Abstract
Objectives
To examine temporal trends in plasma viral load (pVL) suppression and antiretroviral resistance from 1997-2010 in British Columbia (BC), Canada, and determine characteristics, pVL ranges, and resistance profiles of HIV-positive individuals with unsuppressed pVL in 2010.
Methods
HIV-positive individuals ≥19 years old in the provincial database at the BC Centre for Excellence in HIV/AIDS were included. Virologic suppression was defined as two consecutive pVL <500 copies/mL within each calendar year. Temporal trends were evaluated using the Cochran-Armitage test. Persons with suppressed vs. unsuppressed pVL in 2010 were compared using the Pearson χ2 or Fisher’s exact test (categorical variables) and the Wilcoxon rank-sum test (quantitative variables), including unsuppressed individuals only if they were on antiretroviral therapy (ART) in 2010 or their baseline CD4 count was <350 cells/mm3 or <500 cells/mm3, in separate analyses.
Results
The proportion of individuals with suppressed pVL increased from 24% to 80% (p<0.001). In comparative analyses, individuals with unsuppressed pVL (877 of 6142) were more likely to be female (30% vs. 16%), younger (median 43 vs. 48 years), have injection drug use history (38% vs. 30%), report Aboriginal ancestry (30% vs. 16%), and have hepatitis C co-infection (57% vs. 34%) (all p<0.001). Similar patterns were observed using the <500 cells/mm3 CD4 cut-off. The median pVL of all unsuppressed individuals in 2010 was 12,896 copies/mL (IQR 1,495-47,763).
Conclusions
The proportion of individuals achieving pVL suppression in BC has increased markedly since 1997, however further efforts are needed to maximize the individual and societal benefits of modern ART.
Keywords: HIV, viral suppression, antiretroviral therapy, treatment as prevention, antiretroviral resistance, Canada
INTRODUCTION
The introduction of combination antiretroviral therapy (ART) in 1996 represented a pivotal moment in the history of the HIV/AIDS epidemic [1]. ART stops HIV replication on a sustained basis and, as a result, plasma viral load (pVL) becomes undetectable [2,3]. Treatment guidelines uniformly recognize that sustained suppression of pVL is needed to optimize the therapeutic effects of ART [1-4], allowing for immune reconstitution and leading to long-term disease remission and prolonged survival [5-7]. Over the last decade ART has become more efficacious, better tolerated, and less complex [2,3,8], thus improving health outcomes throughout the spectrum of disease severity.
More recently, ART has been shown to greatly reduce the likelihood of transmission by suppressing viral loads below the limit of detection [9-13]. At the population level, expanded access to ART has been shown to be an effective means of decreasing new HIV diagnoses due to reductions in community and aggregate pVL levels [11,14-16].
In response to well-established evidence for the use of ART for individual health as well as the growing body of literature supporting ART use for prevention of transmission at the population level (“treatment as prevention”), the Canadian province of British Columbia (BC) announced the “Seek and Treat for Optimal Prevention of HIV and AIDS” (STOP HIV/AIDS) pilot program in February, 2010 [17]. The program aims to expand HIV testing and ART access to decrease HIV/AIDS-related morbidity and mortality. Secondarily, the program aims to evaluate the impact of ART expansion on new HIV diagnoses, while continuing to promote traditional prevention strategies.
Importantly, much of the program’s potential for success hinges on the accessibility of ART. In accordance with the “cascade of care” concept, HIV-positive persons must be identified, engage in care, initiate treatment, and remain engaged in order for treatment as prevention programs to be effective [18]. Despite the availability of free-of-charge antiretroviral therapy and associated medical monitoring in BC since 1986 [19], treatment remains underutilized, with 40% of HIV-related deaths from 1997-2005 having been among individuals who never accessed treatment [20]. This statistic speaks to a striking disconnect between the availability and actual uptake of services. Identifying characteristics of individuals failing to accrue the maximal benefits of available therapies should help guide the development of targeted engagement and retention strategies, thereby optimizing the individual and societal impact of treatment as prevention initiatives.
This study therefore seeks to inform the expansion of ART, by examining temporal trends in pVL suppression and the prevalence of antiretroviral resistance from 1997-2010, and exploring recent trends by determining the characteristics, pVL ranges, and resistance profiles of HIV-positive individuals with unsuppressed pVL in 2010. Of note, these analyses were conducted in a setting with universal healthcare access, where antiretroviral medications and related medical and laboratory care are fully subsidized.
METHODS
Study setting and participants
Data were obtained from the province-wide registry at the BC Centre for Excellence in HIV/AIDS (BC-CfE) in Vancouver, Canada. This database includes information on all individuals ever having a viral load test in the province; which is also inclusive of all individuals who ever accessed antiretroviral therapy in the province [19]. The BC-CfE and provincial healthcare program offers fully subsidized antiretroviral drugs and clinical and laboratory monitoring (without co-payments or deductibles). The BC-CfE also maintains a set of independently generated HIV/AIDS management guidelines [21], which have remained consistent with the International AIDS Society-USA (IAS-USA) guidelines since 1996 [1,3].
All HIV-positive individuals ≥19 years old ever in the provincial database were eligible for inclusion in this study, regardless of whether or not they ever initiated antiretroviral therapy. The last date of follow-up for the current analysis was 31 December 2010. Ethical approval was obtained from the University of British Columbia Providence Health Care Research Ethics Board.
Primary outcome
The primary outcome of interest was plasma HIV-RNA suppression, defined as two consecutive pVL measures <500 copies/mL within the calendar year of interest. The viral load level of <500 copies/mL was selected to accommodate the temporal changes in pVL assay sensitivities since study initiation in 1997 [11]. Participants with only a single pVL measure in a given year were grouped as having unsuppressed pVL if the measure was ≥500 copies/mL, and removed from the analysis that year if the measure was <500 copies/mL. All viral loads were measured using the Roche Amplicor or Roche TaqMan assays (Roche Diagnostics, Laval, Canada) at the Diagnostic Virology and Reference Laboratory at St. Paul’s Hospital in Vancouver.
Statistical methods
In order to examine temporal trends in pVL suppression, proportions of individuals with pVL suppression were calculated by calendar year for all eligible BC-CfE participants. Participants entered the analysis the year they had their first pVL test and were removed if known to have moved out of the province, died, or were lost to follow-up. Loss to follow-up was defined as no viral load data for at least one year. Mortality data were obtained through linkages with the provincial vital statistics registry. Participants were divided into three mutually exclusive categories for each study year from 1997-2010: 1) unsuppressed pVL off ART; 2) unsuppressed pVL on ART; and, 3) suppressed pVL (as defined above). The first group (unsuppressed pVL off ART) was then further divided by prescription history: unsuppressed previously on ART, and unsuppressed never on ART. A sensitivity analysis then re-categorized individuals who were lost to follow-up as having unsuppressed pVL, and the time since loss to follow-up was assessed. Trends were evaluated using the Cochran-Armitage test.
To determine temporal trends in resistance over time, available drug resistance genotypes (protease/reverse transcriptase) performed on pVL samples from all individuals in the BC-CfE database were examined. Sequences were categorized as resistant (1+ class) or not based on the presence of at least one nucleoside reverse transcriptase inhibitor (NRTI), non-nucleoside reverse transcriptase inhibitor (NNRTI), or protease inhibitor (PI) resistance mutation as defined by a modified 2011 IAS-USA list [22]. Results were then grouped by pVL suppression status each year. Individuals with multiple resistance tests per year were considered resistant if mutations were observed in any sequence collected that year. If in subsequent years a resistance test was not performed, the previous year’s result was carried forward. Thus, individuals were categorized as “never genotyped” in all years preceding their first resistance test, and were assigned to a resistance category for all subsequent years. Trends over time were assessed using the Cochran-Armitage test.
To evaluate recent trends in suppression, descriptive statistics were calculated and compared between individuals with and without pVL suppression in 2010. To avoid the inclusion of participants in the unsuppressed group who were not accessing treatment due to a possible decision or recommendation based on CD4 counts, participants in the unsuppressed pVL group were only included in this comparative analysis if they were on ART in 2010 or if treatment was clinically indicated, defined as having a baseline CD4 count <350 cells/mm3. A second analysis was then conducted raising the CD4 count threshold to <500 cells/mm3, to account for the evolving CD4 count guidelines for treatment initiation, which were updated in the latter half of 2010 [23]. Groups were compared using the Pearson χ2 test or Fisher’s exact test for categorical variables and the Wilcoxon rank-sum test for quantitative variables. Variables of interest for comparison included socio-demographic characteristics (age, sex, Aboriginal ancestry, injection drug use history, and place of residence), clinical indicators (CD4 count, hepatitis C co-infection, and baseline AIDS-defining illnesses), and treatment information (antiretroviral regimens and physician experience, defined as the median number of HIV-positive patients under that physician’s care as of 1 January 2010). Place of residence was categorized as one of five geographically defined provincial health authorities, which are regional divisions that separate the governing and planning of health services [24].
To document the pVL ranges of all individuals with unsuppressed pVL in 2010, we used the last viral load measures of that year to calculate a pooled province-wide measurement, presented as median and interquartile range (IQR). Viral loads in 2010 were measured by the Roche TaqMan v1 assay (dynamic range 40-1,000,000 copies/mL) [25]. Lastly, antiretroviral resistance status among individuals with unsuppressed pVL in 2010 was determined as previously described, and results were grouped by pVL range (500-999 copies/mL, 1,000-9,999 copies/mL, 10,000-99,999 copies/ml, and ≥100,000 copies/mL) and compared using the Pearson χ2 test. All statistical analyses were performed using SAS software (version 9.3).
RESULTS
Temporal trends
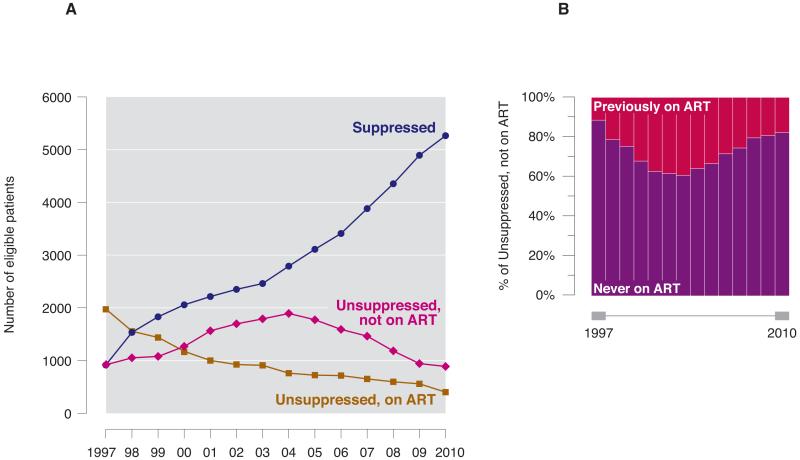
Between 1997 and 2010, the proportion of individuals with pVL suppression increased from 24% to 80% (see Figure 1A; Cochran-Armitage p<0.001). The proportion of unsuppressed individuals on treatment decreased from 52% in 1997 to 6% in 2010 (p<0.001). The majority of individuals with unsuppressed pVL not on ART had never been prescribed antiretroviral therapy in BC, with 157 of 888 individuals with unsuppressed pVL off ART in 2010 (17%) having previously taken treatment (Figure 1B).
Figure 1.
A. Temporal trends in pVL suppression in BC from 1997-2010. Individuals were removed from graph if known to have died, moved out of the province, or were lost to follow-up.
Individuals were removed from graph if known to have died, moved out of province, or were lost to follow-up.
B. Antiretroviral therapy prescription history among individuals in “Unsuppressed, not on ART” from Figure 1A, 1997-2010.
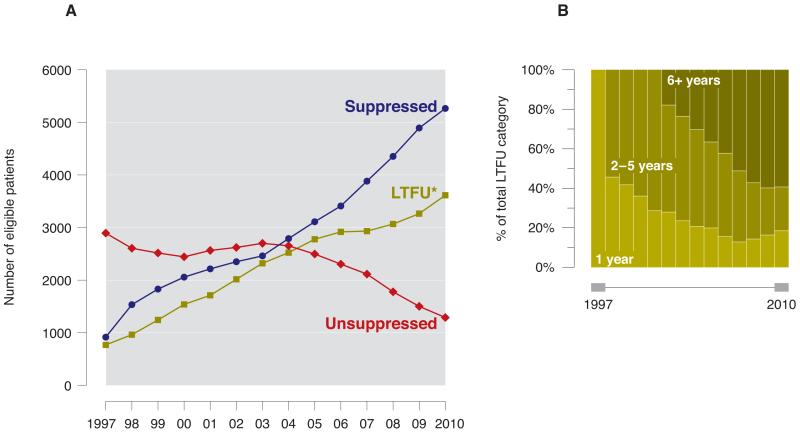
When individuals who were lost to follow-up were re-classified as having unsuppressed pVL in the sensitivity analysis, the proportion of individuals with pVL suppression increased from 20% to 52% over the study period (Figure 2A). Although this improvement is attenuated compared to the primary analysis (an increase from 24% to 80%), the trend remains statistically significant (p<0.001). To note, over half of individuals remaining lost to follow-up in 2010 had been lost for over six years (2144 of 3614 persons, 59%) (Figure 2B).
Figure 2.
A. Temporal trends in pVL suppression in BC from 1997-2010, inclusive of loss to follow-up. Individuals were removed from graph if known to have died or moved out of the province. * LTFU, lost to follow up. Defined as no viral load data for >1 year.
B. Time since loss to follow-up, 1997–2010
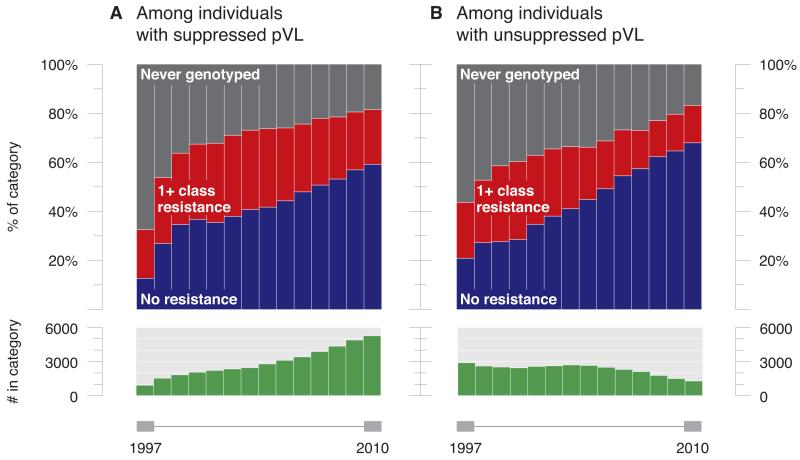
Over the same period, rates of drug resistance testing increased from 41% of patients engaged in care in 1997 to 82% in 2010. Among those tested, the proportion of patients with drug resistant HIV decreased among individuals with both suppressed (61% to 27%; p<0.001) and unsuppressed (52% to 18%; p<0.001) pVL (Figures 3A and 3B).
Figure 3.
A. Temporal trends in cumulative antiretroviral resistance among individuals with suppressed pVL in BC, 1997-2010.
B. Temporal trends in cumulative antiretroviral resistance among individuals with unsuppressed pVL in BC, 1997-2010.
Demographic and clinical characteristics
Table 1 displays demographic and clinical characteristics of persons with unsuppressed pVL in 2010 that were on ART, or clinically indicated for ART, compared to individuals with pVL suppression. Using the CD4 count threshold of <350 cells/mm3, data were available for 6142 participants, 877 (14%) having unsuppressed pVL. Individuals with unsuppressed pVL were significantly more likely to be female (30% vs. 16%), younger (median 43 vs. 48 years), have a history of injection drug use (IDU) (38% vs. 30%), report Aboriginal ancestry (30% vs. 16%), and have hepatitis C co-infection (57% vs. 34%) (all p<0.001). They were less likely to have had a baseline AIDS-defining illness (6% vs. 13%, p<0.001). Suppression proportions also differed significantly by provincial health authority, physician experience, and composition of ART regimen (p<0.001), as shown in Table 1.
Table 1.
Characteristics of individuals with unsuppressed pVL on ART or clinically indicated (based on CD4 count) for ART in 2010 compared to individuals with pVL suppression in British Columbia, Canada.
| Variable | CD4 Cutoff: <350 cells/mm3 | CD4 Cutoff: <500 cells/mm3 | ||||||
|---|---|---|---|---|---|---|---|---|
| Total | Suppressed (n=5265) |
Unsuppressed (n=877) |
p-value | Total | Suppressed (n=5265) |
Unsuppressed (n=1072) |
p-value | |
| Sex | ||||||||
| Male | 5061 | 4446 (84%) | 615 (70%) | <0.001 | 5219 | 4446 (84%) | 773 (72%) | <0.001 |
| Female | 1081 | 819 (16%) | 262 (30%) | 1118 | 819 (16%) | 299 (28%) | ||
| Age | 6142 | 48 (42-55) | 43 (35-49) | <0.001 | 6337 | 48 (42-55) | 43 (35-48) | <0.001 |
| Aboriginal ancestry | ||||||||
| No | 2767 | 2588 (84%) | 179 (70%) | <0.001 | 2792 | 2588 (84%) | 204 (71%) | <0.001 |
| Yes | 558 | 480 (16%) | 78 (30%) | 563 | 480 (16%) | 83 (29%) | ||
| IDU history | ||||||||
| No | 4126 | 3639 (70%) | 487 (62%) | <0.001 | 4285 | 3639 (70%) | 646 (68%) | 0.237 |
| Yes | 1881 | 1587 (30%) | 294 (38%) | 1895 | 1587 (30%) | 308 (32%) | ||
| HCV co-infection | ||||||||
| No | 1855 | 1746 (66%) | 109 (43%) | <0.001 | 1859 | 1746 (66%) | 113 (43%) | <0.001 |
| Yes | 1028 | 886 (34%) | 142 (57%) | 1036 | 886 (34%) | 150 (57%) | ||
| Baseline ADI | ||||||||
| No | 5422 | 4599 (87%) | 823 (94%) | <0.001 | 5616 | 4599 (87%) | 1017 (95%) | <0.001 |
| Yes | 720 | 666 (13%) | 54 (6%) | 721 | 666 (13%) | 55 (5%) | ||
| CD4 count | 5558 | 510 (360-680) | 260 (140-350) | <0.001 | 5753 | 510 (360-680) | 320 (180-430) | <0.001 |
| Health authority | ||||||||
| Unspecified | 520 | 158 (3%) | 362 (41%) | <0.001 | 695 | 158 (3%) | 537 (50%) | <0.001 |
| Interior | 303 | 271 (5%) | 32 (4%) | 303 | 271 (5%) | 32 (3%) | ||
| Fraser | 1344 | 1225 (23%) | 119 (14%) | 1349 | 1225 (23%) | 124 (12%) | ||
| Vancouver Coastal | 3241 | 2982 (57%) | 259 (30%) | 3256 | 2982 (57%) | 274 (26%) | ||
| Vancouver Island | 589 | 518 (10%) | 71 (8%) | 589 | 518 (10%) | 71 (7%) | ||
| Northern | 145 | 111 (2%) | 34 (4%) | 145 | 111 (2%) | 34 (3%) | ||
| MD experience * | 6142 | 171 (76-369) | 94 (2-274) | <0.001 | 6337 | 171 (76-369) | 94 (5-275) | <0.001 |
| ART regimen | ||||||||
| Triple NRTI | 24 | 24 (1%) | 0 (0%) | <0.001 | 24 | 24 (1%) | 0 (0%) | <0.001 |
| Single PI-based | 140 | 133 (3%) | 7 (2%) | 140 | 133 (3%) | 7 (2%) | ||
| Boosted PI-based | 3198 | 2917 (57%) | 281 (70%) | 3198 | 2917 (57%) | 281 (70%) | ||
| NNRTI-based | 1911 | 1821 (36%) | 90 (22%) | 1911 | 1821 (36%) | 90 (22%) | ||
| Other | 215 | 192 (4%) | 23 (6%) | 215 | 192 (4%) | 23 (6%) | ||
Results presented as frequency (%) or median (IQR).
Note: pVL, plasma viral load; IQR, interquartile range; IDU, injection drug use; HCV, hepatitis C virus; ADI, AIDS-defining illness; ART, antiretroviral therapy; NRTI, nucleoside reverse transcriptase inhibitor; PI, protease inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor
Experience of patient’s physician as of 1 January 2010 (median # of HIV-positive patients under physician’s care + IQR)
Using the higher CD4 count threshold of <500 cells/mm3, 6337 individuals were included, of whom 1072 (17%) had unsuppressed pVL. Similar patterns were observed regarding differences in characteristics using this cutoff.
Viral load and antiretroviral resistance among individuals with unsuppressed pVL
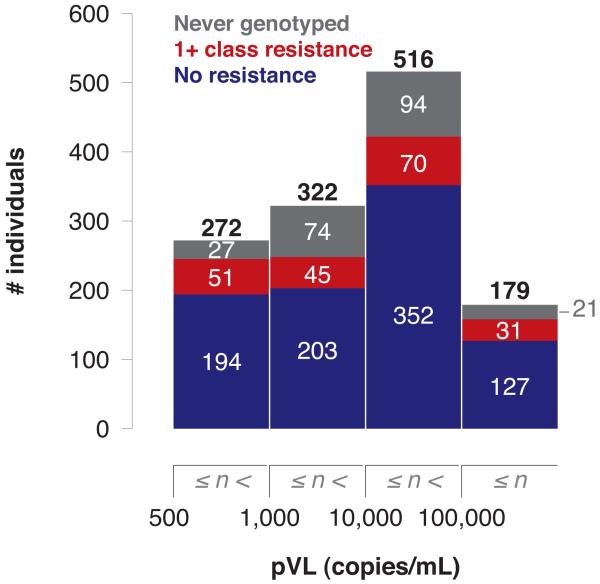
Of the 1289 persons who were virologically unsuppressed in 2010 irrespective of CD4 count and treatment status, the median pVL measure was 12,896 copies/mL (IQR 1,495-47,763), with 21% (n=272) having measures between 500-999 copies/mL; 25% (n=322) 1,000-9,999 copies/mL; 40% (n=516) 10,000-99,999 copies/mL; and 14% (n=179) ≥100,000 copies/mL. Among those tested, the proportion of patients with resistant HIV was comparable across all pVL groups (range 17-21%; p=0.56) (Figure 4). Resistance to the NNRTI class was the most prevalent (10%) among individuals with unsuppressed pVL with available resistance genotypes, followed by NRTI (9%) and PI (3%). No significant differences in rates of resistance between pVL categories were observed in any drug category (all p>0.1).
Figure 4.
Prevalence of antiretroviral resistance among individuals with unsuppressed pVL in BC in 2010, by pVL range (n=1289).
DISCUSSION
Our results demonstrate that while proportions of pVL suppression have significantly improved over the 14-year study period among HIV-positive individuals in BC, disparities in treatment access and success remain. Individuals with unsuppressed pVL were more likely to be female, younger, have a history of injection drug use, report Aboriginal ancestry, and have hepatitis C co-infection. When all individuals with unsuppressed pVL were considered, the median pVL measure in 2010 was 12,896 copies/mL. These findings contribute to a better understanding of the impact of ART expansion in BC, and should also help to inform the implementation of other treatment as prevention strategies.
The population-based registry at the BC-CfE provides a unique opportunity for characterization of the temporal evolution of pVL suppression within a universal healthcare setting with free access to treatment and associated medical and laboratory monitoring. Centralized, linked data collection allows for comprehensive documentation of pVL measures over time. Our results demonstrate a significant increase in the proportion of individuals with pVL suppression from 1997 to 2010, from 24% to 80% if persons lost to follow-up are excluded, and from 20% to 52% if persons lost to follow-up are categorized as having unsuppressed pVL. As 56% of persons remaining lost in 2010 have not had a viral load test in BC in over six years, we postulate that many of these individuals may now be deceased or have moved out of the province.
Over the study period, the proportion of individuals with unsuppressed pVL on treatment decreased from 52% in 1997 to 6% in 2010. This decline is likely explained by advances in ART regimens with respect to efficacy, tolerability, and simplification of drug administration, such as the introduction of fixed-dose combinations [8]. The increased availability of integrative services that facilitate the uptake of and adherence to ART, including directly observed and assisted therapy [26,27], harm reduction strategies such as needle exchange programs and Vancouver’s supervised injection facility [28,29], and linkages to other services such as day programs and methadone maintenance treatment [30-32] has likely also contributed significantly.
The prevalence of antiretroviral resistance among persons with both suppressed and unsuppressed pVL decreased markedly over the study period, even as resistance testing rates increased significantly. The prevalence of resistance in genotyped samples collected from individuals with unsuppressed pVL decreased from 52% in 1997 to 18% in 2010. While it is likely that a substantial portion of this decline could be attributed to increased ART efficacy and tolerability, these results, however, are limited by the availability of data from clinical antiretroviral resistance testing requests. These results therefore assume an equal prevalence of resistance among genotyped and un-genotyped samples. However, changes in clinical genotyping criteria over time may have affected the characteristics of samples tested for resistance. For example, the HIV treatment guidelines in BC did not recommend universal baseline (pre-therapy) resistance testing until 2002. Additionally, since resistance genotyping cannot be consistently performed on samples with low viremia (typically <250 copies/mL), patients’ previous results were carried forward in years where resistance data were not available. As such, the annual prevalence of resistance reported likely represents an overestimate of the true prevalence, particularly among individuals with suppressed pVL.
We acknowledge that trends presented here are not inclusive of individuals who have tested HIV-positive and not sought further care (i.e., pVL or CD4 testing, an HIV physician visit, or initiation of ART); recently estimated to be 811 persons from 1996 to 2009 [33]. These trends also do not encompass individuals with undiagnosed HIV infection. The Public Health Agency of Canada estimates that 26% of HIV-positive Canadians may be unaware of their status [34]. This unmeasured burden of illness may have a clinically important impact on pVL and resistance trends, and ongoing HIV transmission. Importantly, new initiatives in BC aim to normalize HIV testing in effort to achieve more timely diagnosis and subsequent linkage to care, with recent analyses demonstrating substantial reductions in the number of unknown cases across the province since 1996 [33].
While the observed improvements in rates of pVL suppression over time are encouraging, there is no room for complacency. Our analysis identified 877 individuals with unsuppressed pVL in 2010 who have a clinical indication to start treatment (CD4 count <350 cells/mm3), with this number increasing to 1072 persons if a CD4 count threshold of <500 cells/mm3 is considered as the criteria for treatment initiation. More recently, North American guidelines have converged in their recommendation that ART be offered to all HIV-positive individuals regardless of their CD4 count, with the exception of long-term non-progressors or elite controllers [2,3]. This would expand the number of ART-eligible patients from 1072 to 1289 based on the 2010 data, the last year of observation in our study. Moreover, if individuals lost to follow-up in 2010 are re-classified as having unsuppressed pVL and eligible for treatment, this number increases substantially.
Our comparative analysis of individuals with suppressed versus unsuppressed pVL in 2010 highlights the presence of disparities in access to treatment. This analysis demonstrates that in 2010, individuals with unsuppressed pVL on or with an indication for ART were more likely to be female, younger, report Aboriginal ancestry, and have hepatitis C co-infection. These findings are similar to those found in a larger pan-provincial Canadian study, which reported suppression was less likely among women, younger persons, and individuals with IDU history, suggesting these disparities are not unique to BC [35]. We hypothesize a number of potential reasons for these differences, reflective of underlying differences in socioeconomic conditions and other competing life circumstances that may impede optimal access to care and adherence to ART. Differences were also observed by geographic region (health authority). To note, the highest proportion of persons with unsuppressed pVL had missing health authority data; suggesting that these individuals have likely had fewer contacts with the HIV care system and accordingly, limited opportunities to collect this information. Further efforts are thus needed to optimize the potential individual and societal benefits of available therapies.
With respect to the preventive benefits of ART at the population-level, more than half (54%) of individuals with unsuppressed pVL in 2010 had measures ≥10,000 copies/mL; important to note in view of the biologic gradient in the effect of pVL concentration on HIV transmission, shown by Quinn et al. [36] and others [13,37-39]. Indeed, this number grows rapidly when a conservative approach is used, including persons lost to follow-up as having unsuppressed pVL.
The results of this study must be interpreted in the context of several limitations. The analysis was restricted to individuals ≥19 years of age, and the outcomes of persons lost to follow-up are unknown. Data on certain socio-demographic and clinical variables of interest are missing for some participants. In particular, complete data on ethnicity and place of residence would allow for more comprehensive identification of factors associated with disparities in pVL suppression. Finally, as periods of incarceration are not captured in the BC-CfE database, individuals in prison may have been considered lost to follow-up during these periods. Ongoing efforts aim to establish a data linkage to address this limitation.
In conclusion, the data presented here indicate an increase in the proportion of individuals achieving pVL suppression since 1997. However, while the effectiveness of ART is well established, ensuring optimal access to all persons in need remains an unmet challenge in BC despite universal access to free antiretroviral medications and related medical and laboratory care. Our results clearly demonstrate that further efforts are needed if we are to maximize the individual and societal benefits of modern ART.
Acknowledgements
The authors thank the participants in the BC HIV/AIDS Drug Treatment Program; the nurses, physicians, social workers, and volunteers who support them; and David Milan, Benita Yip, James Nakagawa, Guillaume Colley, and Kelly Hsu for their research, technical, and administrative assistance.
Source of Funding:
CJB is supported by a Vanier Canada Graduate Scholarship from the Canadian Institutes of Health Research (CIHR) [CGV-104812]. PRH has received grants from, served as an ad hoc advisor to, or spoke at various events sponsored by Pfizer, Glaxo-Smith Kline, Abbott, Merck, Virco, and Monogram. He is supported by a CIHR/GSK Research Chair in Clinical Virology and has consulted and/or received grant funding from a variety of pharmaceutical diagnostic companies. MH has received grant support from the National Institute on Drug Abuse (NIDA R01DA031043-01) and has received honoraria for speaking engagements and/or consultancy meetings from the following: Bristol-Myers Squibb, Gilead Sciences, Merck, Ortho-Janssen, Pfizer, Vertex Pharmaceuticals, and ViiV. RSH has held grant funding in the last five years from the National Institutes of Health (NIH), CIHR, Health Canada, Merck, and SSHRC. JSGM has received grants from Abbott, Biolytical, Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Janssen, Merck and ViiV Healthcare. He is also supported by the Ministry of Health Services and the Ministry of Healthy Living and Sport, from the Province of British Columbia; through a Knowledge Translation Award from CIHR; and through an Avant-Garde Award (No. 1DP1DA026182-01) from the National Institute on Drug Abuse, at the US National Institutes of Health. He has also received support from the International AIDS Society, United Nations AIDS Program, World Health Organization, National Institute on Drug Abuse, NIH-Office of AIDS Research, National Institute of Allergy & Infectious Diseases, The United States President’s Emergency Plan for AIDS Relief (PEPfAR), Bill & Melinda Gates Foundation, French National Agency for Research on AIDS & Viral Hepatitis (ANRS), and the Public Health Agency of Canada. He has academic partnerships with the University of British Columbia, Simon Fraser University, Providence Health Care, and Vancouver Coastal Health. BN is a CIHR Bisby Fellow and is also funded, in part, by the Michael Smith Foundation for Health Research.
Footnotes
Meetings: Some of the results were presented at the 19th Conference on Retroviruses and Opportunistic Infections (CROI) in Seattle, USA, March 5-8, 2012 (abstract #1116).
Conflicts of Interest
The remaining authors report no disclosures.
Author contributions: The study was conceived and designed by JSGM, AC, JIF, SK, KJL, and MH. Data were analyzed by SK, CJB, and AC, and interpreted by all authors. SK provided statistical expertise. The manuscript was drafted by AC, JSGM, and CJB, and was critically reviewed and subsequently approved by all authors.
REFERENCES
- 1.Carpenter CC, Fischl MA, Hammer SM, et al. Antiretroviral therapy for HIV infection in 1996: Recommendations of an international panel. International AIDS Society-USA. JAMA. 1996;276(2):146–54. [PubMed] [Google Scholar]
- 2.DHHS: Panel on Antiretroviral Guidelines for Adults and Adolescents [Accessed 17 October 2012];Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. 2012 Available from: http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf.
- 3.Thompson MA, Aberg JA, Hoy JF, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA Panel. JAMA. 2012;308(4):387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization [Accessed 13 November 2012];2010 ART guidelines for adults and adolescents - evidence map. 2010 Available from: http://www.who.int/hiv/topics/treatment/evidence/en/index.html.
- 5.Hogg RS, O’Shaughnessy MV, Gataric N, et al. Decline in deaths from AIDS due to new antiretrovirals. Lancet. 1997;349(9061):1294. doi: 10.1016/S0140-6736(05)62505-6. [DOI] [PubMed] [Google Scholar]
- 6.Braitstein P, Brinkhof MW, Dabis F, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367(9513):817–24. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 7.Lima VD, Hogg RS, Harrigan PR, et al. Continued improvement in survival among HIV-infected individuals with newer forms of highly active antiretroviral therapy. AIDS. 2007;21(6):685–92. doi: 10.1097/QAD.0b013e32802ef30c. [DOI] [PubMed] [Google Scholar]
- 8.Boyd MA. Improvements in antiretroviral therapy outcomes over calendar time. Curr Opin HIV AIDS. 2009;4(3):194–9. doi: 10.1097/COH.0b013e328329fc8d. [DOI] [PubMed] [Google Scholar]
- 9.Montaner JS, Hogg R, Wood E, et al. The case for expanding access to highly active antiretroviral therapy to curb the growth of the HIV epidemic. Lancet. 2006;368(9534):531–6. doi: 10.1016/S0140-6736(06)69162-9. [DOI] [PubMed] [Google Scholar]
- 10.Attia S, Egger M, Müller M, Zwahlen M, Low N. Sexual transmission of HIV according to viral load and antiretroviral therapy: systematic review and meta-analysis. AIDS. 2009;23(11):1397–404. doi: 10.1097/QAD.0b013e32832b7dca. [DOI] [PubMed] [Google Scholar]
- 11.Montaner JSG, Lima VD, Barrios R, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010;376(9740):532–9. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donnell D, Baeten JM, Kiarie J, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375(9731):2092–8. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen MS, Chen YQ, McCauley M, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fang CT, Hsu HM, Twu SJ, et al. Decreased HIV transmission after a policy of providing free access to highly active antiretroviral therapy in Taiwan. J Infect Dis. 2004;190(5):879–85. doi: 10.1086/422601. [DOI] [PubMed] [Google Scholar]
- 15.Wood E, Kerr T, Marshall BD, et al. Longitudinal community plasma HIV-1 RNA concentrations and incidence of HIV-1 among injecting drug users: prospective cohort study. BMJ. 2009;338:b1649. doi: 10.1136/bmj.b1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Das M, Chu PL, Santos GM, et al. Decreases in community viral load are accompanied by reductions in new HIV infections in San Francisco. PLoS One. 2010 Jun 10;5(6):e11068. doi: 10.1371/journal.pone.0011068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.British Columbia Centre for Excellence in HIV/AIDS [Accessed 1 October 2012];STOP HIV/AIDS: Seek and Treat for Optimal Prevention of HIV & AIDS. 2012 Available from: www.cfenet.ubc.ca/stop-hiv-aids/
- 18.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hogg RS, Heath KV, Yip B, et al. Improved survival among HIV-infected individuals following initiation of antiretroviral therapy. JAMA. 1998;279(6):450–4. doi: 10.1001/jama.279.6.450. [DOI] [PubMed] [Google Scholar]
- 20.Joy R, Druyts EF, Brandson EK, et al. Impact of neighborhood-level socioeconomic status on HIV disease progression in a universal health care setting. J Acquir Immune Defic Syndr. 2008;47(4):500–5. doi: 10.1097/QAI.0b013e3181648dfd. [DOI] [PubMed] [Google Scholar]
- 21.British Columbia Centre for Excellence in HIV/AIDS [Accessed 13 November 2012];Therapeutic Guidelines. 2011 Available from: http://cfenet.ubc.ca/therapeutic-guidelines/adult.
- 22.Johnson VA, Calvez V, Günthard HF, et al. 2011 update of the drug resistance mutations in HIV-1. Top Antivir Med. 2011;19(4):156–64. [PMC free article] [PubMed] [Google Scholar]
- 23.Thompson MA, Aberg JA, Cahn P, et al. Antiretroviral treatment of adult HIV infection: 2010 recommendations of the International AIDS Society-USA panel. JAMA. 2010;304(3):321–33. doi: 10.1001/jama.2010.1004. [DOI] [PubMed] [Google Scholar]
- 24.British Columbia Ministry of Health [Accessed 2 October 2012];British Columbia Health Authorities. 2011 Available from: www.health.gov.bc.ca/socsec/
- 25.Schumacher W, Frick E, Kauselmann M, Maier-Hoyle V, van der Vliet R, Babiel R. Fully automated quantification of human immunodeficiency virus (HIV) type 1 RNA in human plasma by the COBAS AmpliPrep/COBAS TaqMan system. J Clin Virol. 2007;38(4):304–12. doi: 10.1016/j.jcv.2006.12.022. [DOI] [PubMed] [Google Scholar]
- 26.Tyndall MW, McNally M, Lai C, et al. Directly observed therapy programmes for anti-retroviral treatment amongst injection drug users in Vancouver: access, adherence and outcomes. Int J Drug Policy. 2007;18(4):281–7. doi: 10.1016/j.drugpo.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 27.Parashar S, Palmer AK, O’Brien N, et al. Sticking to it: the effect of maximally assisted therapy on antiretroviral treatment adherence among individuals living with HIV who are unstably housed. AIDS Behav. 2011;15(8):1612–22. doi: 10.1007/s10461-011-0026-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tyndall MW, Wood E, Zhang R, Lai C, Montaner JSG, Kerr T. HIV seroprevalence among participants at a Supervised Injection Facility in Vancouver, Canada: implications for prevention, care and treatment. Harm Reduct J. 2006;3:36. doi: 10.1186/1477-7517-3-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Small W, Van Borek N, Fairbairn N, Wood E, Kerr T. Access to health and social services for IDU: The impact of a medically supervised injection facility. Drug Alcohol Rev. 2009;28(4):341–6. doi: 10.1111/j.1465-3362.2009.00025.x. [DOI] [PubMed] [Google Scholar]
- 30.Krüsi A, Small W, Wood E, Kerr T. An integrated supervised injecting program within a care facility for HIV-positive individuals: a qualitative evaluation. AIDS Care. 2009;21(5):638–44. doi: 10.1080/09540120802385645. [DOI] [PubMed] [Google Scholar]
- 31.Uhlmann S, Milloy MJ, Kerr T, et al. Methadone maintenance therapy promotes initiation of antiretroviral therapy among injection drug users. Addiction. 2010;105(5):907–13. doi: 10.1111/j.1360-0443.2010.02905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Milloy MJ, Kerr T, Buxton J, et al. Social and environmental predictors of plasma HIV RNA rebound among injection drug users treated with antiretroviral therapy. J Acquir Immune Defic Syndr. 2012;59(4):393–9. doi: 10.1097/QAI.0b013e3182433288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nosyk B, Montaner JSG, Colley G, et al. The evolution of the cascade of HIV care in British Columbia, Canada: 1996-2010. submitted. [Google Scholar]
- 34.Public Health Agency of Canada [Accessed 9 October 2012];HIV/AIDS Epi Updates: Chapter 2, Undiagnosed HIV Infections in Canada. 2011 Available from: www.phac-aspc.gc.ca/aids-sida/publication/epi/2010/2-eng.php.
- 35.Cescon AM, Cooper C, Chan K, et al. Factors associated with virological suppression among HIV-positive individuals on highly active antiretroviral therapy in a multi-site Canadian cohort. HIV Med. 2011;12(6):352–60. doi: 10.1111/j.1468-1293.2010.00890.x. [DOI] [PubMed] [Google Scholar]
- 36.Quinn TC, Wawer MJ, Sewankambo N, et al. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Engl J Med. 2000;342(13):921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- 37.Fideli US, Allen SA, Musonda R, et al. Virologic and immunologic determinants of heterosexual transmission of human immunodeficiency virus type 1 in Africa. AIDS. Res Hum Retroviruses. 2001;17(10):901–10. doi: 10.1089/088922201750290023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tovanabutra S, Robison V, Wongtrakul J, et al. Male viral load and heterosexual transmission of HIV-1 subtype E in northern Thailand. J Acquir Immune Defic Syndr. 2002;29(3):275–83. doi: 10.1097/00126334-200203010-00008. [DOI] [PubMed] [Google Scholar]
- 39.Modjarrad K, Chamot E, Vermund SH. Impact of small reductions in plasma HIV RNA levels on the risk of heterosexual transmission and disease progression. AIDS. 2008;22(16):2179–85. doi: 10.1097/QAD.0b013e328312c756. [DOI] [PMC free article] [PubMed] [Google Scholar]