Abstract
Introduction
As mortality and morbidity after a curative resection remains high, it is essential to identify pre-operative factors associated with an early death after a major resection.
Methods
Between 1998 and 2008, we selected a population of 331 patients having undergone a major hepatectomy including segment I with a lymphadenectomy and a common bile duct resection for a proven hilar cholangiocarcinoma in 21 tertiary centres. The study's objective was to identify pre-operative predictors of early death (<12 months) after a resection.
Results
The study cohort consisted of 221 men and 110 women, with a median age of 61 years (range: 24–85). The post-operative mortality and morbidity rates were 8.2% and 61%, respectively. The 1-, 3- and 5-year overall survival rates were 85%, 64% and 53%, respectively. The median tumour size was 23 mm on pathology, ranging from 8 to 40. A tumour size >30 mm [odds ratio (OR) 2.471 (95% confidence interval (CI) 1.136–7.339), P = 0.001] and major post-operative complication [OR 3.369 (95% CI 1.038–10.938), P = 0.004] were independently associated with death <12 months in a multivariate analysis.
Conclusion
The present analysis of a series of 331 patients with hilar cholangiocarcinoma showed that tumour size >30 mm was independently associated with death <12 months.
Introduction
Hilar cholangiocarcinoma is challenging in terms of staging and surgical treatment.1,2 The central location of the tumour in the liver and its close relationship with vascular structures have resulted in a low resectability rate and high morbidity and mortality.3 However, it has long been accepted that a complete surgical resection is the only way to provide patients with a chance of a cure or long-term survival.1,2 Indeed, many surgeons have adopted an aggressive approach combining resection of the main biliary confluence with an extended hepatectomy including the caudate lobe and lymphadenectomy.4–9 This radical treatment is responsible for post-operative mortality and morbidity rates of up to 10–13% and 75%, respectively.5 In spite of a 5-year survival rate in the range of 25–30% being reported for patients who's resection was considered R0,3,4 the high risk of local tumour recurrence is still not satisfactory. In these settings, the utility of surgery with high mortality and morbidity rates can legitimately be questioned, particularly in patients with a dismal prognosis.
Hence, the present study sought to identify pre-operative factors associated with early death (i.e. within 12 months of resection) after a major liver resection for hilar cholangiocarcinoma in a nationwide registry of operated patients (the AFC-HC study group).
Patients and methods
Patient sourcing and selection
The HC-AFC-2009 study group was created under the auspices of the French Association of Surgery (AFC) in 2008 in order to generate a retrospective registry (after comprehensive pathology database screening) of all consecutive patients with hilar cholangiocarcinoma undergoing surgery between January 1998 and January 2008. The protocol was approved by the AFC's investigational review board. Of 710 patients considered for curative treatment, 595 (84%) had undergone a resection. We selected patients having undergone a major hepatectomy (right or left hepatectomy, right or left trisectionectomy and anterior hepatectomy) including segment I with lymphadenectomy and common bile duct resection for proven hilar cholangiocarcinoma. Patients who underwent a resection for intra-hepatic cholangiocarcinoma with secondary hilar extension9 were excluded as well as patients treated with neoadjuvant therapy. Patients undergoing adjacent organ, arterial, a minor resection or a liver resection without segment I were excluded owing to the high rate of R1 resections (51% and 52%, respectively). Three patients having undergone a hilar en bloc resection of extrahepatic bile ducts with the portal vein bifurcation were also not selected. The final selected population comprised 331 patients from 21 surgical centres located throughout France. The flowchart is detailed in Fig. 1.
Figure 1.
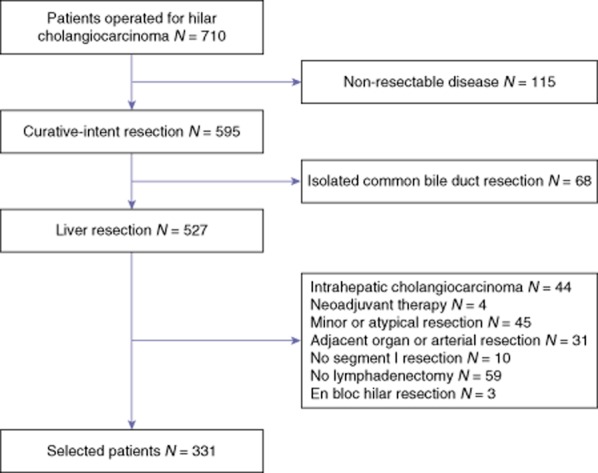
Flowchart
Study criteria
Peri-operative management and surgical strategy have evolved over the past 3 decades in the 21 centres. Portal vein embolization was mainly considered for patients with compromised liver function when the anticipated future remnant volume is <40% of the total liver volume. Pre-operative imaging was used to determine the extent of the tumour precisely and to assess surgical resectability. Pre-operative diagnostic images used included inter alia computed tomography (CT). Since 2003 multidetector row computed tomography (MDCT) and more recently magnetic resonance cholangiography have replaced CT in most centres. Imaging characteristics included tumour location, biliary extension with growth pattern (infiltrative and intraductal, papillary), parenchyma atrophy, portal vein and hepatic artery invasion and lymph node enlargement. Lesions were classified and validated according to both Bismuth–Corlette classification and DeOliveira staging.10,11
In brief, the extrahepatic bile duct of the resected specimen was opened longitudinally from the distal resection margin up to the proximal margin, to accurately evaluate the ductal margin status. All surgical specimen slides were not available for review; however, a copy of the histology report on the tumour specimen was requested for each patient.12 All pathology reports were reviewed by an independent pathologist (D.C.). There was no standardized protocol for pathological analysis of specimens within all 21 tertiary hospitals. A R0 resection was defined as the presence of a macroscopically and microscopically tumour-free resection margin. A R1 resection was defined as microscopic evidence of tumour tissue at the resection margin including biliary, liver parenchyma and adjacent fat tissue. Histological findings were described using the TNM Classification of Malignant Tumors (7th edition, 2009) by the International Union Against Cancer.
Post-operative complications were stratified according to the Clavien–Dindo classification,13 which defines major complications by a score of 3 or more. Specific liver complications were detailed as follows: liver failure defined according to the ‘50–50 criteria’ on post-operative day 5;14 ascites defined as an abdominal drainage output of more than 10 ml per kg per day after the third post-operative day; and biliary leakage defined by a bilirubin concentration in the drainage fluid of more than threefold higher than that in serum.15 Both complications and operative mortality were considered as those occurring within 90 days of surgery, or at any time during the post-operative hospital stay.
Study design
The study's objective was to identify pre-operative predictors of early death, i.e. within 12 months of resection whatever the reason (died of the disease, no evidence of disease, missing data etc). All patients surviving the operation were monitored and the follow-up was updated at the end of 2010. The median follow-up was 42 months (range, 6–235). Variables of interest were systematically double-checked by three surgeons (J.M.R., O.F. and D.F.) and marked as missing as appropriate. Discrepancies in the reported data were corrected after communication between the statistician/data manager and the study centre in question.
Statistical analysis
Data are reported as the prevalence, mean (standard deviation) or median (range). Continuous data were compared by means of the Mann–Whitney U-test and ordinal data were compared in a χ2 test or Fisher's exact test, as appropriate. Tests for independent samples were applied. Statistical analysis was performed using SPSS software (version 20.0; SPSS Inc., Chicago, IL, USA). Predictive factors for early death were determined by uni- and multivariate analysis. For the univariate analysis, the significance level of each factor was tested alone. For the multivariate analysis, a backward-elimination approach in a multiple logistic-regression model was performed. In this model, the significant factors from the univariate analysis were removed one at a time, starting with the factor that had the largest P-value, until all remaining factors had a two-sided P-value of less than 0.10. Odds ratios and P-values were reported for each factor alone and for the factors found to be significant from the backward elimination. All statistical tests were two-sided and the threshold for statistical significance was set to P < 0.05.
Results
Study population
A total of 331 selected patients had resectable hilar cholangiocarcinoma including 221 men and 110 women with a median age of 61 years (range: 24–85). Hilar cholangiocarcinoma was symptomatic in 223 (67%) patients and the most frequent symptom was jaundice (75%). Fourteen (4%) had cholangitis at presentation that was treated either with antibiotic therapy or percutaneous/endoscopic biliary drainage. Two patients had hilar cholangiocarcinoma develop on primary sclerosing cholangitis and one patient had HVC-related cirrhosis. The baseline serum bilirubin level was >50 μmol/l in 72%, > 100 μmol/l in 57% and >200 μmol/l in 37% of patients. At the initial diagnosis, 64 (19%) patients had suspected portal vein involvement on imaging. Pre-operative biliary drainage was performed in 168 of the 248 (68%) jaundiced patients and 62 (19%) patients underwent pre-operative PVE. In patients with available data (n = 93, 28%), the median tumour diameter was 23 mm on pre-operative imaging, ranging from 10 to 40 mm. Two hundred and seventy-two (82%) patients were staged as Bismuth–Corlette type III–IV (Table 1).
Table 1.
Tumour staging (n = 331)
| Tumour staging n (%) | |
|---|---|
| Staging on pre-operative imaging | |
| Median tumour diameter (mm) | 23 (10–40)a |
| Portal vein invasion | 64 (19) |
| Hepatic artery invasion | 16 (5) |
| Lobar atrophy | 58 (17) |
| Lymph node enlargement | 20 (6) |
| Bismuth–Corlette staging | |
| I | 12 (4) |
| II | 47 (14) |
| III | 242 (73) |
| IV | 30 (9) |
| DeOliveira staging | |
| Bile Duct (B) | |
| B1 | 12 (4) |
| B2 | 47 (14) |
| B3-R | 132 (40) |
| B3-L | 110 (33) |
| B4 | 30 (9) |
| Tumour size (T) | |
| T1 | 30 (9) |
| T2 | 126 (38) |
| T3 | 175 (53) |
| Tumour formb | |
| Sclerosing | 111 (43) |
| Mass | 116 (45) |
| Mixed | 21 (8) |
| Polypoid | 10 (4) |
| Involvement of the portal vein (PV) | |
| PV0 | 267 (81) |
| PV1 | 3 (1) |
| PV2 | 12 (3) |
| PV3 | 49 (15) |
| PV4 | 0 (0) |
| Involvement of the hepatic artery (HA)c | |
| HA0 | 331 (100) |
| HA1 | 0 (0) |
| Lymph nodes (n) | |
| N0 | 195 (59) |
| N1 | 136 (41) |
| Metastases (M) | |
| M0 | 331 (100) |
| M1 | 0 (0) |
In patients with available data (n = 238).
The data were not available in 73 (22%) patients.
Patients requiring an arterial resection were excluded from the study.
Surgery and early outcomes
The surgical procedures included 156 (47%) right hepatectomies with resection of segment IVa, 169 (51%) left hepatectomies and 7 (2%) anterior hepatectomies. All patients had a lymphadenectomy including pericholedochal lymph nodes, and lymph nodes around the common hepatic artery and the portal vein. A lymphadenectomy included celiac lymph nodes in 25 (7.6%) patients and posterior pancreaticoduodenal lymph nodes in 20 (6.0%). Five (1.5%) patients had resection of the aortocaval paraaortic lymph nodes. The median number of sampled was 5, ranging from 1 to 34 and the median lymph node ratio was 0.26 (range: 0.05–1). One hundred and twenty (36%) patients had vascular clamping and 144 (43%) patients required an intra-operative transfusion [median number of units of packed blood cells: 3 (range: 1–16)]. Ninety (27%) patients underwent a combined portal vein resection. The median operative time was 376 min (187–900) and the median blood loss was 760 ml (180–3200). One hundred thirty-nine (42%) patients required an intra-operative transfusion. The overall post-operative mortality rate was 8.2% (n = 27) and 202 (61%) patients experienced complications after surgery [Dindo–Clavien I n = 42 (21%), II n = 61 (30%), III n = 79 (39%) and IV n = 20 (10%)]. Causes of death were liver failure (n = 8), sepsis (n = 7), haemorrhage (n = 6), multiple organ failure (n = 3), massive pulmonary embolism (n = 1), acute cardiac failure (n = 1) and portal thrombosis (n = 1)]. The median length of hospitalization was 25 days (range: 9–92).
The median tumour size was 23 mm on pathology, ranging from 8 to 40. The resection margins were R0 in 195 (59%) and R1 in 136 (41%) of the resected patients. Among the 331 patients undergoing a lymphadenectomy, 136 (41%) had lymph node involvement. Perineural invasion was confirmed in 94% of the 331 patients. Microscopic vascular invasion was confirmed in 84%. Fifty-five per cent of the tumours were well differentiated, 36% were moderately differentiated and 9% were poorly differentiated. After resection, 73 (22%) patients received adjuvant therapy. Chemotherapy (based on gemcitabine) was administered to 43 (13%) patients and radiation therapy was administered to 40 (12%) patients. Of these 73 patients, 10 received both chemotherapy and radiation therapy and 9 other patients had adjuvant endobrachytherapy.
Overall survival
The 1-, 3- and 5-year overall survival rates in the 331 selected patients were 85%, 64% and 53%, respectively. The 1-, 3- and 5-year disease-free survival rates were 81%, 49% and 32%, respectively. The 1-, 3- and 5- overall survival rates in the 205 excluded patients were 60%, 30% and 27%, respectively; these values were significantly lower than those of the selected patients (P = 0.0001) (Fig. 2).
Figure 2.
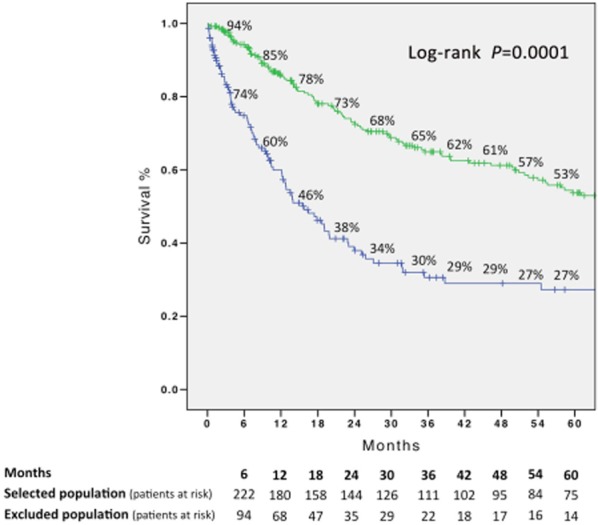
Overall survival in the 331 selected patients and in the 205 excluded patients (Kaplan–Meier) (log-rank P = 0.0001)
Analysis of predictive factors for early death (within 12 months of resection) in selected patients
The numbers of patients who died during the follow-up periods to 6 and 12 months were 24 and 37, respectively. Among these patients, a tumour recurrence was confirmed on follow-up imaging in 20 (55%) patients including the liver (n = 5), lungs (n = 2), peritoneum (n = 7), bone (n = 5) and lymph node (n = 2). For the 17 remaining patients, no data was specified in spite of contacting the centres in question. As detailed in Table 2, symptomatic tumour (P = 0.033), tumour size >30 mm (P = 0.004), lymph node involvement (P = 0.038) and major complications (P = 0.0001) were associated with early death (<12 months) in univariate analysis. A tumour size >30 mm [OR 2.471 (95% CI 1.136–7.339), P = 0.001] and major post-operative complication [OR 3.369 (95% CI 1.038–10.938), P = 0.004] were independently associated with death < 12 months in a multivariate analysis. When considered as continuous variables, age and tumour size were significantly higher (63 versus 59 years, P = 0.03 and 31 versus 22 mm, P = 0.01, respectively) in patients who died at 12 months. In contrast, neither body mass index (24.2 versus 24.2 kg/m2, P = 0.98) nor initial serum bilirubin level (178 versus 164 μmol/l, P = 0.31) were associated with early death.
Table 2.
Identification of variables associated with death within 12 months in the study population (uni- and multivariate)
| Early death (<12 months) | ||||
|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | |||
| OR (95%CI) | P-value | OR (95%CI) | P-value | |
| Patients characteristics | ||||
| Male gender | 1.15 (0.60–2.20) | 0.666 | ||
| Age >70 years | 1.92 (0.99–3.72) | 0.052 | 2.73 (0.76–9.80) | 0.124 |
| Jaundice | 2.04 (0.81–5.13) | 0.132 | ||
| Cholangitis at presentation | 2.13 (0.64–7.14) | 0.219 | ||
| Obese (BMI >30) | 0.73 (0.09–6.17) | 0.774 | ||
| Symptomatic | 2.23 (1.07–4.67) | 0.033 | 1.29 (0.14–12.15) | 0.823 |
| Initial serum bilirubin level >200 μmol/l | 1.42 (0.71–2.83) | 0.312 | ||
| Malnutrition | 1.51 (0.42–5.41) | 0.529 | ||
| Co-morbidities | ||||
| Diabetes mellitus | 1.43 (0.41–5.00) | 0.577 | ||
| Hypertension | 1.04 (0.45–2.41) | 0.922 | ||
| Ischaemic heart disease | 1.83 (0.23–14.71) | 0.568 | ||
| COPD | 4.13 (0.05–7.16) | 0.517 | ||
| Renal failure | 2.59 (0.33–20.41) | 0.364 | ||
| Dyslipidemia | 1.17 (0.25–5.46) | 0.840 | ||
| Alcohol abuse | 2.00 (0.57–6.99) | 0.276 | ||
| Tobacco | 1.62 (0.67–3.94) | 0.288 | ||
| Cancer | 1.01 (0.28–3.66) | 0.988 | ||
| Surgical characteristics | ||||
| Right-sided resection | 0.84 (0.55–1.53) | 0.565 | ||
| Portal vein resection | 1.70 (0.79–3.66) | 0.176 | ||
| Vascular clamping | 0.60 (0.81–3.17) | 0.176 | ||
| Intra-operative transfusion | 0.89 (0.48–1.65) | 0.710 | ||
| Tumour characteristics | ||||
| Tumour size >30 mm | 4.49 (1.64–12.33) | 0.004 | 2.47 (1.14–7.34) | 0.001 |
| Bismuth III/IV | 1.61 (0.59–4.43) | 0.353 | ||
| Microscopic vascular invasion | 1.59 (0.03–29.81) | 1.000 | ||
| T stage | 4.01 (1.13–9.27) | 0.001 | ||
| Lymph node involvement | 3.02 (1.06–8.61) | 0.038 | 1.87 (0.56–6.26) | 0.311 |
| Poor differentiation | 3.12 (0.88–4.20) | 0.106 | ||
| R0 resection | 0.64 (0.26–1.58) | 0.329 | ||
| Post-operative outcomes | ||||
| Complication | 4.51 (1.95–10.42) | 0.0001 | ||
| Major complication | 5.22 (2.65–10.23) | 0.0001 | 3.37 (1.04–10.94) | 0.004 |
| Liver-related complication | 1.58 (0.81–3.08) | 0.175 | ||
| Pulmonary complication | 1.09 (0.47–2.52) | 0.831 | ||
| Renal-related complication | 0.97 (0.48–1.98) | 0.935 | ||
| Post-operative treatment | ||||
| Adjuvant chemo | 0.37 (0.11–1.28) | 0.117 | ||
| Adjuvant EBRT | 0.75 (0.27–2.08) | 0.576 | ||
OR, odds ratio; CI, confidence interval; BMI, body mass index; COPD, chronic obstructive pulmonary disease; EBRT, external beam radiation therapy.
Discussion
To our knowledge, this is the first large study with the purpose of identifying hilar cholangiocarcinoma patients who were more likely to die within 12 months after surgery. Unlike in a recent Italian study including 440 patients with hilar cholangiocarcinoma,16 we deliberately selected patients having undergone a major liver resection of the right or left liver lobe with segment I and bile duct; this criterion excluded nearly 35% of the resected patients. We confirmed that patients with intra-hepatic cholangiocarcinoma and hilar extension, patients treated with neoadjuvant therapy, with minor or atypical resection, with adjacent organ or arterial resection or without segment I resection or without lymphadenectomy had a poorer overall survival than the 331 selected patients. The present series also confirms our previous prospective reports concerning the high rate of post-operative mortality (8.2%) and morbidity (58%) after a major hepatectomy5 and provided data on overall (85%, 64% and 53% at 1-, 3- and 5-years, respectively) and disease-free survival (81%, 49% and 32% at 1-, 3- and 5-years, respectively) in a population of patients having received a theoretical optimal treatment.
To the best of our knowledge, this is the first time a series has identified risk factors for early death (<12 months) after a curative-intent resection. Even although tumour size has hardly ever been noted in analyses of prognostic factors in hilar cholangiocarcinoma (including the Japanese literature),17–22 we found that a tumour size >30 mm on pathology was strongly associated with death within 12 months of surgery in a multivariate analysis. Interestingly, tumour size estimated on pre-operative imaging corroborated tumour size evaluated on pathology in the present study (23 versus 23 mm, P = 0.92). However, this important result requires some caution because the measurement of tumour size on pathology was not standardized in different centres over a long study period. The present series confirms the importance of the new grading system for hilar cholangiocarcinoma proposed by DeOliveira et al.11 In the new system, a tumor >30 mm in size is labelled as T3. The choice of a 30 mm cut-off for T3 was based on a growing body of data indicating a better prognosis for smaller tumours;22,23 this includes excellent outcomes after liver transplantation in the absence of any extra-hepatic spread.24 Furthermore, this cut-off is particularly relevant in patients considered for liver transplantation because the original Mayo Clinic protocol and modified Mayo protocols25,26 exclude all patients with a tumour size >30 mm. Interestingly, our long-term results in resected patients are very similar to overall results for cancers non developed on primary sclerosing cholangitis after liver transplantation, as reported by the group from Mayo Clinic (5-year survival 64%).26
Many previous studies have shown that a R0 resection with adequate margins is an important prognostic factor for survival.17,27,28 In spite of an aggressive resection, patients are frequently left with positive margins because it can be difficult to predict the proximal boundary of the microscopic disease on the basis of the pre-operative imaging data.29 As local resection of the extrahepatic biliary system is associated with an incomplete tumour resection in more than two-thirds of patients, it is generally accepted that an additional, major liver resection of the right or left liver lobe is essential. An additional caudate lobe resection is recommended because direct infiltration along this lobe's bile ducts is reportedly responsible for a high rate of local recurrence.30,31 Our rate of R1 resection was significantly higher in our French series compared with the Italian's after a liver resection (41% versus 21%) and had been recently validated after a meticulous pathological analysis.12 Although we would have expected that tumours >30 mm predict a R1 resection and therefore poor outcomes, multivariate analysis failed to find an association between surgical margins and early deaths. However, a distinction between early deaths owing to aggressive tumour behaviour and early deaths that are the consequence of post-operative outcomes is really difficult. The retrospective design may explain why only 59% of the patients who died within 12 months had proven recurrent disease. Indeed, we did not have complete data about recurrence in patients who died prematurely and recurrence is not always the cause of deaths in this short-run follow-up. We do believe that a significant proportion of these early deaths may in part be related to post-operative outcomes.
We acknowledge that the present study had several limitations which relate to the fact that cases were analysed retrospectively: quality of control of surgical treatment and measurement of tumour size on pathology are very difficult over a long study period, as well as surgical procedure and peri-operative management that were largely different from institute to institute. However, a randomized controlled trial seems hardly feasible and our study included a large number of patients treated with a major liver resection for hilar cholangiocarcinoma provides useful clinical information.
In conclusion, we showed for the first time that a sub-group of patients with a large tumour (>30 mm) on pathology were more likely to die within the 12 months after surgery.
Author contribution
The authors are justifiably credited with authorship, according to the authorship criteria. In detail: J.M.R. – conception, design, analysis and interpretation of data, drafting of the manuscript, final approval given; D.F. – acquisition of data, analysis and interpretation of data, drafting of the manuscript, final approval given; P.P. – acquisition of data, critical revision of manuscript, final approval given; E.B. – acquisition of data, critical revision of manuscript, final approval given; D.C. – acquisition of data, critical revision of manuscript, final approval given; and O.F. – conception, design, analysis and interpretation of data, critical revision of manuscript, final approval given.
Competing interests
The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
References
- Seyama Y, Kubota K, Sano K, Noie T, Takayama T, Kosuge T, et al. Long-term outcome of extended hemihepatectomy for hilar bile duct cancer with no mortality and high survival rate. Ann Surg. 2003;238:73–83. doi: 10.1097/01.SLA.0000074960.55004.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launois B, Reding R, Lebeau G, Buard JL. Surgery for hilar cholangiocarcinoma: French experience in a collective survey of 552 extrahepatic bile duct cancers. J Hepatobiliary Pancreat Surg. 2000;7:128–134. doi: 10.1007/s005340050166. [DOI] [PubMed] [Google Scholar]
- Nimura Y, Kamiya J, Kondo S, et al. Aggressive preoperative management and extended surgery for hilar cholangiocarcinoma: Nagoya experience. J Hepatobiliary Pancreat Surg. 2000;7:155–162. doi: 10.1007/s005340050170. [DOI] [PubMed] [Google Scholar]
- Lee SG, Song GW, Hwang S, Ha TY, Moon DB, Jung DH, et al. Surgical treatment of hilar cholangiocarcinoma in the new era: the Asan experience. J Hepatobiliary Pancreat Surg. 2010;17:476–489. doi: 10.1007/s00534-009-0204-5. [DOI] [PubMed] [Google Scholar]
- Regimbeau JM, Fuks D, Le Treut YP, Bachellier P, Belghiti J, Boudjema K, et al. AFC-HC Study Group. Surgery for hilar cholangiocarcinoma: a multi-institutional update on practice and outcome by the AFC-HC study group. J Gastrointest Surg. 2011;15:480–488. doi: 10.1007/s11605-011-1414-0. [DOI] [PubMed] [Google Scholar]
- Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- Farges O, Fuks D, Boleslawski E, Le Treut YP, Castaing D, Laurent A, et al. Influence of surgical margins on outcome in patients with intrahepatic cholangiocarcinoma: a multicenter study by the AFC-IHCC-2009 study group. Ann Surg. 2011;254:824–829. doi: 10.1097/SLA.0b013e318236c21d. discussion 830. [DOI] [PubMed] [Google Scholar]
- Farges O, Fuks D, Le Treut YP, Azoulay D, Laurent A, Bachellier P, et al. AJCC 7th edition of TNM staging accurately discriminates outcomes of patients with resectable intrahepatic cholangiocarcinoma: by the AFC-IHCC-2009 study group. Cancer. 2011;117:2170–2177. doi: 10.1002/cncr.25712. [DOI] [PubMed] [Google Scholar]
- Sano T, Shimada K, Sakamoto Y, Ojima H, Esaki M, Kosuge T. Prognosis of perihilar cholangiocarcinoma: hilar bile duct cancer versus intrahepatic cholangiocarcinoma involving the hepatic hilus. Ann Surg Oncol. 2008;15:590–599. doi: 10.1245/s10434-007-9687-y. [DOI] [PubMed] [Google Scholar]
- Bismuth H, Nakache R, Diamond T. Management strategies in resection for hilar cholangiocarcinoma. Ann Surg. 1992;215:31–38. doi: 10.1097/00000658-199201000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeOliveira ML, Schulick RD, Nimura Y, Rosen C, Gores G, Neuhaus P, et al. New staging system and a registry for perihilar cholangiocarcinoma. Hepatology. 2011;53:1363–1371. doi: 10.1002/hep.24227. [DOI] [PubMed] [Google Scholar]
- Chatelain D, Farges O, Fuks D, Trouillet N, Pruvot FR, Regimbeau JM. Assessment of pathology reports on hilar cholangiocarcinoma: the results of a nationwide, multicenter survey performed by the AFC-HC-2009 study group. J Hepatol. 2012;56:1121–1128. doi: 10.1016/j.jhep.2011.12.010. [DOI] [PubMed] [Google Scholar]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzan S, Belghiti J, Farges O, Ogata S, Sauvanet A, Delefosse D, et al. The ‘50–50 criteria’ on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg. 2005;242:824–848. doi: 10.1097/01.sla.0000189131.90876.9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149:680–688. doi: 10.1016/j.surg.2010.12.002. [DOI] [PubMed] [Google Scholar]
- Nuzzo G, Giuliante F, Ardito F, Giovannini I, Aldrighetti L, Belli G, et al. Improvement in perioperative and long-term outcome after surgical treatment of hilar cholangiocarcinoma: results of an Italian multicenter analysis of 440 patients. Arch Surg. 2012;147:26–34. doi: 10.1001/archsurg.2011.771. [DOI] [PubMed] [Google Scholar]
- Kondo S, Hirano S, Ambo Y, et al. Forty consecutive resections of hilar cholangiocarcinoma with no postoperative mortality and no positive ductal margins: results of a prospective study. Ann Surg. 2004;240:95–101. doi: 10.1097/01.sla.0000129491.43855.6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena A, Chua TC, Chu FC, Morris DL. Improved outcomes after aggressive surgical resection of hilar cholangiocarcinoma: a critical analysis of recurrence and survival. Am J Surg. 2011;202:310–320. doi: 10.1016/j.amjsurg.2010.08.041. [DOI] [PubMed] [Google Scholar]
- Ramacciato G, Nigri G, Bellagamba R, Petrucciani N, Ravaioli M, Cescon M, et al. Univariate and multivariate analysis of prognostic factors in the surgical treatment of hilar cholangiocarcinoma. Am Surg. 2010;76:1260–1268. [PubMed] [Google Scholar]
- Shimizu H, Kimura F, Yoshidome H, Ohtsuka M, Kato A, Yoshitomi H, et al. Aggressive surgical resection for hilar cholangiocarcinoma of the left-side predominance: radicality and safety of left-sided hepatectomy. Ann Surg. 2010;251:281–286. doi: 10.1097/SLA.0b013e3181be0085. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Uemura K, Sudo T, Hashimoto Y, Nakashima A, Kondo N, et al. Prognostic factors after surgical resection for intrahepatic, hilar, and distal cholangiocarcinoma. Ann Surg Oncol. 2011;18:651–658. doi: 10.1245/s10434-010-1325-4. [DOI] [PubMed] [Google Scholar]
- DeOliveira ML, Cunningham SC, Cameron JL, Kamangar F, Winter JM, Lillemoe KD, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245:755–762. doi: 10.1097/01.sla.0000251366.62632.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarnagin WR, Fong Y, DeMatteo RP, Gonen M, Burke EC, Bodniewicz BJ, et al. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann Surg. 2001;234:507–517. doi: 10.1097/00000658-200110000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito F, Agni R, Rettammel RJ, Been MJ, Cho CS, Mahvi DM, et al. Resection of hilar cholangiocarcinoma: concomitant liver resection decreases hepatic recurrence. Ann Surg. 2008;248:273–279. doi: 10.1097/SLA.0b013e31817f2bfd. [DOI] [PubMed] [Google Scholar]
- Rea DJ, Heimbach JK, Rosen CB, Haddock MG, Alberts SR, Kremers WK, et al. Liver transplantation with neoadjuvant chemoradiation is more effective than resection for hilar cholangiocarcinoma. Ann Surg. 2005;242:451–458. doi: 10.1097/01.sla.0000179678.13285.fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darwish Murad S, Kim WR, Harnois DM, Douglas DD, Burton J, Kulik LM, et al. Efficacy of neoadjuvant chemoradiation, followed by liver transplantation, for perihilar cholangiocarcinoma at 12 US centers. Gastroenterology. 2012;143:88–98. doi: 10.1053/j.gastro.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki S, Imamura H, Kobayashi A, et al. Results of surgical resection for patients with hilar bile duct cancer: application of extended hepatectomy after biliary drainage and hemihepatic portal vein embolization. Ann Surg. 2003;238:84–92. doi: 10.1097/01.SLA.0000074984.83031.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagino M, Kamiya J, Nishio H, et al. Two hundred forty consecutive portal vein embolizations before extended hepatectomy for biliary cancer: surgical outcome and long-term follow-up. Ann Surg. 2006;243:364–372. doi: 10.1097/01.sla.0000201482.11876.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribero D, Amisano M, Lo Tesoriere R, Rosso S, Ferrero A, Capussotti L. Additional resection of an intraoperative margin-positive proximal bile duct improves survival in patients with hilar cholangiocarcinoma. Ann Surg. 2011;254:776–781. doi: 10.1097/SLA.0b013e3182368f85. [DOI] [PubMed] [Google Scholar]
- Mizumoto R, Suzuki H. Surgical anatomy of the hepatic hilum with special reference to the caudate lobe. World J Surg. 1988;12:2–10. doi: 10.1007/BF01658479. [DOI] [PubMed] [Google Scholar]
- Nimura Y, Hayakawa N, Kamiya J, et al. Hepatic segmentectomy with caudate lobe resection for bile duct carcinoma of the hepatic hilus. World J Surg. 1990;14:535–543. doi: 10.1007/BF01658686. [DOI] [PubMed] [Google Scholar]


