Abstract
Background
Clinical trials in children with congenital heart disease are often limited by the absence of: 1) a primary outcome that can be observed in a reasonable period of time; 2) information regarding health-related quality of life; 3) knowledge of the correlation between health status and ventricular function and exercise performance; 4) a sufficient number of children at a single institution to provide adequate statistical power; and 5) procedural and management differences between and within institutions.
Methods
The NHLBI-funded Pediatric Heart Network designed a cross-sectional study of children 6 to 18 years, from 7 pediatric clinical centers, who had undergone a Fontan procedure as treatment for congenital heart disease. Health-related quality of life was measured by the Child Health Questionnaire and the Congenital Heart Adolescent and Teen Questionnaire. Ventricular function was assessed by maximal exercise testing, echocardiography, cardiac magnetic resonance imaging (CMRI) and B-type natriuretic peptide (BNP). The study was designed to detect a correlation of R ≥ 0.30 between health status scores and measures of ventricular function and performance in a sub-cohort with all study measures completed.
Results
A total of 1078 children were screened by chart review; 644 (60%) were eligible. The consent rate was 85% and 546 children were enrolled. Acquisition of echocardiograms, BNP, and health status was ≥ 94%; completion rates were lower for maximal exercise testing (76%) and CMRI (41%).
Conclusions
This large study provides unique information regarding the relationship between health status and clinical measures in post-Fontan patients that will facilitate the design of future randomized trials.
Randomized clinical trials are difficult to perform in children following congenital heart surgery, because few practical yet clinically-significant primary outcome variables can be identified. Discrete clinical outcomes that quantify how a patient feels or functions in daily life are poorly validated in children, and primary events are sufficiently rare to preclude a practical study. Parent-based functional measures and patient laboratory measurements could be used as surrogate endpoints in clinical trials of these patients. The use of a surrogate endpoint in a clinical trial, however, demands sufficient evidence to link changes in the surrogate outcome to a change in clinical outcome. To establish such a link, we performed a cross-sectional observational study designed to estimate the association between clinical outcome or state, measured by a validated quality of life instrument, and measures of cardiac performance or state that can serve as surrogate outcomes in future clinical trials.
Children who have had a Fontan procedure now survive into adulthood and are a good example of a population with congenital heart disease that would benefit from randomized clinical trials. (1,2). The Fontan procedure (total cavopulmonary anastomosis) is usually successful in restoring near-normal systemic oxygen saturation, and extending the lives of patients with even the most complex forms of congenital heart disease. Nevertheless, it results in an abnormal hemodynamic state and is associated with a wide variety of late complications including late ventricular dysfunction (3–6), congestive heart failure leading to a need for cardiac transplantation (7,8), decreased exercise tolerance (9–11), protein-losing enteropathy (12,13), thrombosis and embolism (14,15), and arrhythmias which often require pacemaker implantation (16).
The Fontan Cross-Sectional Study was designed to identify a quantifiable measure of cardiovascular performance that correlates with clinical outcome assessed by a validated, health-related quality of life instrument in children treated with a Fontan procedure. The information gained from this study will provide important information in its own right, as well as provide a basis for endpoint selection in subsequent randomized clinical trials in this population.
Study Design
The Fontan Cross-Sectional Study has a multi-center, cross-sectional, observational design that includes children from 6 to 18 years of age who had undergone a Fontan procedure at least 6 months prior to entry into the study. Prospective data collection for each subject occurred within a 3-month time period and included health status questionnaires completed by a parent and child (10 years and older), cardiac magnetic resonance imaging (CMRI), two-dimensional and Doppler echocardiography, resting serum B-type natriuretic peptide (BNP) and maximal exercise testing. The primary aim was to determine the association between reported health status and laboratory measures of ventricular dimensions and performance, and secondarily to determine the interrelationships among laboratory measures of ventricular size, function, and performance. For each subject, anatomic, clinical and surgical information was collected to determine if differential associations exist between clinical and laboratory measures for patient subgroups.
Inclusion Criteria
Age 6–18 years at enrollment
Fontan surgery of any type at least 6 months prior to enrollment
-
Agreement and ability to have at least the following testing completed:
An echocardiogram at the study center;
Child Health Questionnaire Parent Report (CHQ-PF50);
Serum BNP.
Planned or ongoing cardiac care at the study center allowing completion of study testing within 3 months of study enrollment
Informed consent of a parent or guardian, and assent of the study participant if able to provide it, according to institutional guidelines
Exclusion Criteria
Non-cardiac medical or psychiatric disorder that would prevent successful completion of planned study testing or would invalidate its results
Ongoing or planned participation in another research protocol that would either prevent successful completion of planned study testing or invalidate its results
Lack of reading fluency by the primary caregiver in either English or Spanish, the languages for which the parental report health status questionnaire has been validated
Pregnancy at the time of enrollment or pregnancy planned prior to completion of study testing
Recruitment Protocol
The Data Coordinating Center (DCC) provided each clinical center with a Screening Log to record basic identifying patient information (name, gender, date of birth, date of Fontan completion, medical record number) next to a set of pre-defined study identification numbers. The research coordinator at each site reviewed the clinic or hospital roster to identify all patients who underwent a Fontan procedure and who were believed to be alive and currently followed at the clinical center, and recorded each of these patients in the Screening Log. A Patient Screening Form was completed for each patient by review of the medical record without patient contact and entered into the web-based data management system at the DCC. Based on the screening data, the DCC prepared a list of potentially-eligible patients for each clinical center, defined as those who met inclusion criteria for age, date of surgery, and location of ongoing cardiac care. These lists comprised the sampling frame for the study. Study coordinators then contacted each potential participant and scheduled a visit to obtain written consent and to begin testing for each verbally consenting subject.
To assess the representativeness of the study sample, patients who were study eligible but declined participation were invited to participate as a Partial Protocol participant whose health status and basic demographics would be compared to the sample enrolled in the full protocol. This partial protocol required completion of the Child Health Questionnaire (CHQ) Parent Report only, by mail or at a clinic visit.
Statistical Considerations
To achieve a study sample that representative sample of Fontan survivors, a sampling plan that minimized selection bias was required. Initial estimates of the number of available patients suggested that the number exceeded the target sample size. Random sampling of potentially-eligible subjects at each center was planned as opposed to proceeding sequentially through the patient lists until the target number was achieved. However, the number of patients listed on center rosters and still being followed at the centers was lower than expected; thus a sampling fraction of 1.0 was utilized, as the entire pool of 831 potentially eligible patients was required to achieve target sample size.
Sample Size and Power
The target sample size was determined after consideration of several requirements. First, the study was designed to have 85% power to detect a moderate effect size - a correlation of R=0.3 between health status and clinical measures. For this calculation, the required sample size to detect a correlation of R=0.3 between health status and any of the laboratory measures with 85% power using a two-tailed test and a Type I error rate of 0.05 was 100 (rounded up from 97). Second, because the Congenital Heart Adolescent and Teenager Questionnaire (CHAT) was the only disease-specific instrument in this study, it was necessary to have sufficient power to detect correlations between CHAT scores and the laboratory parameters. Both the CHQ Child Report (CHQ-CF87) and the CHAT questionnaire require that the subject be at least ten years old in order to complete the instrument. It was estimated that 64% of the subjects enrolled in the study would be at least ten years old. Therefore, 157 subjects (100/0.64) were required in order to detect R=0.3 with 85% power and obtain 100 subjects who are at least ten years old. Third, an inflation factor to enable multivariate analysis on 157 subjects who completed all required testing (CMRI and exercise testing in particular were not feasible in many of the 6–8 year olds) was applied. Based on an initial completion rate for all the required testing midway through the study of 30%, the final target sample size was set at 525 subjects.
Outcome Measures and Rationale
The Child Health Questionnaire
The CHQ-PF50 measures the physical and psychosocial (e.g., emotional, behavioral, and social) well-being of children five to eighteen years of age (22). The Child Report (CHQ-CF87) has been validated in children 10–18 years of age and is not applicable to younger children. The CHQ-PF50 instrument provides two summary scores: 1) a measure of physical functioning (mean score and standard deviation for U.S. healthy children 53.0±8.8) and 2) a measure of psychosocial status, (mean score and standard deviation for U.S. healthy children 51.2±9.1). The CHQ User’s Manual provides age- and gender-specific normative data for 379 children in the general U.S. population as well as for six clinical samples of children, including those with chronic illnesses (e.g., asthma and epilepsy) and psychiatric problems (17). Based on these data, 5 to 10 point differences from the normative U.S. sample for either summary score (physical or psychosocial) are considered to represent true disease effects.
Congenital Heart Adolescent and Teenager Questionnaire (CHAT)
The CHAT is a disease-specific quality of life instrument completed by children 10 to 18 years of age. As such it offers the possibility of a stronger relationship with measures of disease severity than those from generic instruments, and may be more likely to be sensitive to changes in health status and provide greater discriminative validity. Further, disease-specific questionnaires often have fewer ceiling effects (where a large number of subjects attain the lowest or highest scores due to their non-healthy state) and thus show more variability in scores, allowing for greater sensitivity and responsiveness to change—a desirable feature when trying to assess the impact of different interventions on health (18).
Two-dimensional (2-D) and Doppler Echocardiography
Each subject underwent a 2-D and Doppler echocardiographic examination to evaluate ventricular diastolic function (9). The available data on diastolic function in patients after the Fontan operation are based on indices derived from Doppler samples of atrioventricular inflow, primarily E:A ratio and rate of deceleration of early inflow. These indices are highly dependent on cardiac loading conditions (19), rendering their accuracy questionable in the Fontan circulation, which is known to be characterized by increased systemic vascular resistance and reduced preload. Furthermore, enhanced chamber compliance, which is characteristic of volume-overloaded ventricles, is not distinguishable from impaired relaxation using this methodology (20). More recently, several promising alternative approaches have been reported (19) that may overcome these limitations, including duration of pulmonary vein flow reversal during atrial systole assessed using pulsed Doppler, atrioventricular valve annulus velocity in early diastole assessed using tissue Doppler, and the rate of ventricular flow propagation using M-mode color Doppler. Image acquisition was performed at the 7 centers according to a common technical protocol. No measurements were done at the time of image acquisition. Echocardiograms were interpreted by a Core Echocardiography Laboratory to minimize bias and inter-observer error. Measurements included valve gradients, vena contracta of regurgitant jets, ventricular volume and ejection fraction using a biplane Simpson’s algorithm, atrioventricular valve inflow Doppler analysis (peak early velocity, peak atrial velocity, early deceleration velocity, and a-wave duration), duration of pulmonary vein flow reversal, tissue Doppler parameters (peak systolic velocity, peak early and late diastolic velocities), and ventricular flow propagation velocity.
Cardiac Magnetic Resonance Imaging
MRI is a highly accurate and a reproducible technique for measurements of ventricular volumes and mass in a variety of disease states, including in those with a complex chamber geometry, such as patients who have undergone a Fontan procedure (21,22). The decreased interstudy variability of CMRI measurements compared with that of echocardiographic measurements may reduce the number of subjects required to achieve a desired level of statistical power if CMRI measures are used as study endpoints.
In this study, CMRI was used to measure: end-diastolic, end-systolic, and stroke volumes, ejection fraction, mass, and mass-to-end-diastolic volume. Secondary variables included localized flow measurements in the superior and inferior vena cavae, branch pulmonary arteries, and the atrioventricular valve(s). The caval flow measures, when summed, estimate systemic flow and provide validation for flow measurement in the ascending aorta. The CMRI data were acquired in each of the 7 participating centers using a 1.5 Tesla MRI scanner, archived on removable storage media and interpreted at a Core CMRI Laboratory.
Exercise Testing
Maximal exercise testing is attractive as an outcome measure because the exercise test itself approximates activities of daily living experienced by a child. Previous studies of exercise performance in Fontan patients show that maximal exercise performance is abnormal and limited by an inability to increase cardiac stroke volume appropriately (9,10). The purpose of including exercise testing in this study was to determine if a correlation exists between functional health status and maximal oxygen consumption, oxygen pulse, anaerobic threshold, chronotropic incompetence, and exercise-related arterial oxygen desaturation.
Subjects were exercised to maximum volition using an electronically braked cycle ergometer. The work rate was increased using a ramp protocol with a slope chosen to achieve the subject’s predicted maximal work rate in 10 to 12 minutes of cycling time. Pulse oximetry, heart rate, blood pressure and VO2 (ml/kg/min) and VCO2 were monitored prior, during and after exercise. This protocol was chosen because it provides direct measurement of work (watts) and it allows the outcome measures maximum VO2, ventilatory anaerobic threshold (VAT), and oxygen pulse to be derived. Sinus node dysfunction was assessed by rhythm on the supine resting electrocardiogram (ECG), baseline and maximum heart rate during exercise, the cardiac rhythm present at the time of the echocardiogram, and the indication for pacing, where applicable.
B-Type Natriuretic Peptide (BNP)
BNP is a neurohormone primarily secreted by the ventricular myocardium in response to volume expansion or pressure overload. Early studies demonstrated a correlation of the plasma BNP level with ventricular dilation in patients with dilated cardiomyopathy (23). Studies in adult patients found plasma BNP to be an accurate predictor of the presence of congestive heart failure (24,25) as well as providing predictive information for use in risk stratification in patients with acute coronary syndromes (26) and chronic heart failure (27). Few studies have been performed measuring plasma BNP concentrations in children with congenital heart disease (28–30). BNP was included in this study to determine if this potential predictor of heart failure in children was correlated with health-related quality of life.
Screening and Test Completion
A total of 1078 children were screened by chart review, and 831 (77%) were potentially eligible. Potentially-eligible children were contacted, and 644 (60%) were fully eligible. The consent rate was 85% of those fully eligible and 546 children were enrolled at age 11.9±3.4 yr with median age at Fontan of 2.8 yr (Table I). Acquisition rates for echocardiograms, BNP, and health status were ≥ 94%; completion rates were lower for maximal exercise testing (76%) and cardiac MRI (41%) (Figure 1). The conduct of this study demonstrated several interesting phenomena that were not expected, and should be considered when planning studies in similar populations and/or with similar study tests: 1) the number of patients who were fully eligible was lower than expected due to the high rate of referrals or migration from some clinical centers; 2) the consent rate for this observational, relatively short-term study (in many cases a single day) was very high (85% actual compared with 70% expected); and 3) the rate of completion for maximal exercise testing and CMRI, even in a sample at least 6 years of age, was lower than expected.
Table I.
Demographic, anatomic, and surgery characteristics of the Pediatric Heart Network Fontan Cross-Sectional Study sample (N=546)
| Number | ||
|---|---|---|
| Age at enrollment (years) | 11.9+3.4 | 546 |
| Male | 60.5% | 330 |
| Race | ||
| White | 85.0% | 464 |
| African American | 11.1% | 61 |
| Asian | 2.8% | 15 |
| Other | 1.1% | 6 |
| Hispanic ethnicity | 4.2% | 23 |
| Ventricular Dominance | ||
| Left Ventricle | 51.8% | 283 |
| Right Ventricle | 41.0% | 224 |
| Neither | 7.0% | 38 |
| Cardiac Diagnosis | ||
| Tricuspid Atresia | 21.9% | 120 |
| Hypoplastic Left Heart Syndrome | 20.6% | 112 |
| Double Inlet Left Ventricle | 15.1% | 82 |
| Heterotaxia | 7.7% | 42 |
| Double Outlet Right Ventricle | 7.5% | 41 |
| Pulmonary Atresia with Intact Ventricular | ||
| Septum | 6.0% | 33 |
| Mitral Atresia | 5.7% | 31 |
| Abnormal Tricuspid Valve | 4.0% | 22 |
| Atrio-Ventricular Canal Defect | 3.9% | 21 |
| Other | 7.0% | 38 |
| Fontan type | ||
| Intra-Cardiac Lateral Tunnel | 60.1% | 328 |
| Extra-Cardiac Lateral Tunnel | 12.7% | 69 |
| Atrio-Pulmonary Connection | 13.1% | 72 |
| Extra-Cardiac Conduit | 12.3% | 67 |
| Atrio-Ventricular Connection | 1.1% | 6 |
| Other | 0.7% | 4 |
Figure 1. Fontan Cross-Sectional Study Flow and Patient enrollment.
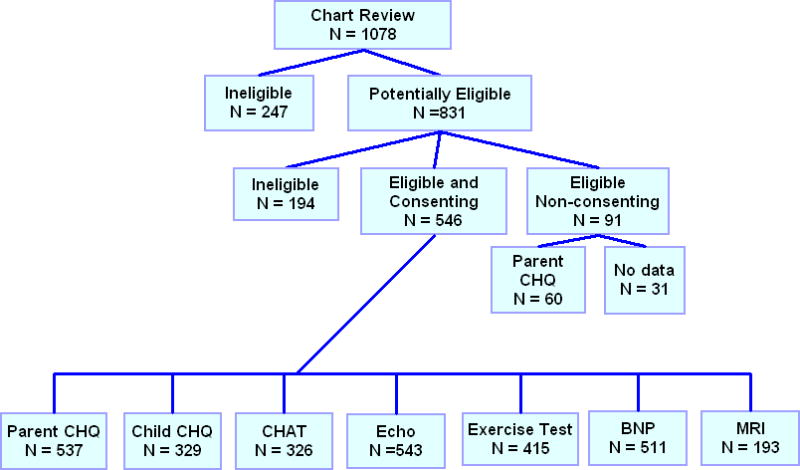
Abbreviations in figure:
CHQ = Child Health Questionnaire
CHAT = Congenital Heart Adolescent and Teenager questionnaire
Echo = Echocardiogram
BNP = B-Type Natriuretic Peptide
MRI = Magnetic Resonance Imaging (cardiac)
Discussion
The Fontan procedure is now commonly employed in the treatment of children with complex congenital heart disease and single ventricle physiology. Based on the incidence of congenital heart disease with single ventricle physiology, approximately 1000–1500 children undergo a Fontan procedure yearly in North America and the application of staged palliation in the treatment of these children can represent up to 30% of all congenital heart surgeries at large pediatric cardiac institutions (31,32).
The current long-term outcome in children with a Fontan procedure is unknown but worrisome because of the multiple late morbidities observed. Some of these complications, such as protein-losing enteropathy, seem unique to children following the Fontan procedure and as yet are poorly understood. Others appear possibly related to the time elapsed since the Fontan procedure was completed. Finally, since the function of the Fontan circuit depends critically on the maintenance of ventricular diastolic compliance, the expected normal decrease in diastolic compliance (increased stiffness) with advancing age is particularly threatening to the well-being of these patients. Ideally, interventions that could offer the possibility of maintaining cardiac function and decreasing the incidence of late morbidity, such as anticipatory pharmacologic management early in the course of the Fontan, could be identified and tested.
Design of appropriate clinical trials for this population is severely hindered by the lack of adequate primary outcome measures and the small number of children available for study at a single institution. The purpose of this study design was to address these two issues by quantifying the association between validated measures of health-related quality of life and measures of ventricular performance. If a significant correlation is found between ventricular function and functional status, it will suggest that both types of measures are suitable as endpoints in clinical trials; more specifically, that changes in ventricular function reflect a corresponding change in the patient’s functional status, and vice versa. If no clinically-significant correlation is identified between ventricular function and functional status, then these endpoints, while providing test-specific standards for a future study, may not be valid surrogates.
In addition to the primary aim of the study, the design will provide two necessary statistical components in preparing for future clinical trials. First, it will provide information regarding standard errors of measure and the distribution of variables compared to normal values for each of the outcomes, thus providing accurate power calculations for the design of future trials. Second, because all measures of performance for a given subject had to be gathered within a 3-month period, meaningful inter-correlations among outcome measures can be estimated, providing information for the design of a future trial with a composite outcome as its primary endpoint.
While not a primary goal of this study, the study design also allowed us to examine present pharmacological treatment of Fontan patients in 7 centers in Canada and the United States. Assessment of the type and frequency of therapies currently in use will help develop hypotheses to be tested in future randomized trials.
Study Limitations
This study has three significant limitations. First, the study was limited to subjects followed at each study center and did not include all children undergoing surgery at the 7 centers in a specified time period. Further, we did not attempt to abstract data from the records of all children who had undergone a Fontan at each center, and thus cannot compare the enrolled population to those children who died after undergoing the Fontan procedure. Similarly, children in the poorest of health may have been less likely to participate. Therefore, the incidence of post-Fontan medical events and complications such as protein-losing enteropathy, stroke, and ventricular dysfunction found in this study are possibly underestimates for the Fontan population as a whole.
A second limitation is the observational cross-sectional design itself, which does not allow for the testing of hypotheses focused on the efficacy of current treatments in children following the Fontan procedure. It should be noted that this was not a goal of the study. The study will, however, generate a significant data set that can be examined for potential relationships between medical history, ventricular performance, and health related quality of life, which can be used to inform hypotheses for future clinical trials.
Third, in a cross-sectional study, data collection is limited to a single time point. Therefore, changes over time cannot be assessed, and estimates of how quickly study measures might respond to therapeutic intervention cannot be provided. This limitation is particularly relevant to research on children after any cardiac surgical procedure, but especially relevant in children following the Fontan procedure, since the limited longitudinal data available suggest a deterioration of cardiac function over time. The important questions related to the relative impact of time since Fontan versus the type of Fontan (which has varied over the last two decades) versus age on ventricular function can only be answered by a longitudinal study. This study, however, lays the logistic and scientific groundwork for implementation of a longitudinal study, and guides the selection of appropriate outcome measures for future clinical trials as well as natural history studies of children with a Fontan procedure.
Acknowledgments
This work was supported by grants from the National Heart, Lung, and Blood Institute, NIH/DHHS, Bethesda, MD, #U01 HL68270 (Sleeper, Geva, Colan), U01 HL068269 (Anderson), U01 HL068292 (Williams), U01 HL068290 (Hsu), U01 HL068288 (McCrindle), U01 HL068285 (Roth), U01 HL068281 (Saul), and U01 HL068279 (Clark)
APPENDIX
National Heart, Lung, and Blood Institute
Gail Pearson, Tracey Hoke, Carl Hunt, Mario Stylianou, Judith Massicot-Fisher, Marsha Mathis, Victoria Pemberton
Data Coordinating Center
New England Research Institutes, Lynn Sleeper, Steven Colan, Paul Mitchell, Gloria Klein, Dianne Gallagher, Patty Connell, Lisa Wruck
Network Chair
Lynn Mahony, University of Texas Southwestern Medical Center
Clinical Site Investigators
Children’s Hospital Boston, Jane Newburger (PI), Stephen Roth, Roger Breitbart, Renee Margossian, Andrew Powell, Jonathan Rhodes, Jodi Elder, Ellen McGrath; Children’s Hospital of New York, Welton M. Gersony (PI), Seema Mital, Beth Printz, Ashwin Prakash, Darlene Servedio; Children’s Hospital of Philadelphia, Victoria Vetter (PI), Bernard J. Clark, Mark Fogel, Steven Paridon, Jack Rychik, Margaret Harkins, Jamie Koh; Duke University, Page A. W. Anderson (PI), Rene Herlong, Lynne Hurwitz, Jennifer S. Li, Ann Marie Nawrocki; Medical University of South Carolina, J. Philip Saul (PI), Andrew M. Atz, Andrew D. Blaufox, Girish Shirali, Jon Lucas, Amy Blevins; Primary Children’s Medical Center, Salt Lake City, Utah, LuAnn Minich (PI), Richard Williams, Linda Lambert, Michael Puchalski; Hospital for Sick Children, Toronto, Brian McCrindle (PI), Timothy Bradley, Kevin Roman, Jennifer Russell, Shi-Joon Yoo, Elizabeth Radojewski, Nancy Slater
Core Laboratories
Cardiac MRI, Children’s Hospital Boston: Tal Geva; Andrew J. Powell
Echocardiography, Children’s Hospital Boston: Steven Colan (Director), Marcy Schwartz, Renee Margossian
Protocol Review Committee
Michael Artman, Chair; Judith Massicot-Fisher, Executive Secretary; Erle Austin, Daniel Bernstein, Timothy Feltes, Julie Johnson, Jeffrey Krischer, G. Paul Matherne, Anne Murphy, ad hoc, Anne Rowley, ad hoc.
Data and Safety Monitoring Board
John Kugler, Chair; Tracey R. Hoke, Executive Secretary; Kathryn Davis, David J. Driscoll, Mark Galantowicz, Sally A. Hunsberger, Thomas J. Knight, Catherine L. Webb, Lawrence Wissow.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marino BS. Outcomes after the Fontan procedure. Curr Opin Pediatr. 2002;14(5):620–626. doi: 10.1097/00008480-200210000-00010. [DOI] [PubMed] [Google Scholar]
- 2.van den Bosch AE, Roos-Hesselink JW, Van Domburg R, et al. Long-term outcome and quality of life in adult patients after the Fontan operation. Am J Cardiol. 2004;93(9):1141–1145. doi: 10.1016/j.amjcard.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 3.Penny DJ, Rigby ML, Redington A. Abnormal patterns of intraventricular flow and diastolic filling after the Fontan operation: evidence of incoordinate ventricular wall motion. Br Heart J. 1991;66:375–378. doi: 10.1136/hrt.66.5.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fogel M, Weinber P, Chin A, Fellows K, et al. Late ventricular geometry and performance changes of functional single ventricle throughout staged Fontan reconstruction assessed by magnetic resonance imaging. J Am Coll Cardiol. 1996;28:212–221. doi: 10.1016/0735-1097(96)00111-8. [DOI] [PubMed] [Google Scholar]
- 5.Fogel MA. Assessment of cardiac function by MRI. Pediatr Cardiol. 2000;21:59–69. doi: 10.1007/s002469910008. [DOI] [PubMed] [Google Scholar]
- 6.Eicken A, Fratz S, Gutfried C, Balling G, et al. Hearts late after Fontan operation have normal mass, normal volume, and reduced systolic function: a magnetic resonance imaging study. J Am Coll Cardiol. 2003;42(6):1061–1065. doi: 10.1016/s0735-1097(03)00986-0. [DOI] [PubMed] [Google Scholar]
- 7.Gamba A, Merlo M, Fiocchi R, Terzi A, et al. Heart transplantation in patients with previous Fontan operations. J Thorac Cardiovasc Surg. 2004;127(2):555–56. doi: 10.1016/j.jtcvs.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Michielon G, Parisi F, Di Carlo D, et al. Orthotopic heart transplantation for failing single ventricle physiology. Eur J Cardiothorac Surg. 2003;24(4):502–510. doi: 10.1016/s1010-7940(03)00342-7. [DOI] [PubMed] [Google Scholar]
- 9.Driscoll D, Danielson G, Puga F, et al. Exercise tolerance and cardiorespiratory response to exercise after the Fontan operation for tricuspid atresia or functional single ventricle. J Am Coll Cardiol. 1986;7:1087–1094. doi: 10.1016/s0735-1097(86)80227-3. [DOI] [PubMed] [Google Scholar]
- 10.Gewillig M, Lundstrom U, Bull C, et al. Exercise responses in patients with congenital heart disease after Fontan repair: Patterns and determinants of Performance. J Am Coll Cardiol. 1990;15:1424–1432. doi: 10.1016/s0735-1097(10)80034-8. [DOI] [PubMed] [Google Scholar]
- 11.Reybrouck T, Rogers R, Weymans M, et al. Serial cardiorespiratory exercise testing in patients with congenital heart disease. Eur J Pediatr. 1995;154:801–806. doi: 10.1007/BF01959785. [DOI] [PubMed] [Google Scholar]
- 12.Feldt R, Driscoll D, Offord K, et al. Protein-losing enteropathy after the Fontan operation. J Thorac Cardiovasc Surg. 1996;112:672–680. doi: 10.1016/S0022-5223(96)70051-X. [DOI] [PubMed] [Google Scholar]
- 13.Mertens L, Hagler DJ, Sauer U, et al. Protein-losing enteropathy after the Fontan operation: an international multicenter study. PLE study group. J Thorac Cardiovasc Surg. 1998;115(5):1063–1073. doi: 10.1016/s0022-5223(98)70406-4. [DOI] [PubMed] [Google Scholar]
- 14.Kaulitz R, Ziemer G, Bergmann F, et al. Atrial thrombus after the Fontan operation: predisposing factors, treatment and prophylaxis. Cardiol Young. 1997;7:37–43. [Google Scholar]
- 15.Monagle P, Karl TR. Thromboembolic problems after the Fontan operation. Semin Thorac Cardiovasc Surg Pediatr Card Surg Ann. 2002;5:36–47. doi: 10.1053/pcsu.2002.29716. [DOI] [PubMed] [Google Scholar]
- 16.Weipert J, Noebauer C, Schreiber C, et al. Occurrence and management of atrial arrhythmia after long-term Fontan circulation. J Thorac Cardiovasc Surg. 2004;127(2):457–464. doi: 10.1016/j.jtcvs.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 17.Landgraf JM, Abetz L, Ware JE. The CHQ User’s Manual, Second Printing. Boston, MA: Health Act; 1999. [Google Scholar]
- 18.Kendall L, Lewin RJ, Parsons JM, et al. Factors associated with self-perceived state of health in adolescents with congenital cardiac disease attending paediatric cardiologic clinics. Cardiol Young. 2001;11:431–438. doi: 10.1017/s1047951101000555. [DOI] [PubMed] [Google Scholar]
- 19.Zile MR, Brutsaert DL. New concepts in diastolic dysfunction and diastolic heart failure: Part I: diagnosis, prognosis, and measurements of diastolic function. Circulation. 2002;105:1387–1393. doi: 10.1161/hc1102.105289. [DOI] [PubMed] [Google Scholar]
- 20.Milanesi O, Stellin G, Colan SD, et al. Systolic and diastolic performance late after the Fontan procedure for a single ventricle and comparison of those undergoing operation at <12 months of age and at >12 months of age. Am J Cardiol. 2002:276–280. doi: 10.1016/s0002-9149(01)02227-5. [DOI] [PubMed] [Google Scholar]
- 21.Germain P, Roul G, Kastler B, et al. Inter-study variability in left ventricular mass measurement. Comparison between M-mode echography and MRI. Eur Heart J. 1992;13(8):1011–1019. doi: 10.1093/oxfordjournals.eurheartj.a060307. [DOI] [PubMed] [Google Scholar]
- 22.Bellenger NG, Davies LC, Frances JM, et al. Reduction in sample size for studies of remodeling in heart failure by the use of cardiovascular magnetic resonance. J Cardiovasc Mag Res. 2000;2:271–278. doi: 10.3109/10976640009148691. [DOI] [PubMed] [Google Scholar]
- 23.Yoshimura M, Yasue H, Okumura K, et al. Different secretion patterns of atrial and natriuretic peptide and brain natriuretic peptide in patients with congestive heart failure. Circulation. 1993;87:464–469. doi: 10.1161/01.cir.87.2.464. [DOI] [PubMed] [Google Scholar]
- 24.Dao Q, Krishnaswamy P, Kazanegra R, et al. Utility of brain natiuretic peptide in the diagnosis of congestive heart failure in an urgent-care setting. J Am Coll Cardiol. 2001;37:379–385. doi: 10.1016/s0735-1097(00)01156-6. [DOI] [PubMed] [Google Scholar]
- 25.Morrison LK, Harrison A, Krishnaswamy P, et al. Utility of a rapid B-Natriuretic peptide assay in differentiating congestive heart failure from lung disease in patients presenting with dyspnea. J Am Coll Cardiol. 2002;39:202–209. doi: 10.1016/s0735-1097(01)01744-2. [DOI] [PubMed] [Google Scholar]
- 26.Lemos J, Morrow D, Bentley J, et al. The prognostic value of brain natriuretic peptide in patients with acute coronary syndromes. N Engl J Med. 2001;345:1014–1021. doi: 10.1056/NEJMoa011053. [DOI] [PubMed] [Google Scholar]
- 27.Tsutamoto T, Wada A, Maeda K, et al. Attenuation of compensation of endogenous cardiac natriuretic peptide system in chronic heart failure: prognostic role of plasma brain natriuretic peptide concentration in patients with chronic symptomatic left ventricular dysfunction. Circulation. 1997;96:509–516. doi: 10.1161/01.cir.96.2.509. [DOI] [PubMed] [Google Scholar]
- 28.Ationu A, Singer D, Smiath A, et al. Studies of cardiopulmonary bypass in children: implications for the regulation of brain natriuretic peptic. Cardiovasc Res. 1993;27:1538–1541. doi: 10.1093/cvr/27.8.1538. [DOI] [PubMed] [Google Scholar]
- 29.Yoshimura N, Yamaguchi M, Oshima Y, et al. Suppression of the secretion of atrial and brain natriuretic peptide after total cavopulmonary connection. J Thorac Cardiovasc Surg. 2000;120:764–769. doi: 10.1067/mtc.2000.108595. [DOI] [PubMed] [Google Scholar]
- 30.Hjortdal V, Stenbog E, Ravn H, et al. Neurohormonal activation late after cavopulmonary connection. Heart. 2000;83:439–443. doi: 10.1136/heart.83.4.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Steinberger EK, Ferencz C, Loffredo CA. Infants with single ventricle: A population-based epidemiological study. Teratology. 2002;65:106–115. doi: 10.1002/tera.10017. [DOI] [PubMed] [Google Scholar]
- 32.Fyler DC, Buckley LP, Hellenbrand W. Report of the New England Regional Infant Cardiac Program. Pediatrics. 1980;65:376–461. [Google Scholar]


