC, min | Duration of ischaemia, min (perfusate) | Duration of reperfusion | Results in preconditioned (non-preconditioned) steatotic livers |
|---|---|---|---|---|---|---|---|---|
| Jimenez-Castro et al.36 | Rat | Genetic | 40–60% | Mixed | 5 + 10a | 360 (UW) | 240 min | ALT: 1100 ± 50 (4200 ± 450 IU/l)b AST: 1200 ± 100 (3300 ± 400 IU/l)b |
| Casillas-Ramirez et al.37 | Rat | Genetic | 40–60% | Mixed | 5 + 10a | 360 (UW) | 240 min | ALT: 1050 ± 100 (4350 ± 500 IU/l)b AST: 1250 ± 150 (3400 ± 550 IU/l)b |
| Carrasco-Chaumel et al.38 | Rat | Genetic | 40–60% | Mixed | 5 + 10a | 360 (UW) | 240 min | ALT: 500 ± 100 (2800 ± 500 U/l)b AST: 500 ± 50 (3250 ± 500 U/l)b |
| Fernandez et al.39 | Rat | Genetic | 40–60% | Mixed | 5 + 10a | 360 (UW) | 240 min | ALT: 600 ± 100 (3000 ± 300 U/l)b AST: 350 ± 50 (4000 ± 600 U/l)b |
5 min total ischaemia + 10 min reperfusion.
P < 0.05 versus non-preconditioned steatotic livers (results shown as mean ± standard error of the mean).
ALT, alanine aminotransferase; AST, aspartate aminotransferase; Mixed, presence of both macrovesicular and microvesicular steatosis; UW, University of Wisconsin solution.
The quality assessments of the included studies for cold IRI are shown in Table S4 (online). The average score reported was 10/14 (74 ± 7%); four studies at the lower end scored 10/14 (71%) and one study achieved a high score of 12/14 (86%). All five studies randomized animals across the treatment groups, but only one reported the concealment of allocation in the study; furthermore, only one study stated that histological assessment was blinded. No animals were excluded from analysis and none of the five studies reported the control of animal body temperature.
Analysis
Impact of IPC in steatotic livers subjected to warm IRI
Both of the studies that reported on mortality demonstrated increased 30-day survival in animals that were subjected to 5 + 10 preconditioning compared with non-preconditioned animals (Table 1).15,27 Histologically, preconditioning was associated with decreased necrosis compared with that in non-preconditioned steatotic livers (Table 2).15,16,24,27–30,33,34 In concordance with histological findings, LFT [alanine aminotransferase/aspartate aminotransferase (ALT/AST)] results were lower in preconditioned animals than in non-preconditioned animals (Table 3).15,24–31,33,34 One study showed no difference in post-reperfusion ALT between animals subjected to 10 + 10 preconditioning and non-preconditioned animals15 and another showed decreased AST in preconditioned animals with MiS but only a trend towards decreased AST in animals with MaS.16 A single study found evidence of increased histological damage and reduced liver function in preconditioned steatotic livers compared with non-preconditioned steatotic livers.32 Funnel plot assessment demonstrated that negative studies appeared to be under-represented for histology and LFT results (data not shown).
Impact of IPC in steatotic livers subjected to cold IRI
In both studies that reported mortality, recipients of preconditioned steatotic grafts achieved significantly improved rates of survival compared with recipients of non-preconditioned steatotic grafts (Table 4).35,36 Likewise, recipients of preconditioned steatotic grafts achieved lower LFT results and showed decreased necrosis on histology (Tables 5 and 6).36–39 There were no negative findings on the effects of IPC on steatotic livers subjected to cold IRI.
Discussion
Ischaemia–reperfusion injury initiates a sequence of events that lead to cellular damage2 and is a major cause of morbidity and mortality in liver surgery. During liver resection, the Pringle manoeuvre is used to decrease blood loss, but it induces IRI that may impair liver regeneration.40 In OLT, it is mandatory for the organ to undergo IRI in the form of cold ischaemia during organ preservation and subsequent warm reperfusion.3 This has led to the development of strategies such as IPC to mitigate the deleterious effects of IRI. Although IPC has been shown to be protective against IRI,1 its impact on steatotic livers subjected to IRI is less evident. Given that hepatic steatosis is associated with consistently poorer functional outcomes following liver surgery and an increased susceptibility to IRI,6,8 there is a need for effective strategies to reduce IRI in steatotic livers. The present review demonstrates that in experimental models of hepatic steatosis, animals with preconditioned livers have improved outcomes following IRI, with increased survival, decreased histological damage and improved liver function.
The majority of studies identified in this review used genetically modified animals to model hepatic steatosis. However, the pathophysiological defect used to induce this condition (leptin or leptin receptor deficiency) does not accurately reflect the aetiology that underpins clinical hepatic steatosis.41 Similarly, animals fed on a choline-deficient diet developed significant weight loss and insulin sensitivity and therefore do not represent a true reflection of this condition.41 It is proposed that a high-fat and high-carbohydrate diet model would most closely emulate steatosis in the clinical setting. Indeed, this was most closely approximated in two of the studies identified, which used a high-cholesterol diet.25,26 However, it is acknowledged that a single model encompassing the full characteristics of human hepatic steatosis remains elusive and future studies must pay close consideration to animal diet to ensure clinical relevance.
Hepatic steatosis is categorized as mild, moderate or severe according to whether < 30%, 30–60% or > 60%, respectively, of hepatocytes contain cytoplasmic fat vacuoles.42 Additionally, hepatic steatosis is described qualitatively according to the type (MaS or MiS) of steatosis present: MaS is thought to be associated with metabolic syndrome or alcohol abuse, and MiS is typically related to toxins or metabolic disorders.42 The presence of MaS alone is rare, and usually both types of steatosis are present.42 In the 18 studies identified, moderate-to-severe steatosis was present in 15 studies and mixed hepatic steatosis was present in 12 studies. Histological heterogeneity among studies makes the generalization of data difficult and there continues to be much controversy on the propriety of individual staining methods and whether histological diagnosis represents a reference-standard method.43 This has remained a major obstacle in efforts to make detailed comparisons between experimental and clinical models of IRI.
The studies referred to in this review used three different durations of IPC, although the majority of studies (n = 12) used a 5 + 10 IPC protocol. This is perhaps not as concerning as the histological differences among the studies because it reflects the variability with which IPC is utilized clinically.12,44 Similarly, the duration of warm ischaemia was variable (45–90 min), but all studies used partial vascular occlusion as the preferred method of inducing warm IRI. Studies that assessed survival used total hepatic ischaemia.15,27 In the clinical setting, total in-flow occlusion is the method most commonly utilized in liver surgery, but this is poorly tolerated in rodents as a result of splanchnic congestion and potential confounding from early bowel ischaemia and consequent haemodynamic instability. Furthermore, there was large variation in the duration of reperfusion in the studies of warm IRI (30 min to 24 h). In studies of cold IRI, there was similar variability in the duration of ischaemia and reperfusion. The differences in duration of IPC, method and duration of IRI appear to be based on laboratory experience and preference, but this precludes the objective comparison of data among studies. Although it would be expedient to standardize IPC and IRI protocols in future studies, it is acknowledged that multiple factors must be considered in the selection of an experimental model of IRI.45
Survival rates of severely steatotic livers subjected to warm IRI were very poor: none survived 60 min of total hepatic ischaemia.15,27 This is consistent with clinical observations, in which patients with > 30% steatosis have an increased risk for postoperative morbidity and mortality.7,8 However, 5 + 10 IPC improved survival rates to 70% in both of the studies that used this protocol,15,27 indicating that IPC was able to decrease liver damage and preserve the functional capacity of the steatotic liver. Moreover, survival rates of recipients of transplanted non-preconditioned steatotic livers were very low and IPC significantly improved survival rates.35,36 This mirrors the findings of Jassem et al.,46 who reported that IPC in deceased donor allografts was associated with a promotion of genes involved in cellular protection and repair.
The use of IPC in steatotic livers was also associated with decreased histological damage and improved liver function. Thirteen studies investigated warm IRI and reported LFT findings. Twelve of these studies reported that IPC was associated with decreases in ALT and AST compared with levels in non-preconditioned steatotic livers15,16,24–31,33,34 and the remaining study found increased transaminases in preconditioned steatotic livers compared with non-preconditioned livers.32 In the four studies of cold IRI in which LFT data were reported, IPC was associated with decreased transaminases in preconditioned steatotic livers.36–39 Interestingly, one study showed that in warm IRI, 10 + 15 and 5 + 10 IPC protocols were associated with lower ALT, but 10 + 10 IPC was not.15 With regard to the impact of IPC on the type of steatosis, Selzner et al. showed a significant benefit of 10 + 10 IPC in MiS, but found the improvement was not statistically significant in MaS.16 Importantly, this study16 was performed using a mouse model; none of the studies made a direct comparison between MiS and MaS using a rat or large animal model. (However, other studies clearly showed a significant benefit of IPC in MaS.24–26) Consistent with these LFT findings, histological assessment showed that IPC led to decreased necrosis post-warm IRI in nine studies15,16,24,27–30,33,34 and one study showed a non-significant trend towards increased hepatic injury.32 Similar findings were reported in preconditioned steatotic livers subjected to cold IRI with decreased necrosis compared with non-preconditioned livers.36–39
The methodological qualities of the animal studies included in this review were comparable with those of a similar recently published systematic review.23 However, there are a few aspects that might be improved to better align with the principles of good scientific practice and to decrease bias. Firstly, a distinct lack of randomization was observed in studies investigating warm IRI, which was compounded by the absence of outcome assessment blinding in both IRI models. There was also no extraneous control of animal body temperature. These issues diminish the scientific and translational value of affected studies. Secondly, there is little consensus on the optimal animal model for this particular field of research, which is clearly apparent in the considerable variation in both the animal models of hepatic steatosis and the histological reporting of steatosis observed in this review. This significant heterogeneity (in animals used, types and models of hepatic steatosis, durations of IPC, ischaemia and reperfusion, and outcome measures) limits the ability to uniformly appraise and meta-analyse data, and instead forces greater focus on study conclusions. There is a clear need for the better reporting of outcomes in animal studies in this field in order to provide a common platform of results and to minimize potential bias in future studies. Finally, the under-representation of negative findings in this field may be attributed to publication bias resulting from a reluctance to publish negative results or the lack of a forum in which to do so. It is hoped that this systematic review will serve as a stimulus for the stipulation that future animal studies should apply methodological standards similar to those required in clinical research.
The articles identified in this systematic review demonstrate that IPC was able to attenuate the negative effects of IRI in steatotic livers as evidenced by decreased histological damage, less hepatocyte injury and improved survival in preconditioned livers. These studies also confirmed that > 30% steatotic livers were more susceptible to warm and cold IRI, which is consistent with clinical observations.6,7 This review demonstrates that IPC may potentially enlarge the available organ donor pool by conferring protective properties to marginal donor grafts. To date, the most commonly used duration of IPC in the clinical environment is 10 + 10 min17 and it is recommended that future experimental studies should use a protocol aligned with this. Similarly, the duration of cold ischaemia in the studies identified was relatively short and a recent study has recommended that organs with > 30% steatosis can be used in OLT if other factors (donor age < 40 years, cold ischaemic time of < 5 h, non-circulatory cause of death) are controlled.47 Although clinical studies have shown minimal to no benefit of IPC in steatotic livers subjected to cold ischaemia of > 6 h,48,49 these findings should be replicated in experimental studies to better elucidate the underlying mechanism of IPC in the prolonged cold storage of steatotic livers. The mechanisms underpinning the effects of IPC have been reviewed extensively elsewhere.1 Both experimental and clinical studies have implicated a complex set of mediators and pathways, but the exact nature of the relationships among these factors remains unknown. Additionally, there have been conflicting clinical results of IPC in warm IRI14,50 and transplantation.51,52 This has led to ongoing debate about the utility of IPC in liver surgery, but the clinical effect of IPC in steatotic livers is not well described. Further studies of IPC in steatotic livers in a clinical context are required and experimental studies should be performed in an appropriate manner in order to increase current understanding of this complex relationship.
Conclusions
The evidence from this systematic review suggests that IPC confers a protective effect in experimental steatotic livers subjected to IRI and results in improved survival, decreased histological injury and an improved liver enzyme profile. Conflicting results with the use of IPC during clinical trials of hepatic surgery should lead to caution in any routine clinical application. Future experimental studies should ideally emulate the clinical environment, in terms of hepatic model and method of performing IRI, to further improve understanding of the mechanism underlying IPC. This may, in turn, lead to the identification of a subset of patients who may particularly benefit from IPC. Concurrent use of pharmacological therapy and IPC may ultimately yield the greatest improvement in patient outcomes, especially in the setting of hepatic steatosis.
Conflicts of interest
None declared.
Supporting Information
Additional supporting information may be found in the online version of this article at the publisher's website.
Table S1. Study details of experimental models of warm ischaemia–reperfusion injury and hepatic steatosis subjected to ischaemic preconditioning.
Table S2. Study details of experimental models of orthotopic liver transplantation and hepatic steatosis subjected to ischaemic preconditioning.
Table S3. Methodological quality of articles examining the effect of ischaemic preconditioning in warm ischaemia–reperfusion injury.
Table S4. Methodological quality of articles examining the effect of ischaemic preconditioning in orthotopic liver transplantation.
References
- Montalvo-Jave EE, Pina E, Montalvo-Arenas C, Urrutia R, Benavente-Chenhalls L, Pena-Sanchez J, et al. Role of ischemic preconditioning in liver surgery and hepatic transplantation. J Gastrointest Surg. 2009;13:2074–2083. doi: 10.1007/s11605-009-0878-7. [DOI] [PubMed] [Google Scholar]
- Fondevila C, Busuttil RW, Kupiec-Weglinski JW. Hepatic ischemia/reperfusion injury – a fresh look. Exp Mol Pathol. 2003;74:86–93. doi: 10.1016/s0014-4800(03)00008-x. [DOI] [PubMed] [Google Scholar]
- de Rougemont O, Dutkowski P, Clavien PA. Biological modulation of liver ischemia–reperfusion injury. Curr Opin Organ Transplant. 2010;15:183–189. doi: 10.1097/MOT.0b013e3283373ced. [DOI] [PubMed] [Google Scholar]
- Pringle JH. Notes on the arrest of hepatic hemorrhage due to trauma. Ann Surg. 1908;48:541–549. doi: 10.1097/00000658-190810000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adam R, Reynes M, Johann M, Morino M, Astarcioglu I, Kafetzis I, et al. The outcome of steatotic grafts in liver transplantation. Transplant Proc. 1991;23(1 Pt 2):1538–1540. [PubMed] [Google Scholar]
- Ploeg RJ, D'Alessandro AM, Knechtle SJ, Stegall MD, Pirsch JD, Hoffmann RM, et al. Risk factors for primary dysfunction after liver transplantation – a multivariate analysis. Transplantation. 1993;55:807–813. doi: 10.1097/00007890-199304000-00024. [DOI] [PubMed] [Google Scholar]
- Belghiti J, Hiramatsu K, Benoist S, Massault PP, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191:38–46. doi: 10.1016/s1072-7515(00)00261-1. [DOI] [PubMed] [Google Scholar]
- Behrns KE, Tsiotos GG, DeSouza NF, Krishna MK, Ludwig J, Nagorney DM. Hepatic steatosis as a potential risk factor for major hepatic resection. J Gastrointest Surg. 1998;2:292–298. doi: 10.1016/s1091-255x(98)80025-5. [DOI] [PubMed] [Google Scholar]
- Zager RA, Baltes LA, Sharma HM, Jurkowitz MS. Responses of the ischemic acute renal failure kidney to additional ischemic events. Kidney Int. 1984;26:689–700. doi: 10.1038/ki.1984.204. [DOI] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Lloris-Carsi JM, Cejalvo D, Toledo-Pereyra LH, Calvo MA, Suzuki S. Preconditioning: effect upon lesion modulation in warm liver ischemia. Transplant Proc. 1993;25:3303–3304. [PubMed] [Google Scholar]
- Clavien PA, Selzner M, Rüdiger HA, Graf R, Kadry Z, Rousson V, et al. A prospective randomized study in 100 consecutive patients undergoing major liver resection with versus without ischemic preconditioning. Ann Surg. 2003;238:843–852. doi: 10.1097/01.sla.0000098620.27623.7d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jassem W, Fuggle SV, Cerundolo L, Heaton ND, Rela M. Ischemic preconditioning of cadaver donor livers protects allografts following transplantation. Transplantation. 2006;81:169–174. doi: 10.1097/01.tp.0000188640.05459.37. [DOI] [PubMed] [Google Scholar]
- Clavien PA, Yadav S, Sindram D, Bentley RC. Protective effects of ischemic preconditioning for liver resection performed under inflow occlusion in humans. Ann Surg. 2000;232:155–162. doi: 10.1097/00000658-200008000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafin A, Rosello-Catafau J, Prats N, Xaus C, Gelpi E, Peralta C. Ischemic preconditioning increases the tolerance of fatty liver to hepatic ischemia–reperfusion injury in the rat. Am J Pathol. 2002;161:587–601. doi: 10.1016/S0002-9440(10)64214-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selzner N, Selzner M, Jochum W, Amann-Vesti B, Graf R, Clavien PA. Mouse livers with macrosteatosis are more susceptible to normothermic ischemic injury than those with microsteatosis. J Hepatol. 2006;44:694–701. doi: 10.1016/j.jhep.2005.07.032. [DOI] [PubMed] [Google Scholar]
- DeOliveira ML, Graf R, Clavien PA. Ischemic preconditioning: promises from the laboratory to patients – sustained or disillusioned? Am J Transplant. 2008;8:489–491. doi: 10.1111/j.1600-6143.2007.02091.x. [DOI] [PubMed] [Google Scholar]
- Gurusamy KS, Kumar Y, Pamecha V, Sharma D, Davidson BR. Ischaemic pre-conditioning for elective liver resections performed under vascular occlusion. Cochrane Database Syst Rev. 2009;(1) doi: 10.1002/14651858.CD007629. CD007629. [DOI] [PubMed] [Google Scholar]
- Gurusamy KS, Kumar Y, Sharma D, Davidson BR. Ischaemic preconditioning for liver transplantation. Cochrane Database Syst Rev. 2008;(1) doi: 10.1002/14651858.CD006315.pub2. CD006315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooijmans CR, Tillema A, Leenaars M, Ritskes-Hoitinga M. Enhancing search efficiency by means of a search filter for finding all studies on animal experimentation in PubMed. Lab Anim. 2010;44:170–175. doi: 10.1258/la.2010.009117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leenaars M, Hooijmans CR, van Veggel N, ter Riet G, Leeflang M, Hooft L, et al. A step-by-step guide to systematically identify all relevant animal studies. Lab Anim. 2012;46:24–31. doi: 10.1258/la.2011.011087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunt EM, Tiniakos DG. Histopathology of nonalcoholic fatty liver disease. World J Gastroenterol. 2010;16:5286–5296. doi: 10.3748/wjg.v16.i42.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wever KE, Menting TP, Rovers M, van der Vliet JA, Rongen GA, Masereeuw R, et al. Ischemic preconditioning in the animal kidney, a systematic review and meta-analysis. PLoS ONE. 2012;7:e32296. doi: 10.1371/journal.pone.0032296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacchini L, Cairo G, De Ponti C, Massip M, Rosello-Catafau J, Peralta C. Upregulation of IL-6 by ischemic preconditioning in normal and fatty rat livers: association with reduction of oxidative stress. Free Radic Res. 2006;40:1206–1217. doi: 10.1080/10715760600885432. [DOI] [PubMed] [Google Scholar]
- Hafez TS, Glantzounis GK, Fusai G, Taanman JW, Wignarajah P, Parkes H, et al. Intracellular oxygenation and cytochrome oxidase C activity in ischemic preconditioning of steatotic rabbit liver. Am J Surg. 2010;200:507–518. doi: 10.1016/j.amjsurg.2009.09.028. [DOI] [PubMed] [Google Scholar]
- Koti RS, Yang W, Glantzounis G, Quaglia A, Davidson BR, Seifalian AM. Effect of ischaemic preconditioning on hepatic oxygenation, microcirculation and function in a rat model of moderate hepatic steatosis. Clin Sci (Colch) 2005;108:55–63. doi: 10.1042/CS20040130. [DOI] [PubMed] [Google Scholar]
- Serafin A, Rosello-Catafau J, Prats N, Gelpi E, Rodes J, Peralta C. Ischemic preconditioning affects interleukin release in fatty livers of rats undergoing ischemia/reperfusion. Hepatology. 2004;39:688–698. doi: 10.1002/hep.20089. [DOI] [PubMed] [Google Scholar]
- Casillas-Ramirez A, Amine-Zaouali M, Massip-Salcedo M, Padrissa-Altes S, Bintanel-Morcillo M, Ramalho F, et al. Inhibition of angiotensin II action protects rat steatotic livers against ischemia–reperfusion injury. Crit Care Med. 2008;36:1256–1266. doi: 10.1097/CCM.0b013e31816a023c. [DOI] [PubMed] [Google Scholar]
- Massip-Salcedo M, Zaouali MA, Padrissa-Altes S, Casillas-Ramirez A, Rodes J, Rosello-Catafau J, et al. Activation of peroxisome proliferator-activated receptor-α inhibits the injurious effects of adiponectin in rat steatotic liver undergoing ischemia–reperfusion. Hepatology. 2008;47:461–472. doi: 10.1002/hep.21935. [DOI] [PubMed] [Google Scholar]
- Massip-Salcedo M, Casillas-Ramirez A, Franco-Gou R, Bartrons R, Ben Mosbah I, Serafin A, et al. Heat shock proteins and mitogen-activated protein kinases in steatotic livers undergoing ischemia–reperfusion: some answers. Am J Pathol. 2006;168:1474–1485. doi: 10.2353/ajpath.2006.050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolo AP, Teodoro JS, Peralta C, Rosello-Catafau J, Palmeira CM. Prevention of I/R injury in fatty livers by ischemic preconditioning is associated with increased mitochondrial tolerance: the key role of ATPsynthase and mitochondrial permeability transition. Transpl Int. 2009;22:1081–1090. doi: 10.1111/j.1432-2277.2009.00916.x. [DOI] [PubMed] [Google Scholar]
- Steenks M, van Baal MCPM, Nieuwenhuijs VB, de Bruijn MT, Schiesser M, Teo MH, et al. Intermittent ischaemia maintains function after ischaemia reperfusion in steatotic livers. HPB. 2010;12:250–261. doi: 10.1111/j.1477-2574.2010.00160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saidi RF, Chang J, Brooks S, Nalbantoglu I, Adsay V, Jacobs MJ. Ischemic preconditioning and intermittent clamping increase the tolerance of fatty liver to hepatic ischemia–reperfusion injury in the rat. Transplant Proc. 2007;39:3010–3014. doi: 10.1016/j.transproceed.2007.09.044. [DOI] [PubMed] [Google Scholar]
- Selzner N, Selzner M, Jochum W, Clavien PA. Ischemic preconditioning protects the steatotic mouse liver against reperfusion injury: an ATP dependent mechanism. J Hepatol. 2003;39:55–61. doi: 10.1016/s0168-8278(03)00147-8. [DOI] [PubMed] [Google Scholar]
- Niemann CU, Hirose R, Liu T, Behrends M, Brown JL, Kominsky DF, et al. Ischemic preconditioning improves energy state and transplantation survival in obese Zucker rat livers. Anesth Analg. 2005;101:1577–1583. doi: 10.1213/01.ANE.0000184897.53609.2A. [DOI] [PubMed] [Google Scholar]
- Jimenez-Castro MB, Casillas-Ramirez A, Massip-Salcedo M, Elias-Miro M, Serafin A, Rimola A, et al. Cyclic adenosine 3’,5'-monophosphate in rat steatotic liver transplantation. Liver Transpl. 2011;17:1099–1110. doi: 10.1002/lt.22359. [DOI] [PubMed] [Google Scholar]
- Casillas-Ramirez A, Alfany-Fernandez I, Massip-Salcedo M, Juan ME, Planas JM, Serafin A, et al. Retinol-binding protein 4 and peroxisome proliferator-activated receptor-γ in steatotic liver transplantation. J Pharmacol Exp Ther. 2011;338:143–153. doi: 10.1124/jpet.110.177691. [DOI] [PubMed] [Google Scholar]
- Carrasco-Chaumel E, Rosello-Catafau J, Bartrons R, Franco-Gou R, Xaus C, Casillas A, et al. Adenosine monophosphate-activated protein kinase and nitric oxide in rat steatotic liver transplantation. J Hepatol. 2005;43:997–1006. doi: 10.1016/j.jhep.2005.05.021. [DOI] [PubMed] [Google Scholar]
- Fernandez L, Carrasco-Chaumel E, Serafin A, Xaus C, Grande L, Rimola A, et al. Is ischemic preconditioning a useful strategy in steatotic liver transplantation? Am J Transplant. 2004;4:888–899. doi: 10.1111/j.1600-6143.2004.00447.x. [DOI] [PubMed] [Google Scholar]
- Selzner M, Camargo CA, Clavien PA. Ischemia impairs liver regeneration after major tissue loss in rodents: protective effects of interleukin-6. Hepatology. 1999;30:469–475. doi: 10.1002/hep.510300215. [DOI] [PubMed] [Google Scholar]
- Larter CZ, Yeh MM. Animal models of NASH: getting both pathology and metabolic context right. J Gastroenterol Hepatol. 2008;23:1635–1648. doi: 10.1111/j.1440-1746.2008.05543.x. [DOI] [PubMed] [Google Scholar]
- McCormack L, Dutkowski P, El-Badry AM, Clavien P-A. Liver transplantation using fatty livers: always feasible? J Hepatol. 2011;54:1055–1062. doi: 10.1016/j.jhep.2010.11.004. [DOI] [PubMed] [Google Scholar]
- El-Badry AM, Breitenstein S, Jochum W, Washington K, Paradis V, Rubbia-Brandt L, et al. Assessment of hepatic steatosis by expert pathologists: the end of a gold standard. Ann Surg. 2009;250:691–697. doi: 10.1097/SLA.0b013e3181bcd6dd. [DOI] [PubMed] [Google Scholar]
- Desai KK, Dikdan GS, Shareef A, Koneru B. Ischemic preconditioning of the liver: a few perspectives from the bench to bedside translation. Liver Transpl. 2008;14:1569–1577. doi: 10.1002/lt.21630. [DOI] [PubMed] [Google Scholar]
- Mendes-Braz M, Elias-Miro M, Jimenez-Castro MB, Casillas-Ramirez A, Ramalho FS, Peralta C. The current state of knowledge of hepatic ischemia–reperfusion injury based on its study in experimental models. J Biomed Biotechnol. 2012;2012:298657. doi: 10.1155/2012/298657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jassem W, Fuggle S, Thompson R, Arno M, Taylor J, Byrne J, et al. Effect of ischemic preconditioning on the genomic response to reperfusion injury in deceased donor liver transplantation. Liver Transpl. 2009;15:1750–1765. doi: 10.1002/lt.21936. [DOI] [PubMed] [Google Scholar]
- Spitzer AL, Lao OB, Dick AAS, Bakthavatsalam R, Halldorson JB, Yeh MM, et al. The biopsied donor liver: incorporating macrosteatosis into high-risk donor assessment. Liver Transpl. 2010;16:874–884. doi: 10.1002/lt.22085. [DOI] [PubMed] [Google Scholar]
- Degli Esposti D, Sebagh M, Pham P, Reffas M, Pous C, Brenner C, et al. Ischemic preconditioning induces autophagy and limits necrosis in human recipients of fatty liver grafts, decreasing the incidence of rejection episodes. Cell Death Dis. 2011;2:e111. doi: 10.1038/cddis.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koneru B, Fisher A, He Y, Klein KM, Skurnick J, Wilson DJ, et al. Ischemic preconditioning in deceased donor liver transplantation: a prospective randomized clinical trial of safety and efficacy. Liver Transpl. 2005;11:196–202. doi: 10.1002/lt.20315. [DOI] [PubMed] [Google Scholar]
- Azoulay D, Lucidi V, Andreani P, Maggi U, Sebagh M, Ichai P, et al. Ischemic preconditioning for major liver resection under vascular exclusion of the liver preserving the caval flow: a randomized prospective study. J Am Coll Surg. 2006;202:203–211. doi: 10.1016/j.jamcollsurg.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Koneru B, Shareef A, Dikdan G, Desai K, Klein KM, Peng B, et al. The ischemic preconditioning paradox in deceased donor liver transplantation – evidence from a prospective randomized single blind clinical trial. Am J Transplant. 2007;7:2788–2796. doi: 10.1111/j.1600-6143.2007.02009.x. [DOI] [PubMed] [Google Scholar]
- Cescon M, Grazi GL, Grassi A, Ravaioli M, Vetrone G, Ercolani G, et al. Effect of ischemic preconditioning in whole liver transplantation from deceased donors. A pilot study. Liver Transpl. 2006;12:628–635. doi: 10.1002/lt.20640. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Study details of experimental models of warm ischaemia–reperfusion injury and hepatic steatosis subjected to ischaemic preconditioning.
Table S2. Study details of experimental models of orthotopic liver transplantation and hepatic steatosis subjected to ischaemic preconditioning.
Table S3. Methodological quality of articles examining the effect of ischaemic preconditioning in warm ischaemia–reperfusion injury.
Table S4. Methodological quality of articles examining the effect of ischaemic preconditioning in orthotopic liver transplantation.


