Abstract
Background
Enhanced recovery after surgery (ERAS) protocols have been shown to reduce hospital stay without compromising outcomes. Attempts to apply ERAS principles in the context of pancreatic surgery have generated encouraging results. A systematic review of the current evidence for ERAS following pancreatic surgery was conducted.
Methods
A literature search of MEDLINE, CINAHL, EMBASE and the Cochrane Library was performed for articles describing postoperative clinical pathways in pancreatic surgery during the years 2000–2013. The keywords ‘clinical pathway’, ‘critical pathway’, ‘fast-track’, ‘pancreas’ and ‘surgery’ and their synonyms were used as search terms. Articles were selected for inclusion based on predefined criteria and ranked for quality. Details of the ERAS protocols and relevant outcomes were extracted and analysed.
Results
Ten articles describing an ERAS protocol in pancreatic surgery were identified. The level of evidence was graded as low to moderate. No articles reported an adverse effect of an ERAS protocol for pancreatic surgery on perioperative morbidity or mortality. Length of stay (LoS) was decreased and readmission rates were found to be unchanged in six of seven studies that compared these outcomes.
Conclusions
Evidence indicates that ERAS protocols may be implemented in pancreatic surgery without compromising patient safety or increasing LoS. Enhanced recovery after surgery programmes in the context of pancreatic surgery should be standardized based upon the best available evidence, and trials of ERAS programmes involving multiple centres should be performed.
Introduction
Pancreatic surgery has undergone immense transformations in the century since it was first performed.1 The initial perioperative mortality rate of 30% has decreased to 2% at high-volume centres, and perioperative morbidity has decreased to 30%.2 Rates of readmission to hospital within 30 days of discharge range from 15% to 59%; one recent publication reported a 30-day readmission rate of 18%.3 Reasons for readmission include postoperative complications, failure to thrive and diagnostic evaluation; the median length of stay (LoS) following readmission is 7 days. One of the factors often credited with improving outcomes following pancreatic surgery has been the regionalization of pancreatectomy to high-volume ‘centres of excellence’. Although the true impact of such regionalization is controversial,4 a recent systematic review by Torre et al. found improved 5-year survival and negative margin status rates at high-volume centres compared with low-volume centres.5 As pancreatic cancer remains the fourth leading cause of cancer death in North America, advances in the field of surgical oncology have generated interest in the management of patients following oncologic resection of the pancreas.6 ‘Enhanced recovery after surgery’ (ERAS) and related programmes, including ‘clinical pathway’, ‘fast track’ and ‘critical pathway’ protocols, are multidisciplinary management plans for patients following surgery. These protocols standardize a patient's course in hospital from surgery until discharge, and dictate when certain events (e.g. catheter removal, dietary advancement) are to occur. By prescribing specific perioperative processes of care, these instruments have the potential to improve quality of care by standardizing perioperative care for patients and thereby decreasing variations in care. An ERAS protocol also facilitates the incorporation of evidence-based practices into clinical care. Furthermore, these tools can improve the efficiency of care delivery, which results in a reduced hospital LoS. Encouraging results of the implementation of ERAS programmes in other areas, particularly in colorectal surgery, have prompted the application of ERAS protocols to myriad operations ranging from cardiovascular to complex cancer procedures such as pancreatectomy.7
Programmes based on ERAS protocols following pancreatic surgery have existed for over a decade.8 Common elements of an ERAS protocol in the context of pancreatic surgery include goal-directed mobilization, early oral intake, and specific instructions for the use and management of surgical drains and nasogastric tubes. The objective of these protocols is to decrease LoS without compromising patient safety and outcomes. Systematic reviews of ERAS programmes in abdominal surgery have recommended their widespread implementation, but uptake to date has been limited.9 To determine the applicability of ERAS protocols in pancreatectomy, a systematic review of the evidence was performed.
Materials and methods
Literature search
Several databases were consulted and search strategies specific to the sources were designed in consultation with an information specialist. The search was designed to identify material published from 2000 to 2013 using the electronic sources MEDLINE, CINAHL, the Cochrane Library (systematic reviews and trials) and EMBASE. The key terms ‘clinical pathway’, ‘critical pathway’, ‘fast track’, ‘enhanced recovery’, ‘pancreas’, ‘surgery’ and their synonyms were used in various combinations. Duplicates were deleted. To ensure that all relevant literature was captured, manual searches of the reference lists of eligible studies were performed.
Selection criteria
Abstracts of identified articles were screened for inclusion based on predefined eligibility criteria. Abstracts were excluded if they: (i) did not describe an ERAS protocol implemented by the author(s); (ii) focused on a single intervention in postoperative care; (iii) measured the impact of intraoperative factors on postoperative outcomes; (iv) did not measure any clinically meaningful outcomes such as LoS, morbidity, mortality, etc.; (v) represented a case series with fewer than 10 patient participants, or (vi) did not represent the most recent publication of a single research project. Lastly, systematic reviews were also excluded from analysis.
Data extraction
A data collection form was developed and pilot-tested. Two reviewers (DJK and MA) independently extracted data from all included articles. Disagreements were resolved through consensus. If consensus could not be reached, an additional reviewer (ACW) was sought to resolve the dispute. Extracted data were tabulated and analysed.
Data analysis
Details regarding the total numbers of eligible and included studies were recorded. These data and the reasons for exclusion were tabulated in a spreadsheet. The methodologic quality of all included studies was assessed using the GRADE guidelines proposed by Balshem et al.10 Qualitative and quantitative information on major outcomes of interest such as LoS, overall morbidity, pancreatic fistulae, delayed gastric emptying, biliary leak, intra-abdominal abscess, wound infections, 60-day mortality rates, 30-day readmission rates, and total hospital costs were recorded, if available. In addition, data on overall findings such as quality of life, patient satisfaction, staff satisfaction and implementation strategies were noted, if reported.
Results
The results of the literature search are presented in Fig. 1. This search strategy yielded a total of 3247 citations. The full-text versions of 59 articles were retrieved. Fourteen articles met the inclusion criteria and underwent data extraction; 10 were primary research studies11–20 and four were systematic reviews.21–24 No additional articles were identified after hand-searching the reference lists of the included articles. Thus, a total of 10 articles met the criteria for analysis.
Figure 1.
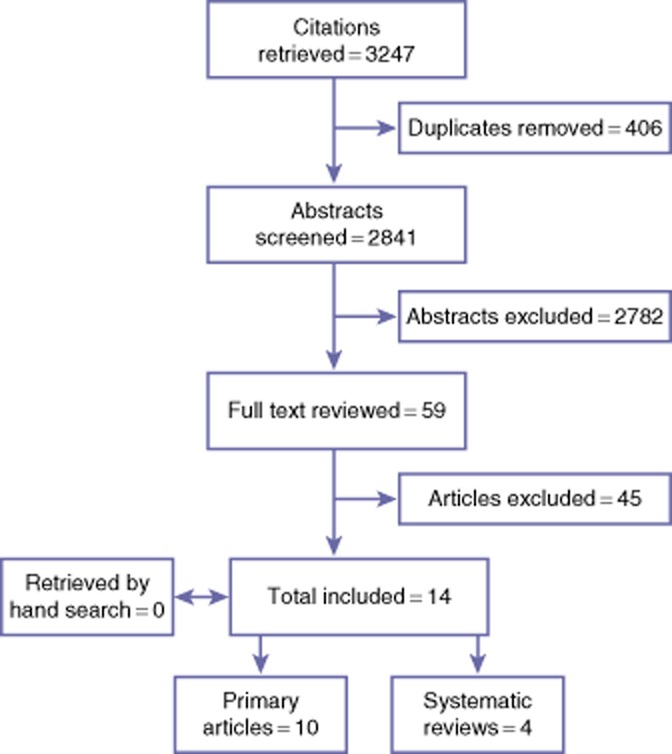
Systematic review search results
The quality of these studies was assessed using GRADE guidelines.10 No high-quality studies were identified. Cohort studies comparing multiple groups were labelled as being of moderate quality. Single-group prospective studies were graded as low quality. Seven articles11–13,15,16,19,20 were deemed to be of moderate quality and three were of low quality.14,17,18 No randomized controlled trials or prospective studies comparing multiple groups were identified.
The characteristics of included studies are presented in Table 1. Of the 10 articles, three were prospective single-group cohort studies14,17,18 and seven were retrospective studies11–13,15,16,19,20 comparing outcomes in patients who underwent surgery after the implementation of an ERAS protocol with those in historical control subjects. Five studies examined exclusively patients undergoing pancreaticoduodenectomy,12,15,18–20 and four studies included patients undergoing other forms of oncologic pancreatic resection.11,13,14,17 One study examined only patients undergoing distal pancreatectomy.16 Notably, each primary research article was restricted in scope to a single institution. No multicentre studies were identified.
Table 1.
Characteristics of included studies of outcomes of enhanced recovery after surgery (ERAS) protocols
| Authors | Year | Journal | Study design | Type of resection | Patients, n | |
|---|---|---|---|---|---|---|
| CC | ERAS | |||||
| Porter et al.11 | 2000 | Annals of Surgical Oncology | Retrospective | Whipple, total pancreatectomy | 68 | 80 |
| Vanounou et al.12 | 2007 | Journal of the American College of Surgeons | Retrospective | Whipple | 64 | 145 |
| Kennedy et al.13 | 2007 | Journal of the American College of Surgeons | Retrospective | Whipple, total pancreatectomy | 44 | 91 |
| Berberat et al.14 | 2007 | Journal of Gastrointestinal Surgery | Prospective | Whipple, distal pancreatectomy, total pancreatectomy, central pancreatectomy, duodenum-preserving pancreatic head resection, segmental pancreatectomy | NA | 255 |
| Balzano et al.15 | 2008 | British Journal of Surgery | Retrospective | Whipple | 252 | 252 |
| Kennedy et al.16 | 2009 | Journal of Gastrointestinal Surgery | Retrospective | Distal pancreatectomy, distal pancreatectomy with splenectomy | 40 | 71 |
| di Sebastiano et al.17 | 2011 | Langenbeck's Archives of Surgery | Prospective | Whipple, distal pancreatectomy, total pancreatectomy, central pancreatectomy | NA | 145 |
| Robertson et al.18 | 2012 | HPB | Prospective | Whipple | NA | 50 |
| Nikfarjam et al.19 | 2013 | Journal of the Pancreas | Retrospective | Whipple | 21 | 20 |
| Abu Hilal et al.20 | 2013 | Pancreatology | Retrospective | Whipple | 24 | 20 |
CC, conventional care; NA, not applicable.
The components of each ERAS protocol described are presented in Table 2. Nine out of 10 studies described protocols containing six or more distinct elements.9–17 One study presented a protocol which specified only four elements, although the description is unclear and the actual protocol implemented may have included more elements.11 The elements most frequently included in the ERAS protocols were: early oral intake; nasogastric tube management; goal-directed mobilization, and surgical drain management. Six of the articles explicitly described discharge planning as a component of the ERAS protocol.13,16–20 Only two protocols included postoperative octreotide administration,14,17 and four protocols included routine administration of prokinetic agents.14,17,19,20 The number of elements in the protocols analysed in these studies ranged from four to 13 and the average number of elements was eight.
Table 2.
Elements included in enhanced recovery after surgery (ERAS) protocols
| Authors | Early oral intake | Goal-directed mobilization | Octreotides | Epidurals/PCA | Surgical drains | NG tubes | Pre-op antibiotics | Foley catheters | Prokinetic agents | Discharge planning | Other | Elements, n |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Porter et al.11 | + | − | − | − | + | + | − | + | − | − | − | 4 |
| Vanounou et al.12 | + | + | − | − | + | + | + | + | − | − | + | 9 |
| Kennedy et al.13 | + | + | − | + | + | + | + | + | − | + | + | 13 |
| Berberat et al.14 | + | − | + | + | − | − | + | − | + | − | + | 6 |
| Balzano et al.15 | + | + | − | + | + | + | − | − | − | − | + | 6 |
| Kennedy et al.16 | + | + | − | + | + | + | + | + | − | + | + | 12 |
| di Sebastiano et al.17 | + | + | + | + | − | + | + | + | + | + | − | 9 |
| Robertson et al.18 | + | + | − | + | + | + | − | + | − | + | + | 10 |
| Nikfarjam et al.19 | + | + | − | − | + | + | + | + | + | + | + | 12 |
| Abu Hilal et al.20 | + | + | − | + | + | + | − | − | + | + | − | 7 |
| Total | 10 | 8 | 2 | 7 | 8 | 9 | 6 | 7 | 4 | 6 | 7 |
+, element explicitly listed in the ERAS protocol; −, element not explicitly listed in the ERAS protocol.
PCA, patient-controlled analgesia; NG, nasogastric.
The outcomes of the analysed studies are displayed in Table 3. Of the seven studies in which outcomes in a control group were compared with those in an ERAS group, six studies reported a significant decrease in postoperative LoS in ERAS patients.11,13,15,16,19,20 The reported LoS ranged from 7 days to 13 days.
Table 3.
Primary outcomes in studies of enhanced recovery after surgery (ERAS) protocols included in this review
| Authors | Length of stay, days Median (range) | Readmission rate n (%) | Morbidity n (%) | Reoperation rate n (%) | Mortality n (%) | Total cost, US$ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC | ERAS | CC | ERAS | CC | ERAS | CC | ERAS | CC | ERAS | CC | ERAS | |
| Porter et al.11 | 15 | 12a | 10 (15%) | 9 (11%) | 20 (29%) | 24 (30%) | 1 (2%) | 1 (1%) | 2 (3%) | 1 (1%) | 47 515 | 36 627a |
| Vanounou et al.12 | 8 | 8 | 4 (6%) | 13 (9%) | 40 (62%) | 77 (54%) | 4 (6%) | 7 (5%) | 1 (2%) | 2 (1%) | 23 112 | 19 561 |
| Kennedy et al.13 | 13 | 7a | 3 (7%) | 7 (8%) | 19 (44%) | 34 (37%) | – | – | 1 (2%) | 1 (1%) | 240 242 | 126 566a |
| Berberat et al.14 | – | 10 (4–115) | – | 9 (4%) | – | 105 (41%) | – | 23 (9%) | – | 5 (2%) | – | – |
| Balzano et al.15 | 15 (7–102) | 13 (7–110)a | 16 (6%) | 18 (7%) | 148 (59%) | 119 (47%)a | 20 (8%) | 17 (7%) | 7 (3%) | 9 (4%) | – | – |
| Kennedy et al.16 | 10 | 7a | 10 (25%) | 5 (7%)a | 15 (38%) | 11 (16%) | – | – | 1 (2%) | 1 (1%) | 26 393 | 22 806 |
| di Sebastiano et al.17 | – | 10 (6–69) | – | 9 (6%) | – | 56 (39%) | – | 11 (8%) | – | 4 (3%) | – | – |
| Robertson et al.18 | – | 10 (8–17) | – | 2 (4%) | – | 23 (46%) | – | 5 (10%) | – | 2 (4%) | – | – |
| Nikfarjam et al.19 | 14 (8–29) | 8 (7–16)a | 0 (0%) | 3 (15%) | – | – | – | – | – | – | – | – |
| Abu Hilal et al.20 | 13 (11–20) | 8 (7–13)a | 2 (10%) | 1 (4%) | 16 (67%) | 8 (40%) | 3 (12%) | 1 (5%) | 0 (0%) | 0 (0%) | – | – |
Significant difference: P < 0.05.
CC, conventional care.
Rates of 30-day readmission did not differ significantly between conventional and ERAS care in six of the seven studies.11,13–15,17–20 Among ERAS patients, rates of readmission ranged from 3.5% to 15%.
Balzano et al., whose study on post-pancreaticoduodenectomy ERAS protocols included the largest number of patients, reported a decrease in postoperative morbidity among patients treated according to the protocol.15 The other five studies assessing postoperative complications found no significant change in overall morbidity among ERAS patients.11–13,16,20 No study found an increased rate of complications after implementing an ERAS protocol.
All studies assessing postoperative mortality found equivalent rates across patients treated conventionally and those treated with an ERAS protocol.11–18,20 Mortality rates ranged from 1% to 4%.
Four studies examined costs associated with postoperative care following pancreatic surgery.11–13,16 Two of these studies found a decrease in cost following the implementation of an ERAS protocol11,12 and two studies found no significant change.13,16
Discussion
Since their introduction by Kehlet in the 1990s, postoperative ERAS programmes have been extensively studied.25 Although randomized trials and meta-analyses have consistently reported an advantage to ERAS over conventional care, these studies have been performed predominantly in colorectal surgery patients.26 The results of this systematic review reveal that only a handful of studies have examined ERAS programmes in the context of pancreatic surgery. None of these studies reported primary data. At present, recommendations are based on evidence of low and moderate quality.
The results of 10 primary clinical studies demonstrate that ERAS protocols in pancreatic surgery result in unchanged or decreased LoS, overall morbidity, perioperative mortality, and rates of readmission to hospital. Thus, based on the results of this systematic review and reviews conducted by others, it appears that ERAS programmes in pancreatic surgery are safe and effective, and do not compromise the aforementioned outcomes.8,22,24 Studies examining the impact of implementing an ERAS protocol in the context of pancreatic surgery in terms of reductions in the cost of care reported results in favour of ERAS; however, it is worth noting the substantial disparity between studies in absolute values of perioperative costs, which emphasizes the need to standardize cost reporting.11–13,16
This study also revealed substantial heterogeneity in the content of ERAS protocols in pancreatic surgery. For example, whereas most protocols included some form of early goal-directed mobilization, what this entailed differed between protocols. Di Sebastiano et al. recommended mobilization for 4 h on the first postoperative day,17 whereas Abu Hilal et al. recommended that the patient should sit out of bed for 2 h and undertake 30 s of marching on the spot on postoperative day 1.20 Similar differences between protocols exist in many other elements. Thus, it is difficult to ascribe any beneficial outcome to a particular ERAS element and impossible to recommend any one specific intervention as optimal. Notably, in studies examining more than one type of pancreatectomy, the ERAS protocols implemented were identical in different operations,8,10,11,14 despite differences in resection and reconstruction. Kennedy et al. implemented extremely similar protocols in patients undergoing Whipple and total pancreatectomy in one study,13 and distal pancreatectomy in another,16 which suggests that similar ERAS protocols can produce benefits when applied to different operations. Moreover, the evidence for and rationale underpinning each element are not reported in most studies examining ERAS programmes in pancreatic surgery. The ERAS Society recently published guidelines for perioperative care after pancreaticoduodenectomy27 and made 27 evidence-based recommendations. Future protocols should adhere to these guidelines.
The routine intraoperative placement of intraperitoneal drains is controversial, with multiple publications suggesting that intraoperative drain placement does not improve outcomes and may actually worsen morbidity.28,29 Conversely, a recent randomized prospective multicentre trial of pancreaticoduodenectomy with and without routine drain placement was stopped early because mortality increased from 3% to 12% in patients without intraperitoneal drainage.30 In the context of pancreatic surgery, ERAS protocols often include algorithms for drain management which may indicate that drain amylase should be measured on a specific postoperative day and the drain removed if the result is below a specific threshold. Based on the evidence reviewed here, this strategy is appropriate and is likely to evolve as future studies clarify the role of intraoperative drain placement during pancreatectomy.
Emphasis on discharge planning varied substantially among ERAS protocols. Some studies included explicit criteria to be met prior to discharge, whereas others projected discharge on a specific postoperative day. What is not reported is the degree to which patients and allied health care professionals (HCPs) were involved in discharge planning. Future protocols might explicitly include specific patient-centred discharge planning interventions.
Although ERAS protocols have the potential to improve outcomes for patients undergoing pancreatic resection, their impact is limited by variable implementation strategies. Simply developing evidence-based protocols is not sufficient to change practice. Simultaneous strategies such as the initiation of patient education, audit and feedback systems, and adherence to standards of desired practice are required to ensure the effective implementation of ERAS protocols. In general, the reporting of implementation strategies was poor. Only three studies included standardized sets of doctors' orders as part of their implementation process.11,13,16 Other implementation strategies reported include: formal and informal education sessions for HCPs; patient education sessions, and the use of pilot project results to inform the future implementation of ERAS programmes. Although the beneficial effects of audit and feedback on improving the practice of HCPs and ameliorating patient outcomes have been demonstrated,31 only one of the studies covered by this review included audit and feedback as part of the ERAS implementation process.13 This shortcoming has been highlighted in other reviews on ERAS programmes in hepatopancreatobiliary surgery.24
It must be noted that the introduction of ERAS programmes in all of the studies included was limited to a single academic institution. This limits the external validity of these studies and their applicability to other health care contexts. Interventions developed and tested in a single clinical environment are not necessarily generalizable. This highlights the need for multicentre trials of ERAS programmes in pancreatic surgery. Multicentre implementations of ERAS programmes have been reported in other areas of general surgery; for example, the synchronous implementation of an ERAS programme for elective colonic surgery at 33 hospitals in the Netherlands resulted in a reduction in median postoperative LoS from 9 days to 6 days.32 In addition to demonstrating the feasibility of multicentre ERAS implementation, this article provides strategies to encourage compliance with multicentre ERAS programmes, which are highly applicable to ERAS initiatives in the context of pancreatic surgery.
The present review is limited by its inability to pool the data extracted using meta-analysis. Because of substantial clinical and statistical heterogeneity, high-quality meta-analysis was not feasible for any of the outcomes measured. Despite this limitation, these findings remain meaningful for clinicians and eliminate some of the contradiction among the findings of individual studies. Most importantly, the preponderance of evidence favours the standardization of postoperative care following pancreatic surgery, in which severe complications are both frequent and variable. Although postoperative complications necessitate deviation from ERAS protocols, establishing a baseline pattern of care to be followed in patients without complications is safe and effective, and has the potential to shorten hospitalization and improve postoperative outcomes. Future studies on ERAS protocols following pancreatectomy should be randomized multicentre trials, and should include data from both high- and low-volume centres in their analysis.
Conclusions
This systematic review identified 10 primary studies of ERAS programmes in elective pancreatic surgery. Although the quality of evidence was low to moderate, ERAS protocols in pancreatic surgery result in equivalent or better outcomes in terms of LoS, morbidity, mortality and hospital readmission rates without evidence of harm. Thus, ERAS programmes have the potential to improve the quality and efficiency of perioperative care following pancreatectomy. Future efforts should be directed towards developing ERAS protocols based on the best available evidence, incorporating multifaceted implementation strategies, and evaluating the impact of ERAS programmes in a multicentre framework at both high- and low-volume centres.
Acknowledgments
The authors would like to acknowledge Marina Englesakis and the University Health Network Health Sciences Library for invaluable assistance in generating and executing the search strategies for this systematic review.
Conflicts of interest
None declared.
References
- Siquini W. Surgical Treatment of Pancreatic Diseases. Milan: Springer; 2009. pp. 1–2. [Google Scholar]
- Cameron JL, Riall TS, Coleman J, Belcher K. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CC, Gondek SP, Vollmer CM, Callery MP, Kent TS. Readmission following pancreatectomy: what can be improved? HPB. 2013;15:703–708. doi: 10.1111/hpb.12036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simunovic M, Urbach D, Major D, Sutradhar R, Baxter N, To T, et al. Assessing the volume–outcome hypothesis and region-level quality improvement interventions: pancreas cancer surgery in two Canadian provinces. Ann Surg Oncol. 2010;17:2537–2544. doi: 10.1245/s10434-010-1114-0. [DOI] [PubMed] [Google Scholar]
- Torre ML, Nigri G, Ferrari L, Cosenza G, Ravaioli M, Ramacciato G. Hospital volume, margin status, and longterm survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am Surg. 2012;78:225–229. [PubMed] [Google Scholar]
- Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183:630–641. doi: 10.1016/s0002-9610(02)00866-8. [DOI] [PubMed] [Google Scholar]
- Coolsen MM, van Dam RM, van der Wilt AA, Slim K, Lassen K, Dejong CH. Systematic review and meta-analysis of enhanced recovery after pancreatic surgery with particular emphasis on pancreaticoduodenectomies. World J Surg. 2013;37:1909–1918. doi: 10.1007/s00268-013-2044-3. [DOI] [PubMed] [Google Scholar]
- Ronellenfitsch U, Rössner E, Jakob J, Post S, Hohenberger P, Schwarzbach M. Clinical pathways in surgery – should we introduce them into clinical routine? A review article. Langenbecks Arch Surg. 2008;393:449–457. doi: 10.1007/s00423-008-0303-9. [DOI] [PubMed] [Google Scholar]
- Balshem H, Helfand M, Schunemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. 2011;64:401–406. doi: 10.1016/j.jclinepi.2010.07.015. [DOI] [PubMed] [Google Scholar]
- Porter GA, Pisters PWT, Mansyur C, Bisanz A, Reyna K, Stanford P, et al. Cost and utilization impact of a clinical pathway for patients undergoing pancreaticoduodenectomy. Ann Surg Oncol. 2000;7:484–489. doi: 10.1007/s10434-000-0484-0. [DOI] [PubMed] [Google Scholar]
- Vanounou T, Pratt W, Fischer JE, Vollmer JCM, Callery MP. Deviation-based cost modelling: a novel model to evaluate the clinical and economic impact of clinical pathways. J Am Coll Surg. 2007;204:570–579. doi: 10.1016/j.jamcollsurg.2007.01.025. [DOI] [PubMed] [Google Scholar]
- Kennedy EP, Rosato EL, Sauter PK, Rosenberg LM, Doria C, Marino IR, et al. Initiation of a critical pathway for pancreaticoduodenectomy at an academic institution – the first step in multidisciplinary team building. J Am Coll Surg. 2007;204:917–923. doi: 10.1016/j.jamcollsurg.2007.01.057. [DOI] [PubMed] [Google Scholar]
- Berberat P, Ingold H, Gulbinas A, Kleeff J, Müller M, Gutt C, et al. Fast track – different implications in pancreatic surgery. J Gastrointest Surg. 2007;11:880–887. doi: 10.1007/s11605-007-0167-2. [DOI] [PubMed] [Google Scholar]
- Balzano G, Zerbi A, Braga M, Rocchetti S, Beneduce AA, Di Carlo V. Fast-track recovery programme after pancreatico-duodenectomy reduces delayed gastric emptying. Br J Surg. 2008;95:1387–1393. doi: 10.1002/bjs.6324. [DOI] [PubMed] [Google Scholar]
- Kennedy E, Grenda T, Sauter P, Rosato E, Chojnacki K, Rosato JF, et al. Implementation of a critical pathway for distal pancreatectomy at an academic institution. J Gastrointest Surg. 2009;13:938–944. doi: 10.1007/s11605-009-0803-0. [DOI] [PubMed] [Google Scholar]
- di Sebastiano P, Festa L, De Bonis A, Ciuffreda A, Valvano M, Andriulli A, et al. A modified fast-track programme for pancreatic surgery: a prospective single-centre experience. Langenbecks Arch Surg. 2010;396:345–351. doi: 10.1007/s00423-010-0707-1. [DOI] [PubMed] [Google Scholar]
- Robertson N, Gallacher PJ, Peel N, Garden OJ, Duxbury M, Lassen K, et al. Implementation of an enhanced recovery programme following pancreaticoduodenectomy. HPB. 2012;14:700–708. doi: 10.1111/j.1477-2574.2012.00521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikfarjam M, Weinberg L, Low N, Fink MA, Muralidharan V, Houli N, et al. A fast track recovery programme significantly reduces hospital length of stay following uncomplicated pancreaticoduodenectomy. JOP. 2013;14:63–70. doi: 10.6092/1590-8577/1223. [DOI] [PubMed] [Google Scholar]
- Abu Hilal M, Di Fabio F, Badran A, Alsaati H, Clarke H, Fecher I, et al. Implementation of enhanced recovery programme after pancreatoduodenectomy: a single-centre UK pilot study. Pancreatology. 2013;13:58–62. doi: 10.1016/j.pan.2012.11.312. [DOI] [PubMed] [Google Scholar]
- Olsén MF, Wennberg E. Fast-track concepts in major open upper abdominal and thoracoabdominal surgery: a review. World J Surg. 2011;35:2586–2593. doi: 10.1007/s00268-011-1241-1. [DOI] [PubMed] [Google Scholar]
- Spelt L, Ansari D, Sturesson C, Tingstedt B, Andersson R. Fast-track programmes for hepatopancreatic resections: where do we stand? HPB. 2011;13:833–838. doi: 10.1111/j.1477-2574.2011.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ypsilantis E, Praseedom RK. Current status of fast-track recovery pathways in pancreatic surgery. JOP. 2009;10:646–650. [PubMed] [Google Scholar]
- Hall TC, Dennison AR, Bilku DK, Metcalfe MS, Garcea G. Enhanced recovery programmes in hepatobiliary and pancreatic surgery: a systematic review. Ann R Coll Surg Engl. 2012;94:318–326. doi: 10.1308/003588412X13171221592410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehlet H. Multimodal approach to control postoperative pathophysiology and rehabilitation. Br J Anaesth. 1997;78:606–617. doi: 10.1093/bja/78.5.606. [DOI] [PubMed] [Google Scholar]
- Lemmens L, van Zelm R, Borel Rinkes I, van Hillegersberg R, Kerkkamp H. Clinical and organizational content of clinical pathways for digestive surgery: a systematic review. Dig Surg. 2009;26:91–99. doi: 10.1159/000206142. [DOI] [PubMed] [Google Scholar]
- Lassen K, Coolsen MM, Slim K, Carli F, de Aguilar-Nascimento JE, Schäfer M, et al. Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr. 2012;31:817–830. doi: 10.1016/j.clnu.2012.08.011. [DOI] [PubMed] [Google Scholar]
- Mehta VV, Fisher SB, Maithel SK, Sarmiento JM, Staley CA, Kooby DA. Is it time to abandon routine operative drain use? A single institution assessment of 709 consecutive pancreaticoduodenectomies. J Am Coll Surg. 2013;216:635–642. doi: 10.1016/j.jamcollsurg.2012.12.040. [DOI] [PubMed] [Google Scholar]
- Conlon K, Labow D, Leung D, Smith A, Jarnagin W, Coit DG, et al. Prospective randomized clinical trial of the value of intraperitoneal drainage after pancreatic resection. Ann Surg Oncol. 2001;234:487–493. doi: 10.1097/00000658-200110000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Buren G, Bloomston M, Hughes SJ, Winter J, Behrman SW, Zyromski NJ, et al. A randomized prospective multicentre trial of pancreaticoduodenectomy with and without routine intraperitoneal drainage. Ann Surg. 2014;259:605–612. doi: 10.1097/SLA.0000000000000460. [DOI] [PubMed] [Google Scholar]
- Ivers N, Jamtvedt G, Flottorp S, Young JM, Odgaard-Jensen J, French SD, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;(6) doi: 10.1002/14651858.CD000259.pub3. CD000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillissen F, Hoff C, Maessen J, Winkens B, Teeuwen JH, von Meyenfeldt MF, et al. Structured synchronous implementation of an enhanced recovery programme in elective colonic surgery in 33 hospitals in the Netherlands. World J Surg. 2013;37:1082–1093. doi: 10.1007/s00268-013-1938-4. [DOI] [PubMed] [Google Scholar]


